Rapid Prediction of Nutrient Concentration in Citrus Leaves Using Vis-NIR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Spectral Acquisition
2.3. Foliar Ionomic Analysis
2.4. Chemometric Analysis
3. Results and Discussion
3.1. Descriptive Statistics of the Foliar Macro and Micronutrient Concentrations
3.2. PLS-R Models for Macro and Micronutrients Estimation
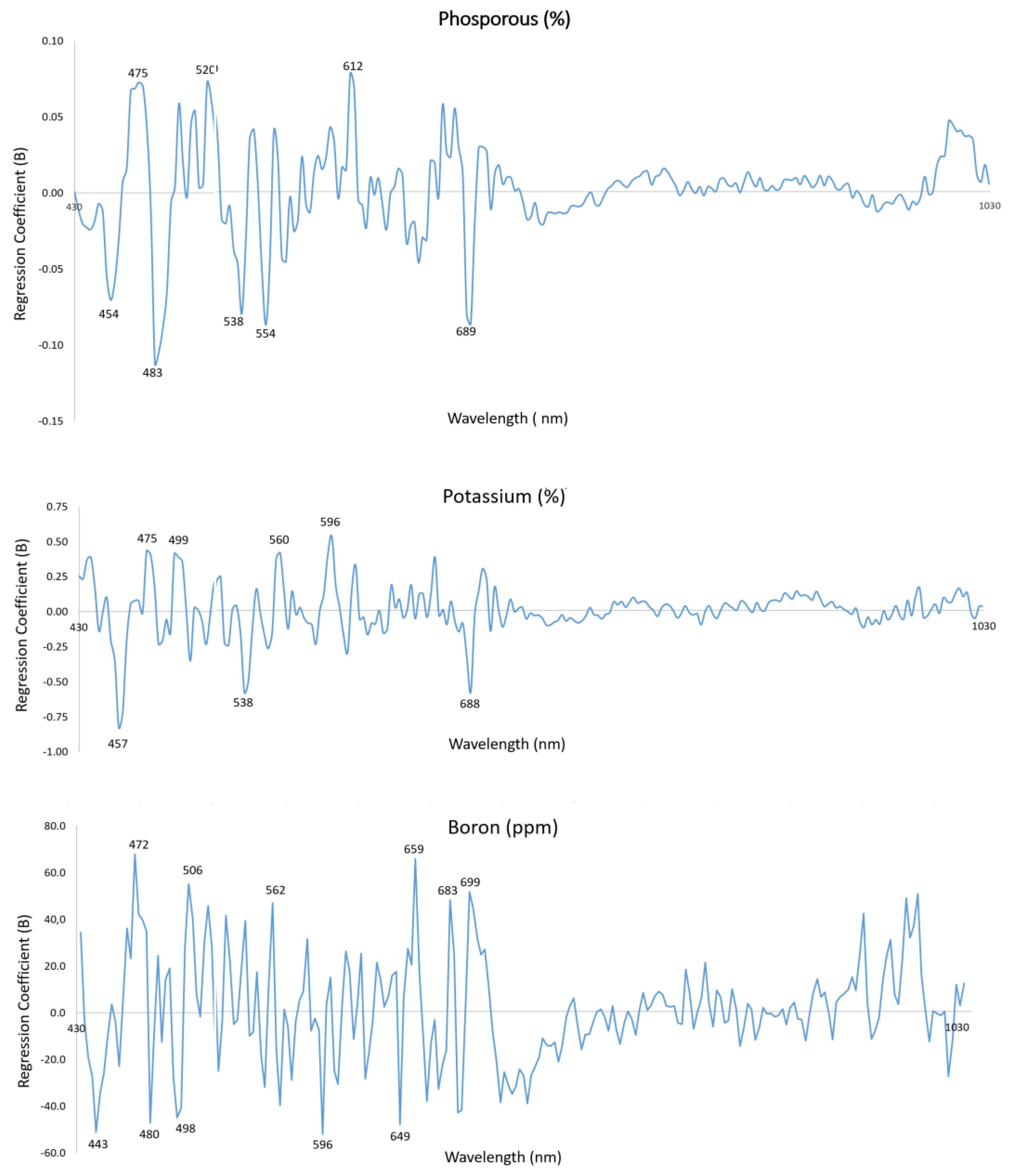
3.3. Evaluation of Relevant Wavelengths for Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Artificial neural networks (ANN) | Molybdenum (Mo) |
| Boron (B) | Multiple linear regression (MLR) |
| Calcium (Ca) | Near infrared spectroscopy (NIRS) |
| Carbon (C) | Nitrogen (N) |
| Carbon dioxide (CO2) | Nickel (Ni) |
| Chlorine (Cl) | Oxygen (O) |
| Chlorophyll a (Chl-a) | Partial least squares (PLS) |
| Chlorophyll b (Chl-b) | PLS-regression (PLS-R) |
| Copper (Cu) | Phosphorus (P) |
| Cross-validation (CV) | Principal component regression (PCR) |
| Emission spectrometry (ICP-OES) | Random forest (RF) |
| First derivative (1D) | Root mean square error (RMSE) |
| Fourier Transform NIR (FT-NIR) | Standard normal variate (SNV) |
| Hydrogen (H) | Sulphur (S) |
| Iron (Fe) | Support vector machine (SVM) |
| Latent variables (LV) | Variable importance in projection (VIP) |
| Magnesium (Mg) | Visible (Vis) |
| Manganese (Mn) | Weight regression coefficients (BW) |
| Mean centre (MC) | Zinc (Zn) |
References
- Food and Agriculture Organization (FAO). Citrus Fruit. Fresh and Processed Statistical Bulletin 2020. Market and Trade Commodities. Available online: https://www.fao.org/markets-and-trade/commodities/citrus/en (accessed on 7 July 2023).
- United States Department of Agriculture (USDA). Citrus: World Market and Trade. Office of Global Analysis. Foreign Agriculture Service. Available online: https://www.fas.usda.gov/data/citrus-world-markets-and-trade (accessed on 7 July 2023).
- United States Department of Agriculture (USDA). Citrus Annual. Foreign Agricultural Service. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Citrus%20Annual_Madrid_European%20Union_E42023-0001.pdf (accessed on 7 July 2023).
- Marschner, P. Rhizosphere Biology. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 369–388. [Google Scholar]
- Millard, P. Ecophysiology of the Internal Cycling of Nitrogen for Tree Growth. Z. Pflanzenernahr. Bodenkd. 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen Supply Influences Photosynthesis Establishment along the Sugarcane Leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, R.J.; Dise, N.B.; Field, C.D.; Dore, A.J.; Caporn, S.J.M.; Stevens, C.J. Nitrogen Deposition and Plant Biodiversity: Past, Present, and Future. Front. Ecol. Environ. 2017, 15, 431–436. [Google Scholar] [CrossRef]
- Quinones, A.; Martínez-Alcántara, B.; Primo-Millo, E.; Legaz, F. Abonado de Los Cítricos. In Guía Práctica de la Fertilización Racional de los Cultivos en España; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2010; pp. 193–204. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O.; Hoover, M.R. Analysis of Forages by Infrared Reflectance. J. Dairy Sci. 1979, 62, 807–812. [Google Scholar] [CrossRef]
- Mcvicar, T.R.; Briggs, P.R.; King, E.A.; Raupach, M.R. A Review of Predictive Modelling from a Natural Resource Management Perspective: The Role of Remote Sensing of the Terrestrial Environment By CSIRO; CSIRO Earth Observation Centre: Canberra, Australia, 2003. [Google Scholar]
- Carter, G.A. Reflectance Wavebands and Indices for Remote Estimation of Photosynthesis and Stomatal Conductance in Pine Canopies. Remote Sens. Environ. 1998, 63, 61–72. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘Point’ Spectroscopy in Postharvest Fruit and Vegetable Assessment: The Science behind Three Decades of Commercial Use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Guo, T.; Tan, C.; Li, Q.; Cui, G.; Li, H. Estimating Leaf Chlorophyll Content in Tobacco Based on Various Canopy Hyperspectral Parameters. J. Ambient. Intell. Humaniz. Comput. 2019, 10, 3239–3247. [Google Scholar] [CrossRef]
- Ling, B.; Goodin, D.G.; Raynor, E.J.; Joern, A. Hyperspectral Analysis of Leaf Pigments and Nutritional Elements in Tallgrass Prairie Vegetation. Front. Plant Sci. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Nanni, M.R.; Cezar, E.; dos Santos, G.L.A.A.; Reis, A.S.; de Oliveira, K.M.; de Oliveira, R.B. Vis–NIR Spectroscopy: From Leaf Dry Mass Production Estimate to the Prediction of Macro- and Micronutrients in Soybean Crops. J. Appl. Remote Sens. 2020, 14, 044505. [Google Scholar] [CrossRef]
- Dos Santos, G.L.A.A.; Reis, A.S.; Besen, M.R.; Furlanetto, R.H.; Rodrigues, M.; Crusiol, L.G.T.; de Oliveira, K.M.; Falcioni, R.; de Oliveira, R.B.; Batista, M.A.; et al. Spectral Method for Macro and Micronutrient Prediction in Soybean Leaves Using Interval Partial Least Squares Regression. Eur. J. Agron. 2023, 143, 126717. [Google Scholar] [CrossRef]
- Menesatti, P.; Pallottino, F.; Antonucci, F.; Roccuzzo, G.; Intrigliolo, F.; Costa, C. Non-Destructive Proximal Sensing for Early Detection of Citrus Nutrient and Water Stress. In Advances in Citrus Nutrition; Springer: Dordrecht, The Netherlands, 2012; pp. 113–123. [Google Scholar] [CrossRef]
- Galvez-Sola, L.; García-Sánchez, F.; Pérez-Pérez, J.G.; Gimeno, V.; Navarro, J.M.; Moral, R.; Martínez-Nicolás, J.J.; Nieves, M. Rapid Estimation of Nutritional Elements on Citrus Leaves by near Infrared Reflectance Spectroscopy. Front. Plant Sci. 2015, 6, 571. [Google Scholar] [CrossRef] [Green Version]
- Phanomsophon, T.; Jaisue, N.; Tawinteung, N.; Khurnpoon, L.; Sirisomboon, P. Classification of N, P, and K Concentrations in Durian (Durio Zibethinus Murray CV. Mon Thong) Leaves Using near-Infrared Spectroscopy. Eng. Appl. Sci. Res. 2022, 49, 127–132. [Google Scholar]
- Cuq, S.; Lemetter, V.; Kleiber, D.; Levasseur-Garcia, C. Assessing Macro- (P, K, Ca, Mg) and Micronutrient (Mn, Fe, Cu, Zn, B) Concentration in Vine Leaves and Grape Berries of Vitis Vinifera by Using near-Infrared Spectroscopy and Chemometrics. Comput. Electron. Agric. 2020, 179, 105841. [Google Scholar] [CrossRef]
- Ayala-Silva, T.; Beyl, C.A. Changes in Spectral Reflectance of Wheat Leaves in Response to Specific Macronutrient Deficiency. Adv. Space Res. 2005, 35, 305–317. [Google Scholar] [CrossRef]
- Johnson, J.M.; Sila, A.; Senthilkumar, K.; Shepherd, K.D.; Saito, K. Application of Infrared Spectroscopy for Estimation of Concentrations of Macro- and Micronutrients in Rice in Sub-Saharan Africa. Field Crops Res. 2021, 270, 108222. [Google Scholar] [CrossRef]
- Yarce, C.J.; Rojas, G. Near Infrared Spectroscopy for the Analysis of Macro and Micro Nutrients in Sugarcane Leaves. Zuckerindustrie 2012, 137, 707–710. [Google Scholar] [CrossRef]
- Chen, M.; Glaz, B.; Gilbert, R.A.; Daroub, S.H.; Barton, F.E.; Wan, Y. Near-Infrared Reflectance Spectroscopy Analysis of Phosphorus in Sugarcane Leaves. Agron. J. 2002, 94, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Acosta, M.; Visconti, F.; Quiñones, A.; Blasco, J.; de Paz, J.M. Estimation of Macro and Micronutrients in Persimmon (Diospyros Kaki L.) cv. ‘Rojo Brillante’ Leaves through Vis-NIR Reflectance Spectroscopy. Agronomy 2023, 13, 1105. [Google Scholar] [CrossRef]
- Acosta, M.; Rodríguez-Carretero, I.; Blasco, J.; de Paz, J.M.; Quiñones, A. Non-Destructive Appraisal of Macro- and Micronutrients in Persimmon Leaves Using Vis/NIR Hyperspectral Imaging. Agriculture 2023, 13, 916. [Google Scholar] [CrossRef]
- Bodenkunde, I. Inorganic Forms of Nitrogen in Soil. Nitrogen Agric. Soils 1982, 13, 43–66. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Elsevier: Cambridge, MA, USA, 1985; pp. 274–282. [Google Scholar]
- Ulissi, V.; Antonucci, F.; Benincasa, P.; Farneselli, M.; Tosti, G.; Guiducci, M.; Tei, F.; Costa, C.; Pallottino, F.; Pari, L.; et al. Nitrogen Concentration Estimation in Tomato Leaves by VIS-NIR Non-Destructive Spectroscopy. Sensors 2011, 11, 6411–6424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Alchanatis, V.; Schmilovitch, Z.; Meron, M. In-Field Assessment of Single Leaf Nitrogen Status by Spectral Reflectance Measurements. Precis. Agric. 2005, 6, 25–39. [Google Scholar] [CrossRef]
- Furlanetto, R.H.; Moriwaki, T.; Falcioni, R.; Pattaro, M.; Vollmann, A.; Sturion Junior, A.C.; Antunes, W.C.; Nanni, M.R. Hyperspectral Reflectance Imaging to Classify Lettuce Varieties by Optimum Selected Wavelengths and Linear Discriminant Analysis. Remote Sens. Appl. Soc. Environ. 2020, 20, 100400. [Google Scholar] [CrossRef]
- Lindgren, F.; Geladi, P.; Wold, S. The Kernel Algorithm for PLS. J. Chemom. 1993, 7, 45–59. [Google Scholar] [CrossRef]
- Dimov, I.; Georgieva, R.; Todorov, V. Balancing of Systematic and Stochastic Errors in Monte Carlo. In Algorithms for Integral Equations; Springer: Cham, Switzerland, 2015; pp. 44–51. [Google Scholar] [CrossRef]
- Todorov, V.; Dimov, I. Efficient Stochastic Approaches for Multidimensional Integrals in Bayesian Statistics; Springer: Cham, Switzerland, 2020; pp. 454–462. [Google Scholar] [CrossRef]
- Cawley, G.C.; Talbot, N.L.C. Efficient Leave-One-out Cross-Validation of Kernel Fisher Discriminant Classifiers. Pattern Recognit. 2003, 36, 2585–2592. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A Review of Variable Selection Methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Frenich, A.G.; Jouan-Rimbaud, D.; Massart, D.L.; Kuttatharmmakul, S.; Galera, M.M.; Vidal, J.L.M. Wavelength Selection Method for Multicomponent Spectrophotometric Determinations Using Partial Least Squares. Analyst 1995, 120, 2787. [Google Scholar] [CrossRef]
- Osco, L.P.; Ramos, A.P.M.; Pinheiro, M.M.F.; Moriya, É.A.S.; Imai, N.N.; Estrabis, N.; Ianczyk, F.; de Araújo, F.F.; Liesenberg, V.; de Castro Jorge, L.A.; et al. A Machine Learning Framework to Predict Nutrient Content in Valencia-Orange Leaf Hyperspectral Measurements. Remote Sens. 2020, 12, 906. [Google Scholar] [CrossRef] [Green Version]
- Gates, D. Energy, Plants and Ecology. Ecology 1965, 46, 1–13. [Google Scholar] [CrossRef]
- Jacquemoud, S.U.S. Modeling Leaf Optical Properties. Photobiological. Photobiological Sciences Online. Environmental Photobiology. Available online: http://www.photobiology.info/#environ (accessed on 27 November 2018).
- Sonobe, R.; Sano, T.; Horie, H. Using Spectral Reflectance to Estimate Leaf Chlorophyll Content of Tea with Shading Treatments. Biosyst. Eng. 2018, 175, 168–182. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.M.; Iii, W.W.A. In Vivo Functions of Carotenoids in Higher Plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Azadnia, R.; Rajabipour, A.; Jamshidi, B.; Omid, M. New Approach for Rapid Estimation of Leaf Nitrogen, Phosphorus, and Potassium Contents in Apple-Trees Using Vis/NIR Spectroscopy Based on Wavelength Selection Coupled with Machine Learning. Comput. Electron. Agric. 2023, 207, 107746. [Google Scholar] [CrossRef]

| Annual Dose Chemical Compound (kg/ha) | |
|---|---|
| N | 240 |
| P2O5 | 80 |
| K2O | 140 |
| MgO | 180 |
| Fe | 1 |
| N | P | K | Ca | Mg | Na | S | Fe | Cu | Mn | Zn | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| November | 1.94 | 0.11 | 0.83 | 5.65 | 0.52 | 0.03 | 0.33 | 83.13 | 3.03 | 25.1 | 26.7 | 46.61 |
| Mean | 2.10 (D) | 0.13 (L) | 1.00 (O) | 4.01 (H) | 0.41 (H) | 0.03 (O) | 0.28 (O) | 72.26 (O) | 3.95 (D) | 22.32 (O) | 28.51 (O) | 41.88 (O) |
| Max | 2.78 | 0.22 | 1.71 | 6.57 | 0.68 | 0.06 | 0.37 | 149.9 | 14.8 | 54.93 | 57.64 | 94.9 |
| Min | 1.48 | 0.05 | 0.36 | 1.05 | 0.12 | 0.01 | 0.16 | 28.9 | 0.75 | 6.34 | 3.48 | 21.57 |
| SD | 0.29 | 0.04 | 0.35 | 1.36 | 0.13 | 0.01 | 0.05 | 27.65 | 2.77 | 11.44 | 14.25 | 12.12 |
| Median | 2.06 | 0.14 | 0.96 | 4.18 | 0.41 | 0.03 | 0.29 | 65.75 | 3.19 | 20.98 | 27.48 | 41.13 |
| Nutrient | Pre-Treatment | LVs | Calibration | Cross-Validation | Test Set | |||
|---|---|---|---|---|---|---|---|---|
| RMSE | R2 | RMSE | R2 | RMSE | R2 | |||
| N | MC | 10 | 0.18 | 0.58 | 0.18 | 0.55 | 0.19 | 0.57 |
| P | MC | 10 | 0.02 | 0.69 | 0.02 | 0.66 | 0.02 | 0.60 |
| K | MC + SNV | 12 | 0.21 | 0.65 | 0.23 | 0.58 | 0.22 | 0.63 |
| Ca | MC + 1D | 7 | 0.65 | 0.67 | 0.69 | 0.63 | 0.73 | 0.53 |
| Mg | Raw | 9 | 0.08 | 0.52 | 0.08 | 0.47 | 0.08 | 0.47 |
| S | MC | 11 | 0.02 | 0.52 | 0.03 | 0.48 | 0.03 | 0.44 |
| Fe | MC | 7 | 24.93 | 0.48 | 24.93 | 0.46 | 24.39 | 0.48 |
| Cu | Raw | 9 | 0.93 | 0.33 | 0.95 | 0.29 | 0.93 | 0.31 |
| Mn | MC + SNV | 12 | 7.73 | 0.53 | 8.42 | 0.44 | 8.07 | 0.49 |
| Zn | MC + 1D | 7 | 9.94 | 0.50 | 10.52 | 0.44 | 10.25 | 0.46 |
| B | MC + 1D | 7 | 5.26 | 0.70 | 5.75 | 0.64 | 5.83 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, M.; Quiñones, A.; Munera, S.; de Paz, J.M.; Blasco, J. Rapid Prediction of Nutrient Concentration in Citrus Leaves Using Vis-NIR Spectroscopy. Sensors 2023, 23, 6530. https://doi.org/10.3390/s23146530
Acosta M, Quiñones A, Munera S, de Paz JM, Blasco J. Rapid Prediction of Nutrient Concentration in Citrus Leaves Using Vis-NIR Spectroscopy. Sensors. 2023; 23(14):6530. https://doi.org/10.3390/s23146530
Chicago/Turabian StyleAcosta, Maylin, Ana Quiñones, Sandra Munera, José Miguel de Paz, and José Blasco. 2023. "Rapid Prediction of Nutrient Concentration in Citrus Leaves Using Vis-NIR Spectroscopy" Sensors 23, no. 14: 6530. https://doi.org/10.3390/s23146530
APA StyleAcosta, M., Quiñones, A., Munera, S., de Paz, J. M., & Blasco, J. (2023). Rapid Prediction of Nutrient Concentration in Citrus Leaves Using Vis-NIR Spectroscopy. Sensors, 23(14), 6530. https://doi.org/10.3390/s23146530








