A Portable Laser Spectroscopic System for Measuring Nitrous Oxide Emissions on Fertilized Cropland
Abstract
:1. Introduction
1.1. N2O from Agriculture—A Major Greenhouse Gas
1.2. State of the Art of N2O Measurement
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Laboratory Setup
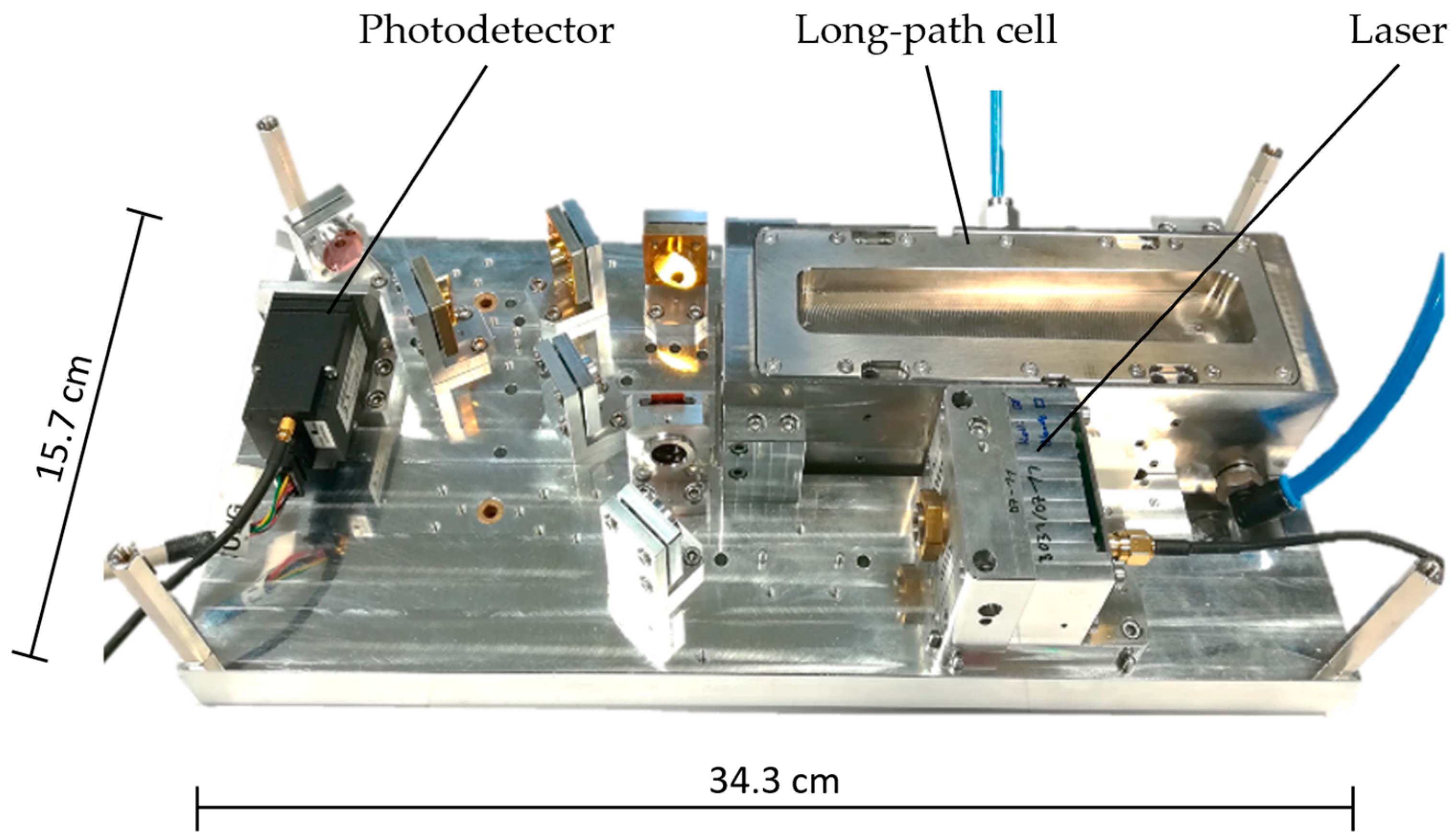
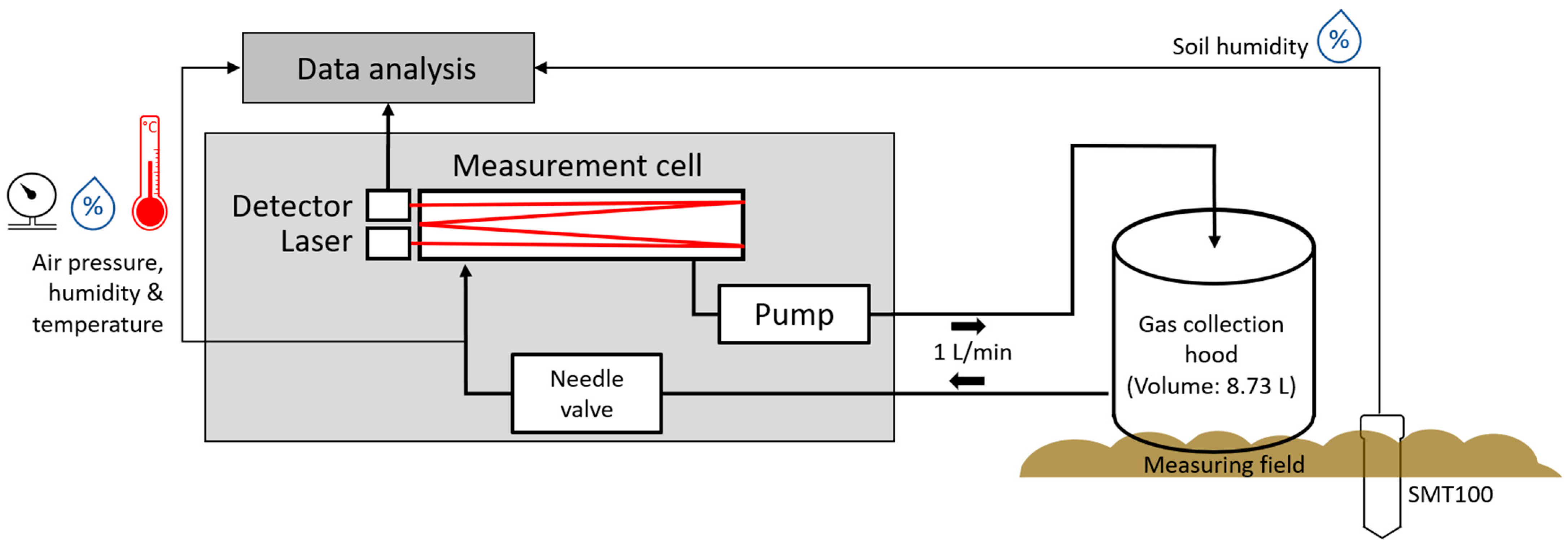
2.1.2. Portable System
2.2. Measurement Method
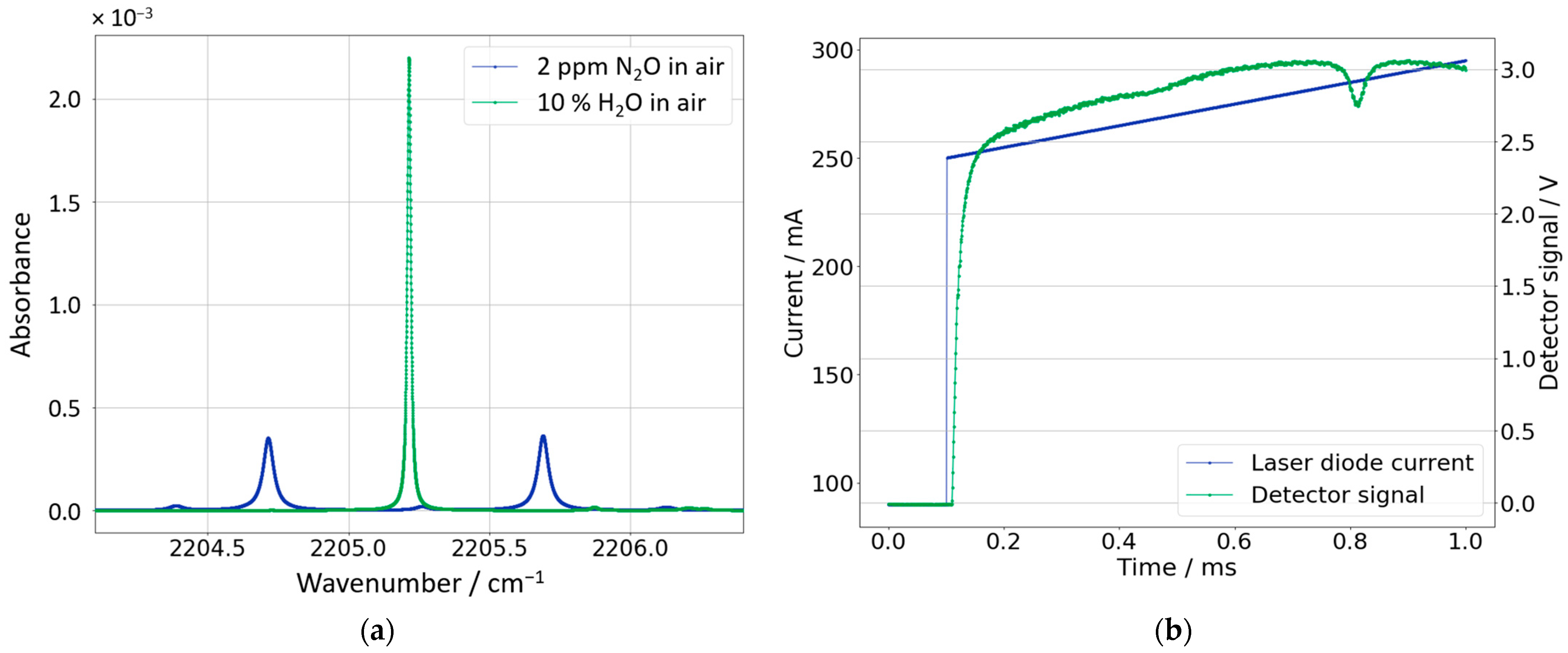
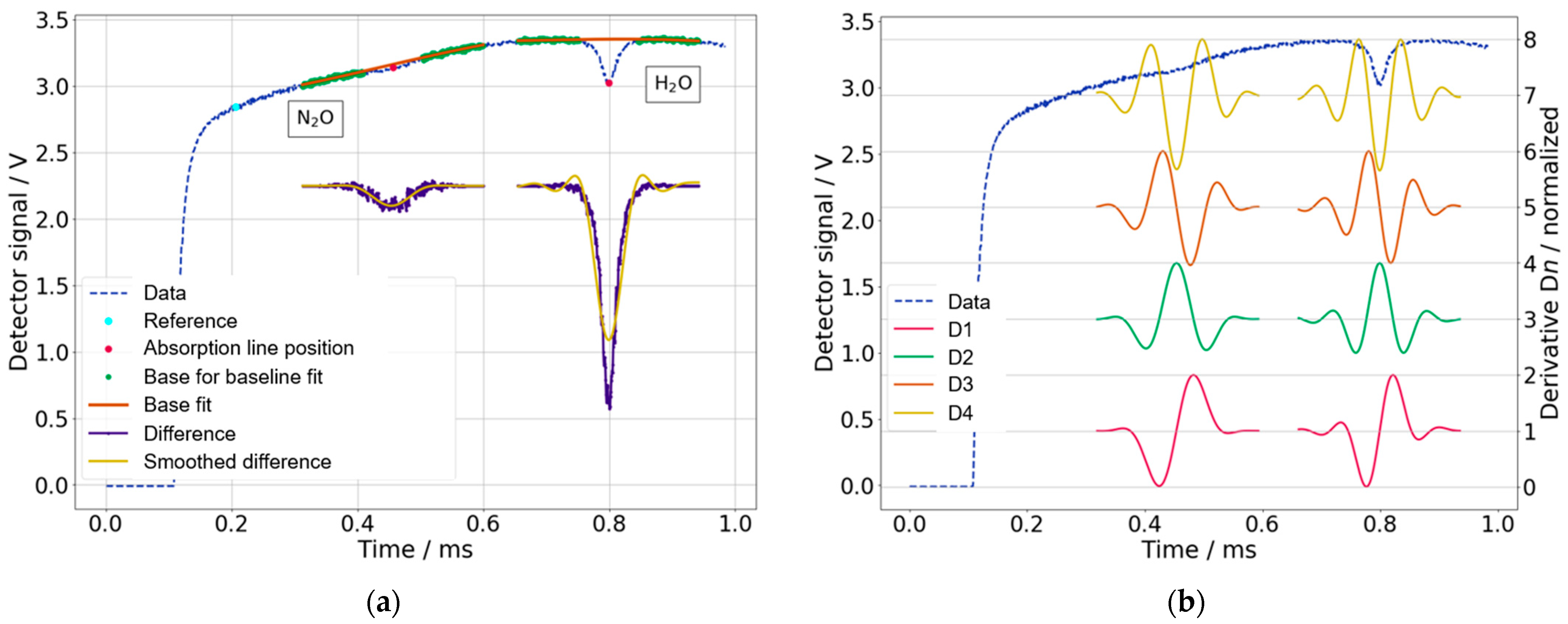
2.3. Data Analysis
3. Results
3.1. Measurement Results with Laboratory Setup
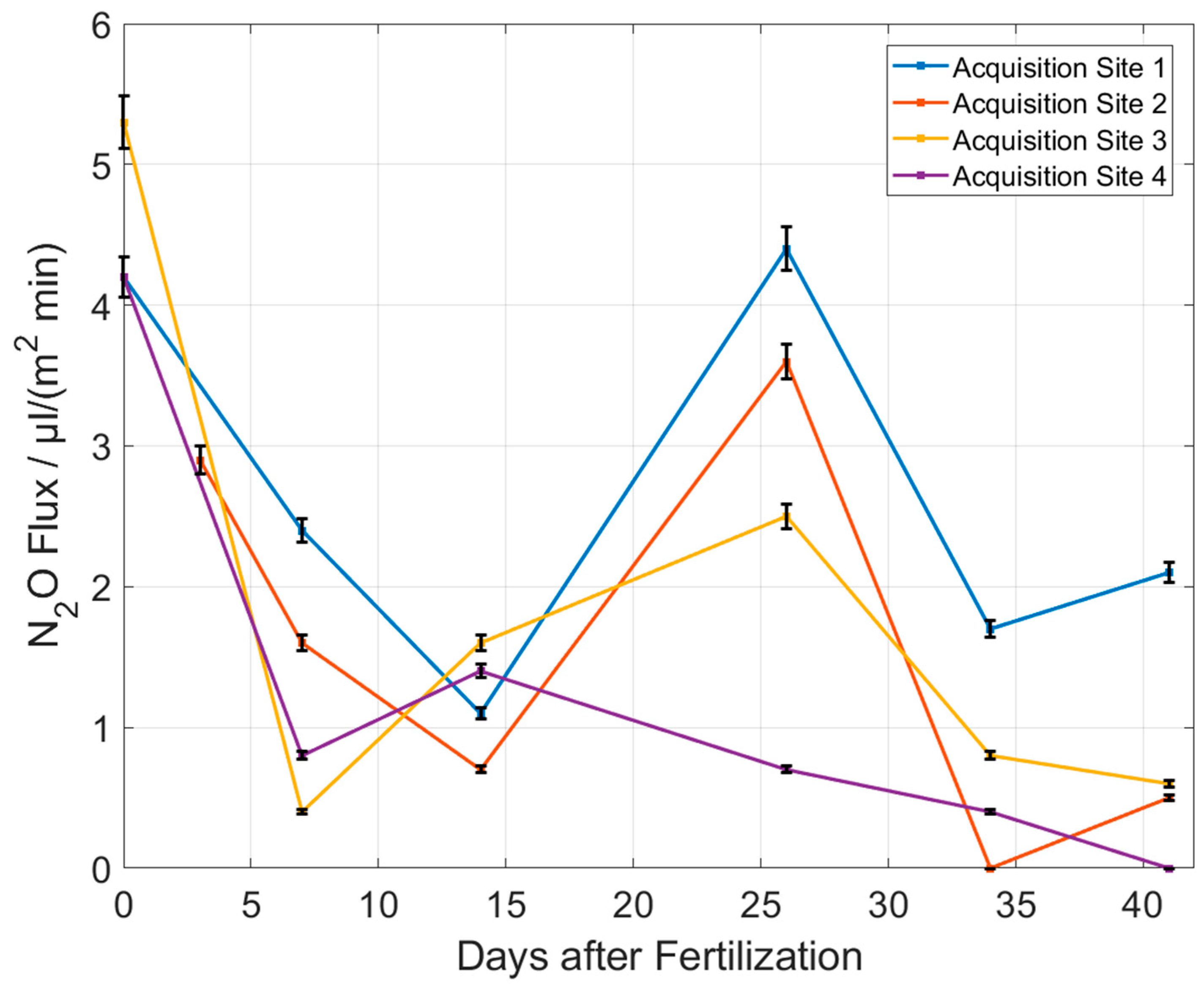
3.2. Measurement Results with Portable Setup
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaman, M.; Heng, L.; Müller, C. Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Official Journal of the European Union. UN Climate Change Conference 2022 in Sharm-el-Sheikh, Egypt (COP27): European Parliament Resolution of 20 October 2022 on the 2022 UN Climate Change Conference in Sharm El-Sheikh, Egypt (COP27); P9_TA(2022)0373; European Parliament 2019–2024: Brussels, Belgium, 2022. [Google Scholar]
- Rees, R.M.; Augustin, J.; Alberti, G.; Ball, B.C.; Boeckx, P.; Cantarel, A.; Castaldi, S.; Chirinda, N.; Chojnicki, B.; Giebels, M.; et al. Nitrous oxide emissions from European agriculture—An analysis of variability and drivers of emissions from field experiments. Biogeosciences 2013, 10, 2671–2682. [Google Scholar] [CrossRef] [Green Version]
- Global Monitoring Laboratory—Earth System Research Laboratories. Nitrous Oxide (N2O): Combined Dataset. Available online: https://gml.noaa.gov/hats/combined/N2O.html (accessed on 6 April 2023).
- Friedl, J.; Cardenas, L.M.; Clough, T.J.; Dannenmann, M.; Hu, C.; Scheer, C. Measuring denitrification and the N2O:(N2O + N2) emission ratio from terrestrial soils. Curr. Opin. Environ. Sustain. 2020, 47, 61–71. [Google Scholar] [CrossRef]
- Rapson, T.D.; Dacres, H. Analytical techniques for measuring nitrous oxide. TrAC Trends Anal. Chem. 2014, 54, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.; Kjær, T.; Revsbech, N.P. An oxygen insensitive microsensor for nitrous oxide. Sens. Actuator B-Chem. 2001, 81, 42–48. [Google Scholar] [CrossRef]
- Tallec, T.; Brut, A.; Joly, L.; Dumelié, N.; Serça, D.; Mordelet, P.; Claverie, N.; Legain, D.; Barrié, J.; Le Dantec, V.; et al. N2O flux measurements over an irrigated maize crop: A comparison of three methods. Agric. For. Meteorol. 2019, 264, 56–72. [Google Scholar] [CrossRef]
- Picarro, Inc. Picarro_G5310 Analyzer Datasheet. Available online: https://www.picarro.com/support/library/documents/g5310_analyzer_datasheet# (accessed on 5 April 2023).
- Gasera ONE GHG. Gasera_Photoacoustic Gas Analyzer Datasheet. Available online: https://www.gasera.fi/wp-content/uploads/2021/11/Gasera_brochure_GHG_11112021_web.pdf (accessed on 5 April 2023).
- Brümmer, C.; Lyshede, B.; Lempio, D.; Delorme, J.P.; Ruffer, J.J.; Fuss, R.; Moffat, A.M.; Hurkuck, M.; Ibrom, A.; Ambus, P.; et al. Gas chromatography vs. quantum cascade laser-based N2O flux measurements using a novel chamber design. Biogeosciences 2017, 14, 1365–1381. [Google Scholar] [CrossRef] [Green Version]
- Maier, M.; Longdoz, B.; Laemmel, T.; Schack-Kirchner, H.; Lang, F. 2D profiles of CO2, CH4, N2O and gas diffusivity in a well aerated soil: Measurement and Finite Element Modeling. Agric. For. Meteorol. 2017, 247, 21–33. [Google Scholar] [CrossRef]
- ABB Inc. Measurement & Analytics. LGR-ICOS™ GLA151-N2OCM: N2O & CO Analyzer—QC Portable. Available online: https://search.abb.com/library/Download.aspx?DocumentID=3KXG167022R1001&LanguageCode=en&DocumentPartId=&Action=Launch (accessed on 6 April 2023).
- LI-COR. Soil Gas Flux Measurement Solutions: Complete Systems Guided by Scientific. 2022. Available online: https://www.licor.com/env/products/soil_flux/?gclid=Cj0KCQjw8NilBhDOARIsAHzpbLBF2FBt70XpRzMlMXF_TCB22CBAz8PP8WHvLj8-ArHO42PVRDO5u-caAmNpEALw_wcB (accessed on 15 May 2023).
- Burba, G.; Begashaw, I.; Leggett, G.; Minish, K.; Xu, L. Measuring Very Small Soil Fluxes of N2O & CH4 Using a New OF-CEAS Technology 2021. In Proceedings of the AGU Fall Meeting, New Orleans, LA, USA, 13–17 December 2021. [Google Scholar]
- White, J.U. Long Optical Paths of Large Aperture. J. Opt. Soc. Am. 1942, 32, 285–288. [Google Scholar] [CrossRef]
- Herriott, D.R.; Schulte, H.J. Folded Optical Delay Lines. Appl. Opt. 1965, 4, 883–889. [Google Scholar] [CrossRef]
- AVL. AVL QCL i60 N2O Analyzer. Available online: https://www.avl.com (accessed on 23 March 2020).
- Fetzner, S.; Brunner, R.; Ulmer., U. Device for determining the concentration of at least one gas in a sample gas flow by means of infrared absorption spectroscopy. U.S. Patent 9995675B2, 12 June 2018. [Google Scholar]
- Rothman, L.S.; Gordon, I.E. The HITRAN Molecular Database. AIP Conf. Proc. 2013, 1545, 223–231. [Google Scholar] [CrossRef] [Green Version]
- AdTech Optics. 4.52 μm DISTRIBUTED FEEDBACK (DFB) QCL. Available online: http://www.atoptics.com/pdf/TO3VHL/Specs%20TO3%204.52um.pdf (accessed on 5 April 2023).
- Nanoplus Nanosystems and Technologies GmbH. TOP Wavelengths—DFB: 4524 nm & 4534 nm. Available online: https://nanoplus.com/fileadmin/user_upload/Data_sheets/nanoplus_DFB_TOP_WL_4524_nm.pdf (accessed on 5 April 2023).
- Stemmler, S.; Wiedenmann, D. Multi-Sensor Data Acquisition for Assessing the Condition of Vegetation; SPIE Remote Sensing: Bellingham, WA, USA, 2021; Volume 11856. [Google Scholar] [CrossRef]
- Umweltbundesamt. Atmosphärische Treibhausgas-Konzentrationen. Available online: https://www.umweltbundesamt.de/daten/klima/atmosphaerische-treibhausgas-konzentrationen#lachgas (accessed on 5 April 2023).
- IAS GmbH. HovaCAL: Prüfgasgenerator zur Erzeugung Hochgenauer Gasdampfgemische. Available online: https://hovacal.de/downloads/datenblaetter/IAS_Datenblatt_HovaCAL.pdf (accessed on 11 April 2023).


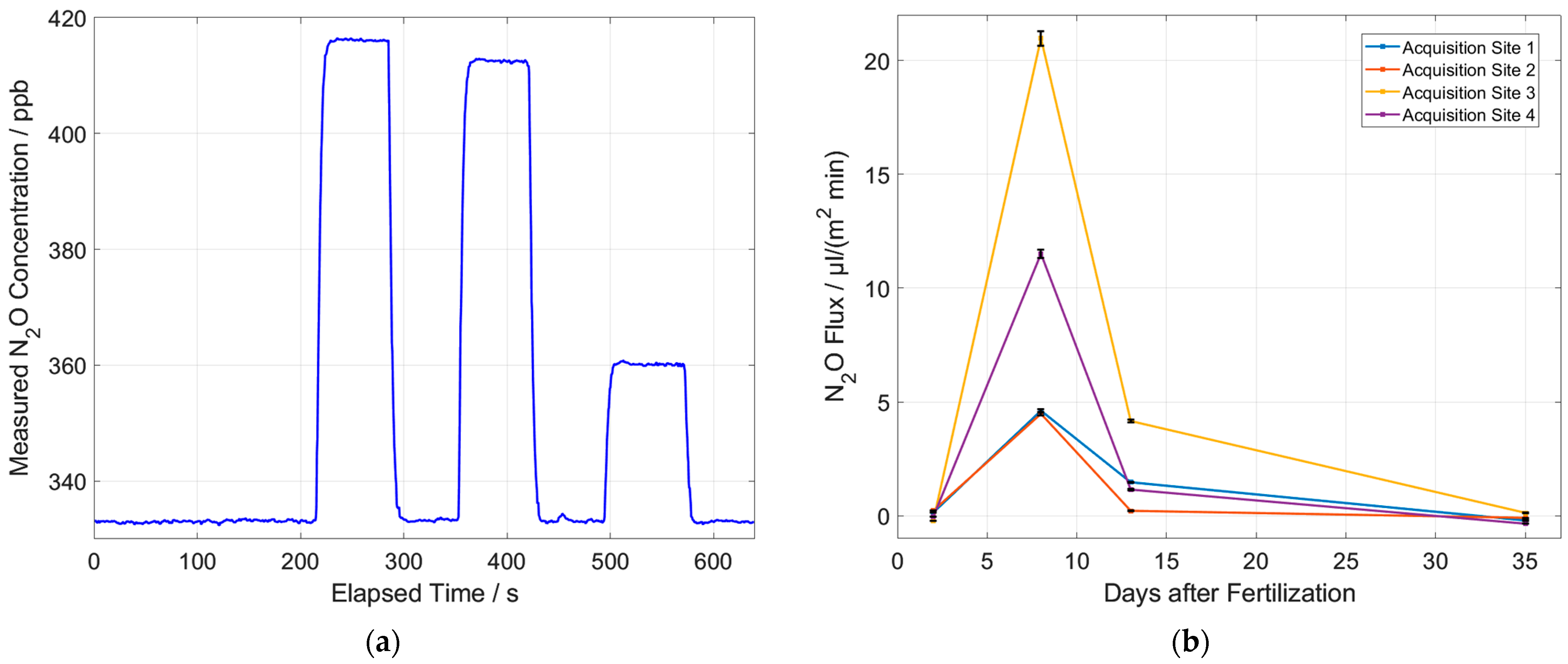


| Lab. Setup (amb. Air) | Lab. Setup (1st Sample) | Port. Setup (amb. Air) | Port. Setup (Ref.: 500 ppb) | |
|---|---|---|---|---|
| Mean Value | 333.0 ppb | 416.1 ppb | 354.3 ppb | 497. 5 ppb |
| Standard Deviation | 0.2 ppb | 0.2 ppb | 8.4 ppb | 8.2 ppb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiefvater, G.; Hespos, Y.; Wiedenmann, D.; Lambrecht, A.; Brunner, R.; Wöllenstein, J. A Portable Laser Spectroscopic System for Measuring Nitrous Oxide Emissions on Fertilized Cropland. Sensors 2023, 23, 6686. https://doi.org/10.3390/s23156686
Stiefvater G, Hespos Y, Wiedenmann D, Lambrecht A, Brunner R, Wöllenstein J. A Portable Laser Spectroscopic System for Measuring Nitrous Oxide Emissions on Fertilized Cropland. Sensors. 2023; 23(15):6686. https://doi.org/10.3390/s23156686
Chicago/Turabian StyleStiefvater, Gerrit, Yvonne Hespos, Dominic Wiedenmann, Armin Lambrecht, Raimund Brunner, and Jürgen Wöllenstein. 2023. "A Portable Laser Spectroscopic System for Measuring Nitrous Oxide Emissions on Fertilized Cropland" Sensors 23, no. 15: 6686. https://doi.org/10.3390/s23156686






