Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications
Abstract
1. Introduction
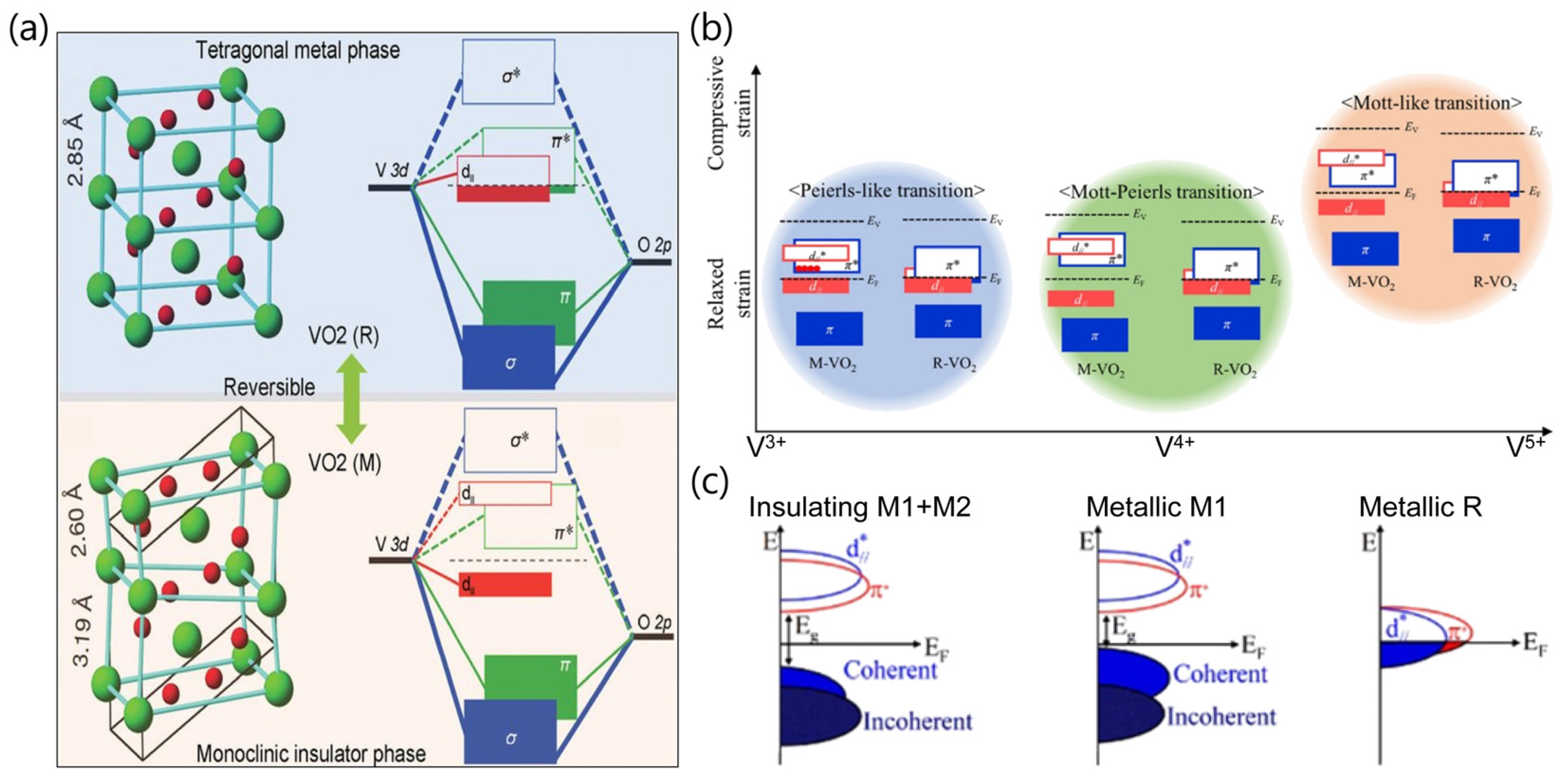
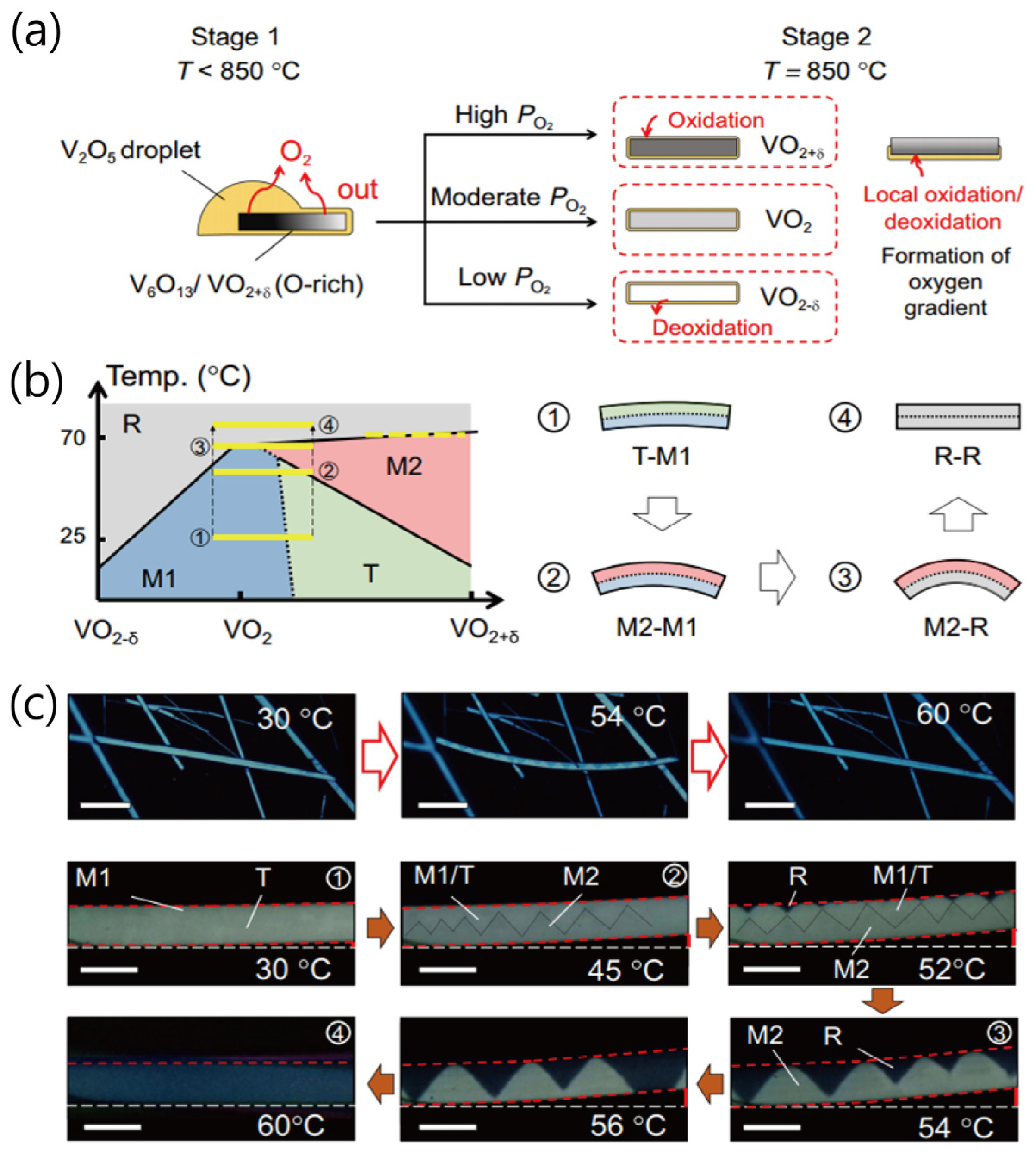
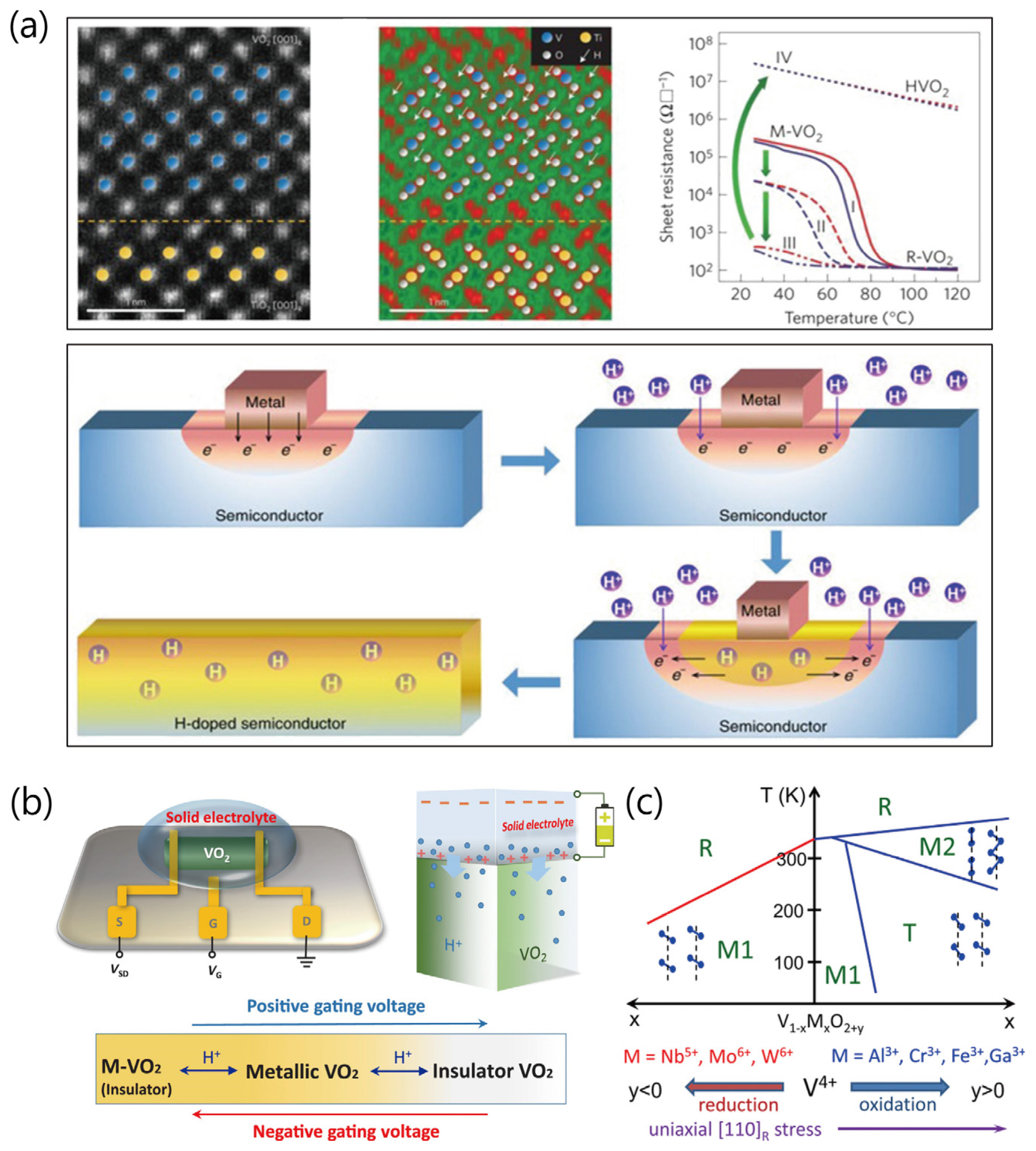
2. Synthesis of Nanostructured VO2 Materials and Modulation of Their Properties
2.1. Synthesis Methods of Nanostructured VO2
2.2. Modulation of Physical Properties of Nanostructured VO2

3. Optical Sensing Applications
3.1. Detection Mechanisms for VO2-Based Photodetectors
3.1.1. Light-Induced Phase Transition
3.1.2. Photoconductive Effect
3.1.3. Photogating Effect
3.1.4. Photovoltaic Effect
3.1.5. Photobolometric Effect
3.2. VO2-Based Photodetectors
3.2.1. Ultraviolet (UV) Photodetection
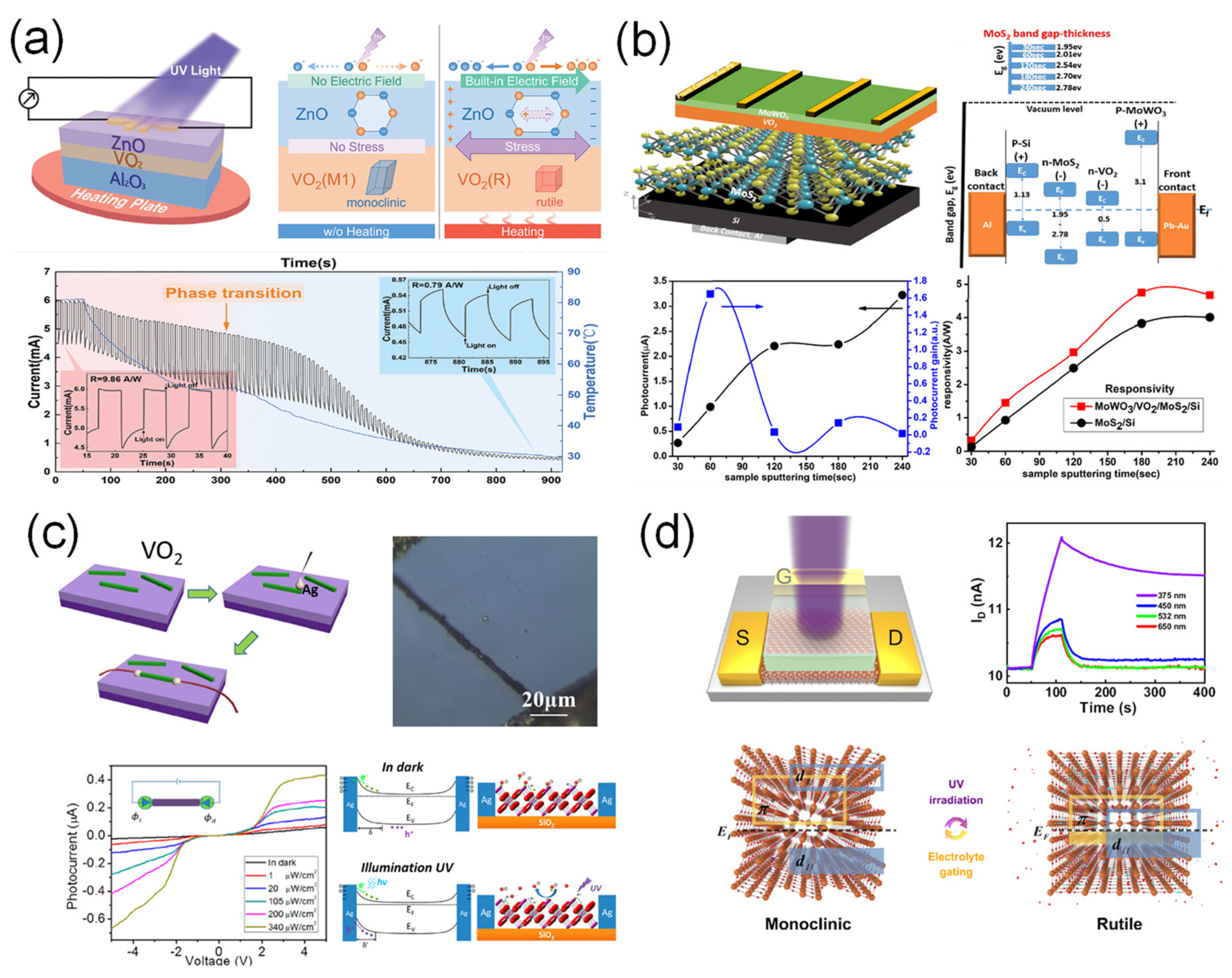
3.2.2. Visible Photodetection
3.2.3. Near-IR (NIR) Photodetection
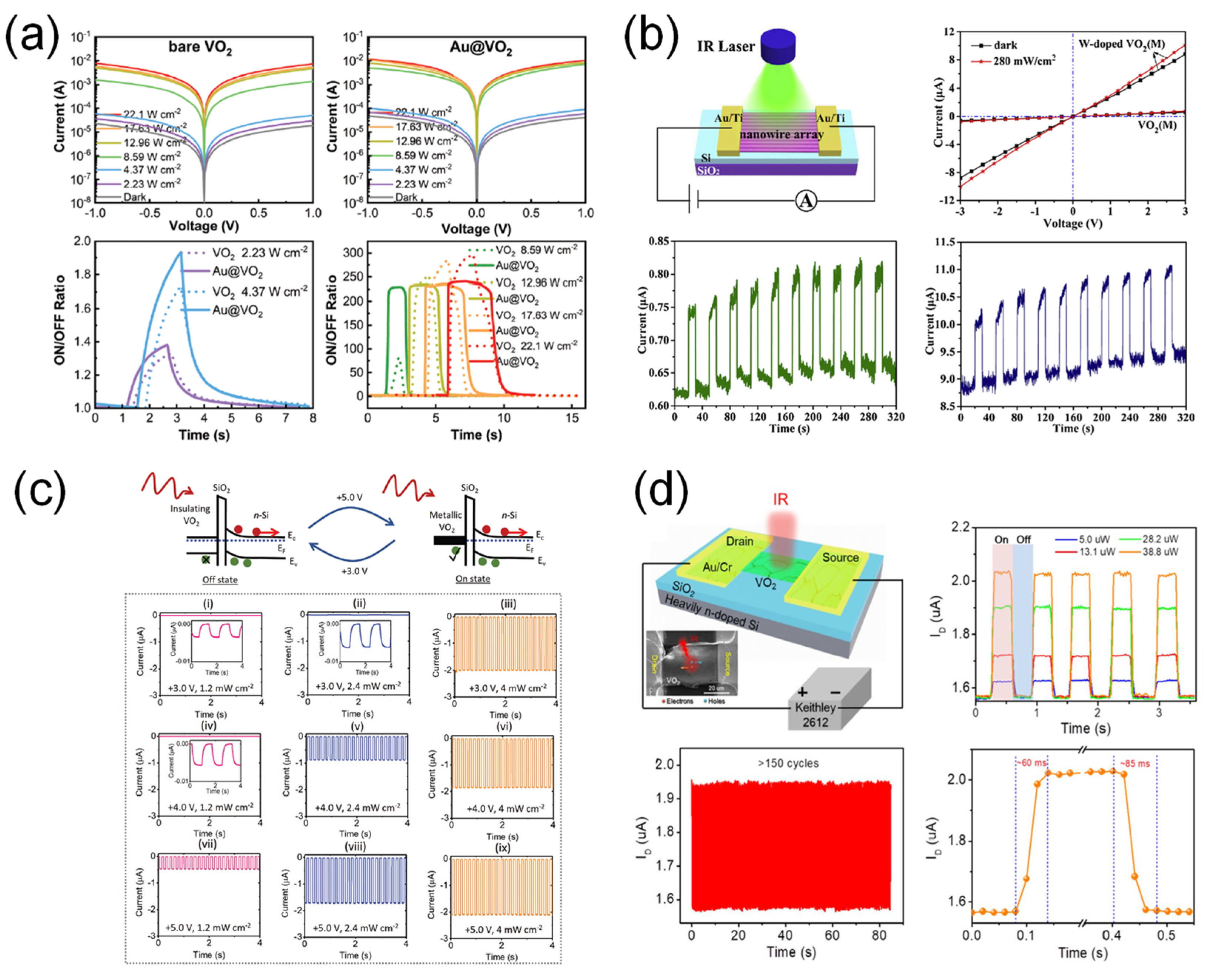
3.2.4. IR Photodetection
3.2.5. Broadband Photodetection
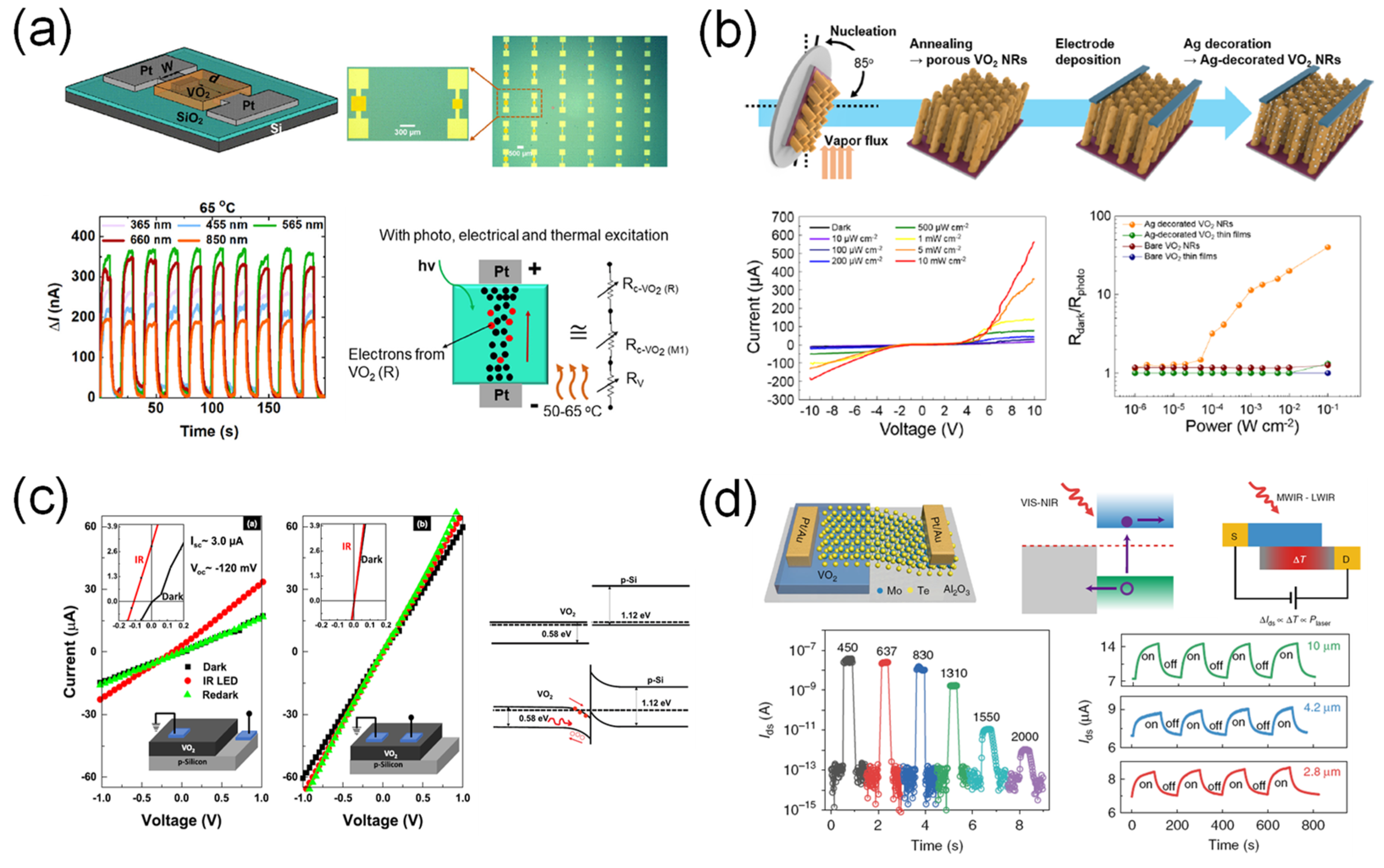
3.3. Summary of VO2-Based Photodetectors
| Materials Type | λ (nm) | Methodology | R (A/W) | D * (Jones) | Ref. |
|---|---|---|---|---|---|
| VO2 | 375 | PLD | - | - | [36,37] |
| VO2/Nb-doped TiO2 | 254/405 | DC sputter | 15.7 (@ 405 nm) 35.6 (@254 nm) | - | [58] |
| MoWO3/VO2/ MoS2/Si | 365 | RF sputter | 4.7 | 4.3 × 108 | [40] |
| VO2 (1D) | 360–400 | CVD | 7069 | 1.5 × 1014 | [41] |
| ZnO/VO2 | 365 | PLD | 10.07 | 1.2 × 1010 | [39] |
| VO2/MoS2 | 500–700 | DC Sputter | 1.25 | - | [42] |
| VO2/WSe2 | 532 | DC Sputter | 2.4 (@ RT) 6.6 (@ 90 °C) | 1.9 × 1013 (@ RT) 1.8 × 1011 (@ 90 °C) | [43] |
| Au/VO2 | 808 | DC Sputter | 0.26 | 1.14 × 1011 | [44] |
| W-doped VO2 (1D) | 980 | Hydrothermal | 0.02 | - | [45] |
| VO2/n-Si | 940 | ALD | 0.001 | 1.0 × 1012 | [48] |
| H-doped VO2 nanoparticles | 780 | Sol-gel | 3.6 × 104 | 1.1 × 1013 | [59] |
| VO2/p-Si | 850 | RF sputter | 14.8 | 7.0 × 1012 | [60] |
| VO2 | 850 | CVD | 0.02 | - | [49] |
| VO2 | 1550 | CVD | 7.1 × 10−5 | 1.1 × 1011 | [50] |
| VO2 | 1064/1550 | DC sputter | 0.014 (@ 1064 nm) | 1.7 × 1012 (@ 1064 nm) | [61] |
| VO2 (1D)/CNT | IR | Hydrothermal | 0.6 × 10−3 | - | [51] |
| VO2/ZnO | 365/525/1064 | Hydrothermal | 0.5 × 10−3 (@ 365 nm) | 2.7 × 109 (@ 365 nm) | [62] |
| VO2 | 365–850 | DC sputter | 0.9 (@ 565 nm, M1) 2.1 (@ 850 nm, R) | 9.4 × 109 (@ 565 nm, M1) 4.6 × 109 (@ 850 nm, M1) | [53] |
| VO2/Si | 650/980 | PLD | 0.35 (@ 650 nm) | - | [54] |
| VO2/MoTe2 | 450–2000, 2800–10,000 | DC sputter | 0.22 (@ 830 nm) | 3.0 × 1010 (@ 830 nm) | [57] |
| VO2/p-Si | 456,515,950 | PLD | 2.0 × 10−5 (@ 950 nm) | - | [56] |
| Ag/VO2 (1D) | 400–1000 | E-beam evaporation | 4.1 × 103 | 1.4 × 1014 | [55] |
3.4. Optical Switching and Color Modulator Applications


4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brahlek, M.; Zhang, L.; Lapano, J.; Zhang, H.-T.; Engel-Herbert, R.; Shukla, N.; Datta, S.; Paik, H.; Schlom, D.G. Opportunities in vanadium-based strongly correlated electron systems. MRS Commun. 2017, 7, 27–52. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal synthesis of VO2 polymorphs: Advantages, challenges and prospects for the application of energy efficient smart windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef]
- Devthade, V.; Lee, S. Synthesis of vanadium dioxide thin films and nanostructures. J. Appl. Phys. 2020, 128, 231101. [Google Scholar] [CrossRef]
- Liu, K.; Lee, S.; Yang, S.; Delaire, O.; Wu, J. Recent progresses on physics and applications of vanadium dioxide. Mater. Today 2018, 21, 875–896. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, X.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef]
- Lee, D.; Yang, D.; Kim, H.; Kim, J.; Song, S.; Choi, K.S.; Bae, J.-S.; Lee, J.; Lee, J.; Lee, Y.; et al. Deposition-temperature-mediated selective phase transition mechanism of VO2 films. J. Phys. Chem. C 2020, 124, 17282–17289. [Google Scholar] [CrossRef]
- Zhou, Y.; Ramanathan, S. Mott memory and neuromorphic devices. Proc. IEEE 2015, 103, 1289–1310. [Google Scholar] [CrossRef]
- Lee, D.; Min, T.; Lee, G.; Kim, J.; Song, S.; Lee, J.; Bae, J.-S.; Kang, H.; Lee, J.; Park, S. Understanding the phase transition evolution mechanism of partially M2 phased VO2 film by hydrogen incorporation. J. Phys. Chem. Lett. 2020, 11, 9680–9688. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, S.; Liu, G.; Li, M.; White, T.J.; Long, Y. Vanadium dioxide: The multistimuli responsive material and its applications. Small 2018, 14, 1802025. [Google Scholar]
- Mao, F.; Fan, X.; Long, L.; Li, Y.; Chen, H.; Zhou, W. Constructing 3D hierarchical CNTs/VO2 composite microspheres with superior electromagnetic absorption performance. Ceram. Int. 2023, 49 Pt A, 16924–16931. [Google Scholar] [CrossRef]
- Mao, F.; Long, L.; Zeng, G.; Chen, H.; Li, Y.; Zhou, W. Achieving excellent electromagnetic wave absorption property by constructing VO2 coated biomass carbon heterostructures. Diam. Relat. Mater. 2022, 130, 109422. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, W.; Chen, W.; Zheng, Y. Recent progress on vanadium dioxide nanostructures and devices: Fabrication, properties, applications and perspectives. Nanomaterials 2021, 11, 338. [Google Scholar] [CrossRef]
- Wang, S.; Owusu, K.A.; Mai, L.; Ke, Y.; Zhou, Y.; Hu, P.; Magdassi, S.; Long, Y. Vanadium dioxide for energy conservation and energy storage applications: Synthesis and performance improvement. Appl. Energy 2018, 211, 200–217. [Google Scholar]
- Zeng, W.; Chen, N.; Xie, W. Research progress on the preparation methods for VO2 nanoparticles and their application in smart windows. CrystEngComm 2020, 22, 851–869. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Tian, Z.; Mei, Y. Low-dimensional vanadium dioxide nanomaterials: Fabrication, properties and applications. J. Phys. Mater. 2020, 3, 032007. [Google Scholar]
- Shi, R.; Chen, Y.; Cai, X.; Lian, Q.; Zhang, Z.; Shen, N.; Amini, A.; Wang, N.; Cheng, C. Phase management in single-crystalline vanadium dioxide beams. Nat. Commun. 2021, 12, 4214. [Google Scholar] [CrossRef]
- Hong, B.; Yang, Y.; Hu, K.; Dong, Y.; Zhou, J.; Zhang, Y.; Zhao, W.; Luo, Z.; Gao, C. Strain engineering on the metal-insulator transition of VO2/TiO2 epitaxial films dependent on the strain state of vanadium dimers. Appl. Phys. Lett. 2019, 115, 251605. [Google Scholar] [CrossRef]
- Ma, H.; Xiao, X.; Wang, Y.; Sun, Y.; Wang, B.; Gao, X.; Wang, E.; Jiang, K.; Liu, K.; Zhang, X. Wafer-scale freestanding vanadium dioxide film. Sci. Adv. 2021, 7, eabk3438. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ertekin, E.; Srinivasan, V.; Fan, W.; Huang, S.; Zheng, H.; Yim, J.W.L.; Khanal, D.R.; Ogletree, D.F.; Grossman, J.C.; et al. Strain engineering and one-dimensional organization of metal–insulator domains in single-crystal vanadium dioxide beams. Nat. Nanotechnol. 2009, 4, 732–737. [Google Scholar] [CrossRef] [PubMed]
- White, S.T.; Thompson, E.A.; Brown, P.F.; Haglund, R.F. Substrate chemistry and lattice effects in vapor transport growth of vanadium dioxide microcrystals. Cryst. Growth Des. 2021, 21, 3770–3778. [Google Scholar] [CrossRef]
- Breckenfeld, E.; Kim, H.; Burgess, K.; Charipar, N.; Cheng, S.-F.; Stroud, R.; Piqué, A. Strain effects in epitaxial VO2 thin films on columnar buffer-layer TiO2/Al2O3 virtual substrates. ACS Appl. Mater. Interfaces 2017, 9, 1577–1584. [Google Scholar] [CrossRef]
- Shin, K.H.; Bae, J.Y.; Lee, S.Y.; Ahn, D.; Cho, J.; Yoon, J.; Hong, W.-K.; Sohn, J.I. Core-shell heterostructure-enabled stress engineering in vanadium dioxide nanobeams. Appl. Mater. Today 2021, 25, 101244. [Google Scholar] [CrossRef]
- Deng, S.; Yu, H.; Park, T.J.; Islam, A.N.M.N.; Manna, S.; Pofelski, A.; Wang, Q.; Zhu, Y.; Sankaranarayanan, S.K.R.S.; Sengupta, A.; et al. Selective area doping for Mott neuromorphic electronics. Sci. Adv. 2023, 9, eade4838. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Ren, H.; Chen, Y.; Yan, W.; Wang, C.; Li, B.; Jiang, J.; Zou, C. Gate-controlled VO2 phase transition for high-performance smart windows. Sci. Adv. 2019, 5, eaav6815. [Google Scholar] [CrossRef]
- Strelcov, E.; Tselev, A.; Ivanov, I.; Budai, J.D.; Zhang, J.; Tischler, J.Z.; Kravchenko, I.; Kalinin, S.V.; Kolmakov, A. Doping-based stabilization of the M2 phase in free-standing VO2 nanostructures at room temperature. Nano Lett. 2012, 12, 6198–6205. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Choi, M.; Lim, T.-W.; Kwon, H.; Ihm, K.; Kim, J.K.; Choi, S.-Y.; Son, J. Reversible phase modulation and hydrogen storage in multivalent VO2 epitaxial thin films. Nat. Mater. 2016, 15, 1113–1119. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Chen, S.; Ren, H.; Wang, L.; Zhang, G.; Lu, Y.; Jiang, J.; Zou, C.; Luo, Y. Non-catalytic hydrogenation of VO2 in acid solution. Nat. Commun. 2018, 9, 818. [Google Scholar] [CrossRef]
- Guo, H.; Chen, K.; Oh, Y.; Wang, K.; Dejoie, C.; Asif, S.A.S.; Warren, O.L.; Shan, Z.W.; Wu, J.; Minor, A.M. Mechanics and dynamics of the strain-induced M1–M2 structural phase transition in individual VO2 nanowires. Nano Lett. 2011, 11, 3207–3213. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Chen, K.; Shen, H.; Wang, Y.-C.; Hedhill, M.N.; Zhang, X.; Li, J.; Shan, Z.-W. Achieving room-temperature M2-phase VO2 nanowires for superior thermal actuation. Nano Res. 2021, 14, 4146–4153. [Google Scholar] [CrossRef]
- Marezio, M.; McWhan, D.B.; Remeika, J.P.; Dernier, P.D. Structural aspects of the metal-insulator transitions in Cr-doped VO2. Phys. Rev. B 1972, 5, 2541–2551. [Google Scholar] [CrossRef]
- Brückner, W.; Gerlach, U.; Thuss, B. Phase diagram of V1-xAlxO2. Phys. Status Solidi A 1977, 40, K131–K134. [Google Scholar] [CrossRef]
- Zha, J.; Luo, M.; Ye, M.; Ahmed, T.; Yu, X.; Lien, D.-H.; He, Q.; Lei, D.; Ho, J.C.; Bullock, J.; et al. Infrared photodetectors based on 2D materials and nanophotonics. Adv. Funct. Mater. 2022, 32, 2111970. [Google Scholar]
- Cavalleri, A.; Tóth, C.; Siders, C.W.; Squier, J.A. Femtosecond structural dynamics in VO2 during an ultrafast solid-solid phase transition. Phys. Rev. Lett. 2001, 87, 237401. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Yang, D.-S.; Zewail, A.H. 4D visualization of transitional structures in phase transformations by electron diffraction. Science 2007, 318, 788–792. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Guo, L.; Stone, G.; Zhang, L.; Zheng, Y.-X.; Freeman, E.; Keefer, D.W.; Chaudhuri, S.; Paik, H.; Moyer, J.A.; et al. Imprinting of local metallic states into VO2 with ultraviolet light. Adv. Funct. Mater. 2016, 26, 6612–6618. [Google Scholar] [CrossRef]
- Li, G.; Xie, D.; Zhang, Z.; Zhou, Q.; Zhang, H.; Ni, H.; Wang, J.; Guo, E.-J.; He, M.; Wang, C.; et al. Flexible VO2 films for in-sensor computing with ultraviolet light. Adv. Funct. Mater. 2022, 32, 2203074. [Google Scholar] [CrossRef]
- Li, G.; Xie, D.; Zhong, H.; Zhang, Z.; Fu, X.; Zhou, Q.; Li, Q.; Ni, H.; Wang, J.; Guo, E.-J.; et al. Photo-induced non-volatile VO2 phase transition for neuromorphic ultraviolet sensors. Nat. Commun. 2022, 13, 1729. [Google Scholar] [CrossRef]
- Richards, P.L. Bolometers for infrared and millimeter waves. J. Appl. Phys. 1994, 76, 1–24. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, L.; Ruan, S.; Ye, Z.; Zeng, Y.-J. Phase-transition-induced superior ultraviolet photodetection of a ZnO/VO2 bilayer. J. Mater. Chem. C 2020, 8, 11399–11406. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Shaban, M.; Eker, Y.R.; Yilmaz, M. Efficient MoWO3/VO2/MoS2/Si UV Schottky photodetectors; MoS2 optimization and monoclinic VO2 surface modifications. Sci. Rep. 2020, 10, 15926. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E. Ultrahigh responsivity and external quantum efficiency of an ultraviolet-light photodetector based on a single VO2 microwire. ACS Appl. Mater. Interfaces 2014, 6, 14286–14292. [Google Scholar] [CrossRef]
- Oliva, N.; Casu, E.A.; Yan, C.; Krammer, A.; Rosca, T.; Magrez, A.; Stolichnov, I.; Schueler, A.; Martin, O.J.F.; Ionescu, A.M. Van der Waals MoS2/VO2 heterostructure junction with tunable rectifier behavior and efficient photoresponse. Sci. Rep. 2017, 7, 14250. [Google Scholar] [CrossRef]
- Luo, H.; Wang, B.; Wang, E.; Wang, X.; Sun, Y.; Li, Q.; Fan, S.; Cheng, C.; Liu, K. Phase-transition modulated, high-performance dual-mode photodetectors based on WSe2/VO2 heterojunctions. Appl. Phys. Rev. 2019, 6, 041407. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, L.; Zhen, W.; Lin, Y.; Wang, C.; Pan, T.; Li, L.; Du, G.; Lu, L.; Cao, X.; et al. Phase-transition-induced VO2 thin film IR photodetector and threshold switching selector for optical neural network applications. Adv. Electron. Mater. 2021, 7, 2001254. [Google Scholar] [CrossRef]
- Xie, B.H.; Fu, W.B.; Fei, G.T.; Xu, S.H.; Gao, X.D.; Zhang, L.D. Preparation and enhanced infrared response properties of ordered W-doped VO2 nanowire array. Appl. Surf. Sci. 2018, 436, 1061–1066. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhai, Z.H.; Shi, Q.W.; Zhu, L.G.; Li, J.; Huang, W.X.; Yue, F.; Hu, Y.Y.; Peng, Q.X.; Li, Z.R. Ultrafast terahertz modulation characteristic of tungsten doped vanadium dioxide nanogranular film revealed by time-resolved terahertz spectroscopy. Appl. Phys. Lett. 2015, 107, 031906. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Deng, S.; Zheng, M.; Wang, Y.; van Kan, J.-A.; Tang, S.H.; Zhang, X.; Sow, C.H.; Mhaisalkar, S.G. Highly sensitive and multispectral responsive phototransistor using tungsten-doped VO2 nanowires. Nanoscale 2014, 6, 7619–7627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Lim, S.; Kim, J.; Seo, H. Picoampere dark current and electro-opto-coupled sub-to-super linear response from Mott-transition enabled infrared photodetector for near-sensor vision processing. Adv. Mater. 2023, 35, 221907. [Google Scholar] [CrossRef]
- Guo, X.; Tan, Y.; Hu, Y.; Zafar, Z.; Liu, J.; Zou, J. High quality VO2 thin films synthesized from V2O5 powder for sensitive near-infrared detection. Sci. Rep. 2021, 11, 21749. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, B.; Tadeo, I.J.; Urnarji, A.M. IR photoresponsvie VO2 thin films and electrically assisted transition prepared by single-step chemical vapor deposition. J. Mater. Chem. C 2020, 8, 12543–12550. [Google Scholar] [CrossRef]
- Fu, W.B.; Ma, H.; Wei, Y.; Jiang, K.; Fei, G.T.; Zhang, L.D. Preparation and infrared response properties of vanadium dioxide nanowire/carbon nanotube composite film. J. Mater. Sci. 2017, 52, 7224–7231. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Fu, Y.; Zhang, X. A bottom-up strategy toward a flexible vanadium dioxide/silicon nitride composite film with infrared sensing performance. Nanoscale 2020, 12, 11865–11867. [Google Scholar]
- Kabir, S.; Nirantar, S.; Zhu, L.; Ton-That, C.; Jain, S.K.; Kayani, A.B.A.; Murdoch, B.J.; Sriram, S.; Walia, S.; Bhaskaran, M. Phase change vanadium dioxide light sensors. Appl. Mater. Today 2020, 21, 100833. [Google Scholar] [CrossRef]
- Umar, Z.A.; Ahmed, R.; Asghar, H.; Liaqat, U.; Fayyaz, A.; Baig, M.A. VO2 thin film based highly responsive and fast VIS/IR photodetector. Mater. Chem. Phys. 2022, 290, 126655. [Google Scholar]
- Hong, K.T.; Moon, C.W.; Suh, J.M.; Lee, T.H.; Kim, S.-I.; Lee, S.; Jang, H.W. Daylight-induced metal-insulator transition in Ag decorated vanadium dioxide nanorod arrays. ACS Appl. Mater. Interfaces 2019, 11, 11568–11578. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Siddique, R.; Sajjad, S.A.; Umer, Z.A.; Bukhari, S.; Anwar-UI-Haq, M.; Rafique, M.; Raza, S.R.A. Self-powered and temperature-tunable infrared-visible photodetector based on a VO2/Si heterojunction. J. Phys. D Appl. Phys. 2021, 54, 165109. [Google Scholar] [CrossRef]
- Jiang, W.; Zheng, T.; Wu, B.; Jiao, H.; Wang, X.; Chen, Y.; Zhang, X.; Peng, M.; Wang, H.; Lin, T.; et al. A versatile photodetector assisted by photovoltaic and bolometric effect. Light Sci. Appl. 2020, 9, 160. [Google Scholar] [PubMed]
- Creeden, J.A.; Madaras, S.E.; Beringer, D.B.; Novikova, I.; Lukaszew, R.A. Growth and characterization of vanadium dioxide/niobium doped titanium dioxide heterostructures for ultraviolet detection. Adv. Opt. Mater. 2019, 7, 1901143. [Google Scholar] [CrossRef]
- Kim, M.-W.; Jo, Y.-R.; Lee, C.; Moon, W.-J.; Shim, J.H.; Kim, B.-J. Ultrafast infrared photoresponse from heavily hydrogen-doped VO2 Single Crystalline Nanoparticles. Nano Lett. 2020, 20, 2733–2740. [Google Scholar] [CrossRef]
- Selman, A.M.; Kadhim, M.J. Fabrication of high sensitivity and fast response IR photodetector based on VO2 nanocrystalline thin films prepared on the silicon substrate. Opt. Mater. 2022, 131, 112664. [Google Scholar] [CrossRef]
- Tadeo, I.J.; Krupanidhi, S.B.; Umarji, A.M. Enhanced phase transition and infrared photoresponse characteristics in VO2(M1) thin films synthesized by DC reactive sputtering on different substrates. Mater. Adv. 2021, 2, 3726–3735. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, X.; An, J.; Chen, G.; Bao, J.; Xu, X. UV-vis-IR broad spectral photodetectors based on VO2-ZnO nanocrystal films. ACS Omega 2022, 7, 37078–37084. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Balin, I.; Wang, N.; Lu, Q.; Tok, A.I.Y.; White, T.J.; Magdassi, S.; Abdulhalim, I.; Long, Y. Two-dimensional SiO2/VO2 photonic crystals with statically visible and dynamically infrared modulated for smart window deployment. ACS Appl. Mater. Interfaces 2016, 8, 33112–33120. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Yang, H. Dual-band modulation of visible and near-infrared light transmittance in an all-solution-processed hybrid micro-nano composite film. ACS Appl. Mater. Interfaces 2017, 9, 40810–40819. [Google Scholar] [CrossRef]
- Wan, C.; Horak, E.H.; King, J.; Salman, J.; Zhang, Z.; Zhou, Y.; Roney, P.; Gundlach, B.; Ramanathan, S.; Goldsmith, R.H.; et al. Limiting optical diodes enabled by the phase transition of vanadium dioxide. ACS Photonics 2018, 5, 2688–2692. [Google Scholar] [CrossRef]
- Shu, F.-Z.; Yu, F.-F.; Peng, R.-W.; Zhu, Y.-Y.; Xiong, B.; Fan, R.-H.; Wang, Z.-H.; Liu, Y.; Wang, M. Dynamic plasmonic color generation based on phase transition of vanadium dioxide. Adv. Opt. Mater. 2018, 6, 1700939. [Google Scholar] [CrossRef]
- Duan, X.; White, S.T.; Cui, Y.; Neubrech, F.; Gao, Y.; Haglund, R.F.; Liu, N. Reconfigurable multistate optical systems enabled by VO2 phase transitions. ACS Photonics 2020, 7, 2958–2965. [Google Scholar] [CrossRef]
- In, S.; Cho, J.; Park, J.; Kim, S.Y.; Kim, H.-T.; Noh, T.W.; Park, N. Self-organized gold network-vanadium dioxide hybrid film for dynamic modulation of visible-to-near-infrared light. Adv. Photonics Res. 2020, 1, 2000050. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, L.; Mukherjee, D.; Zheng, Y.-X.; Haislmaier, R.C.; Alem, N.; Engel-Herbert, R. Wafer-scale growth of VO2 thin films using a combinatorial approach. Nat. Commun. 2015, 6, 8475. [Google Scholar] [CrossRef]
- Goi, E.; Zhang, Q.; Chen, X.; Luan, H.; Gu, M. Perspective on photonic memristive neuromorphic computing. PhotoniX 2020, 1, 3. [Google Scholar] [CrossRef]
- Yuan, R.; Duan, Q.; Tiw, P.J.; Li, G.; Xiao, Z.; Jing, Z.; Yang, K.; Liu, C.; Ge, C.; Huang, R.; et al. A calibratable sensory neuron based on epitaxial VO2 for spike-based neuromorphic multisensory system. Nat. Commun. 2022, 13, 3973. [Google Scholar] [CrossRef]
- Gea, C.; Lia, G.; Zhou, Q.-l.; Du, J.-y.; Guo, E.-j.; He, M.; Wang, C.; Yang, G.-z.; Jin, K.-j. Gating-induced reversible HxVO2 phase transformations for neuromorphic computing. Nano Energy 2020, 67, 104268. [Google Scholar] [CrossRef]
- Oh, S.; Shi, Y.; del Valle, J.; Salev, P.; Lu, Y.; Huang, Z.; Kalcheim, Y.; Schuller, I.K.; Kuzum, D. Energy-efficient Mott activation neuron for full-hardware implementation of neural networks. Nat. Nanotechnol. 2021, 16, 680–687. [Google Scholar] [CrossRef]
- Nikoo, M.S.; Soleimanzadeh, R.; Krammer, A.; Marega, G.M.; Park, Y.; Son, J.; Schueler, A.; Kis, A.; Moll, P.J.W.; Matioli, E. Electrical control of glass-like dynamics in vanadium dioxide for data storage and processing. Nat. Electron. 2022, 5, 596–603. [Google Scholar] [CrossRef]
- Tang, K.; Dong, K.; Li, J.; Gordon, M.P.; Reichertz, F.G.; Kim, H.; Rho, Y.; Wang, Q.; Lin, C.-Y.; Grigoropoulos, C.P.; et al. Temperature-adaptive radiative coating for all-season household thermal regulation. Science 2021, 374, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, T.; Yang, Y.M.; Tan, G.; Long, Y. Scalable thermochromic smart windows with passive radiative cooling regulation. Science 2021, 374, 1501–1504. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Li, L.; Li, L.; Zhao, S.; Lu, K.; Chen, K.; Zhu, J.; Zhou, T.; Hu, C.; et al. Continuous photothermal and radiative cooling energy harvesting by VO2 smart coatings with switchable broadband infrared emission. ACS Nano 2023, 17, 9501–9509. [Google Scholar] [CrossRef]




| Synthesis Method | Advantages | Limitations |
|---|---|---|
| Sol-gel |
|
|
| Hydrothermal synthesis |
|
|
| PLD 1 |
|
|
| Sputtering |
|
|
| CVD 2 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Hong, W.-K.; Kim, Y.; Park, S.-Y. Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications. Sensors 2023, 23, 6715. https://doi.org/10.3390/s23156715
Yoon J, Hong W-K, Kim Y, Park S-Y. Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications. Sensors. 2023; 23(15):6715. https://doi.org/10.3390/s23156715
Chicago/Turabian StyleYoon, Jongwon, Woong-Ki Hong, Yonghun Kim, and Seung-Young Park. 2023. "Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications" Sensors 23, no. 15: 6715. https://doi.org/10.3390/s23156715
APA StyleYoon, J., Hong, W.-K., Kim, Y., & Park, S.-Y. (2023). Nanostructured Vanadium Dioxide Materials for Optical Sensing Applications. Sensors, 23(15), 6715. https://doi.org/10.3390/s23156715







