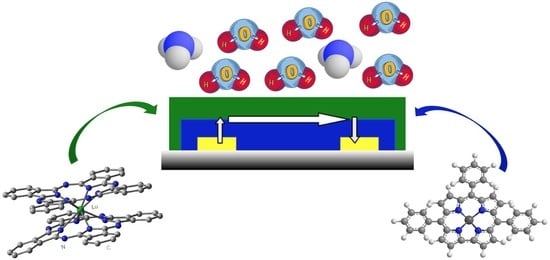
Ammonia and Humidity Sensing by Phthalocyanine–Corrole Complex Heterostructure Devices
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Syntheses
2.2. Cyclic Voltammetry
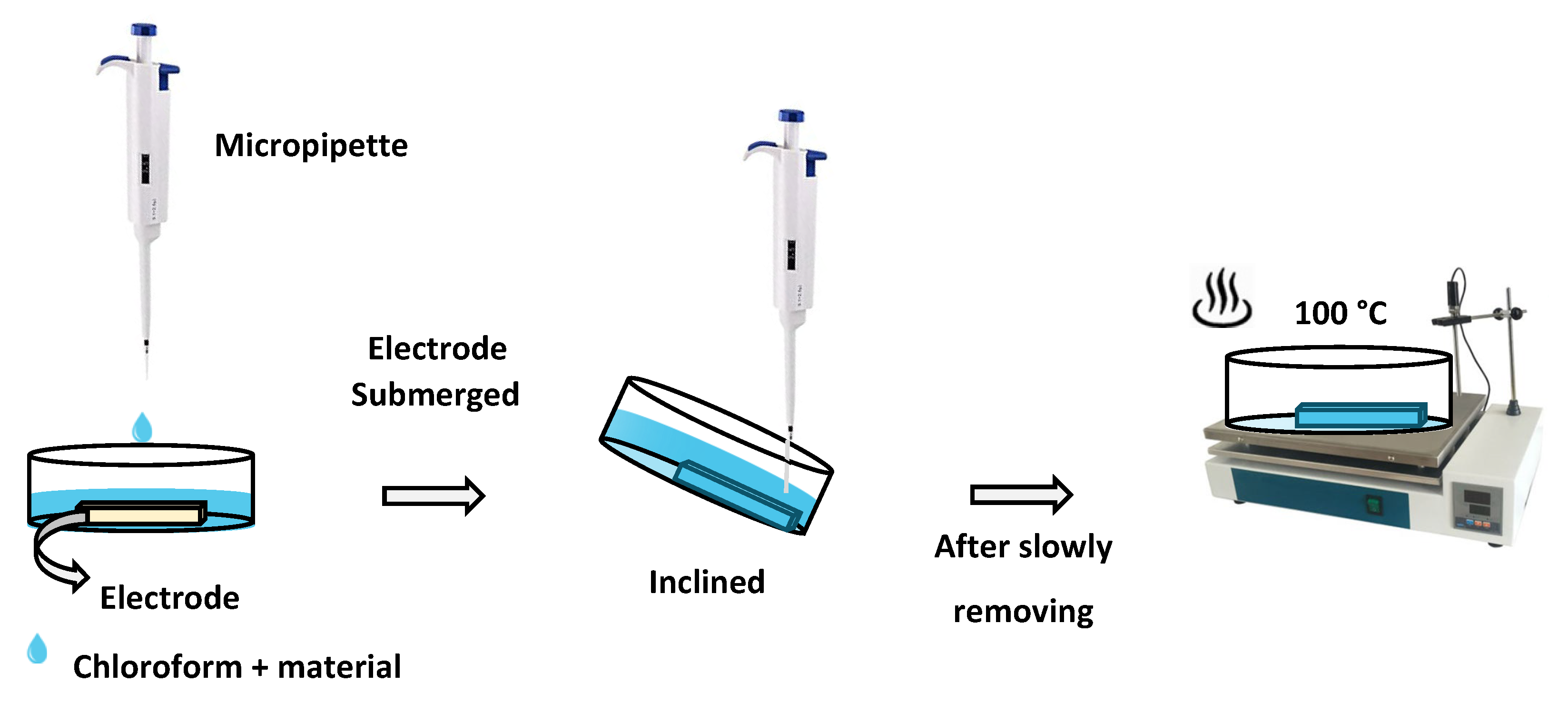
2.3. Sample Preparation
2.4. Spectroscopic Characterization of the Devices
2.5. Electrical and Gas-Sensing Measurements
3. Results and Discussion
3.1. Syntheses
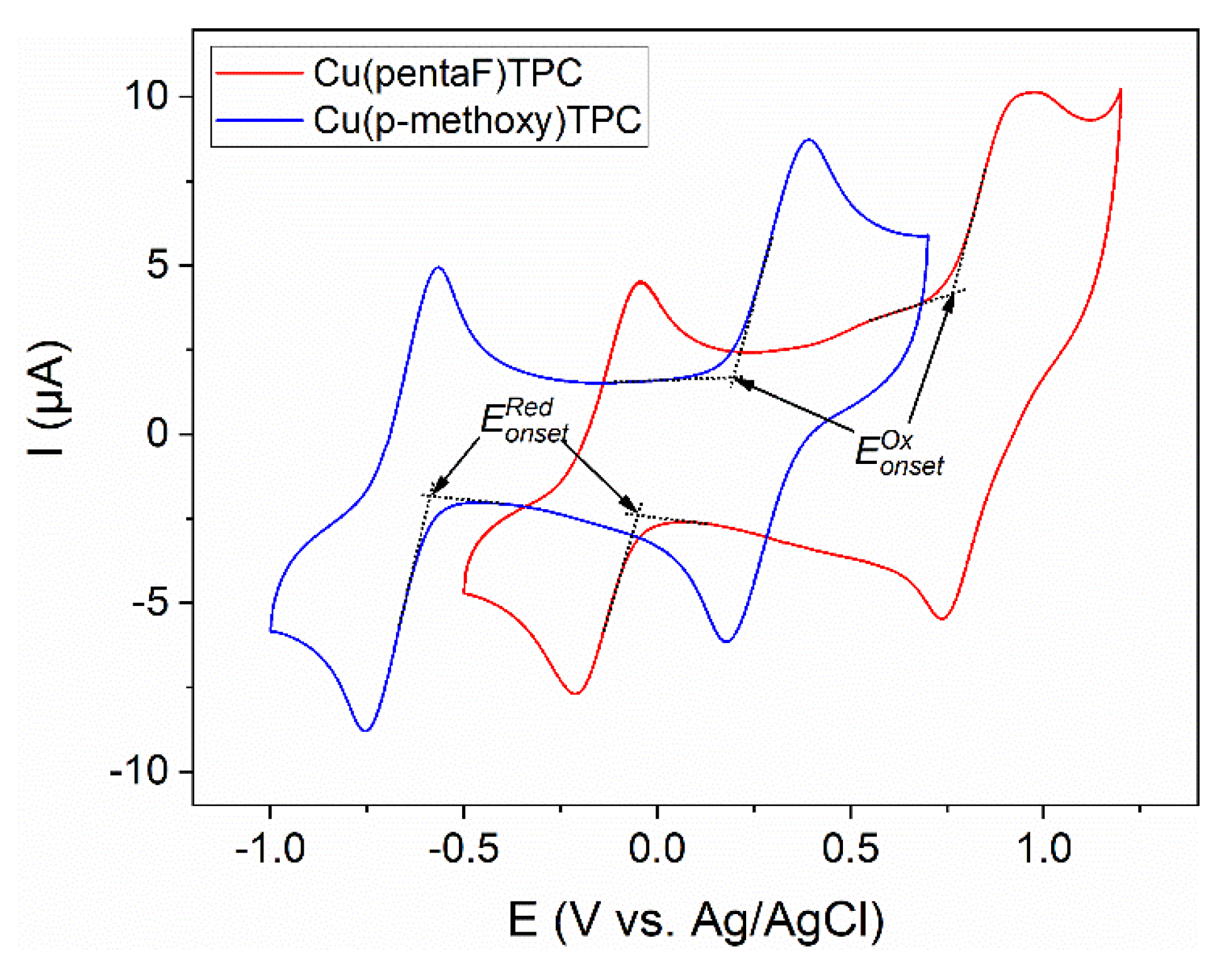
3.2. Electrochemical Characterization
3.3. Device Characterization
3.3.1. Spectroscopic Characterization
3.3.2. Morphological Characterization
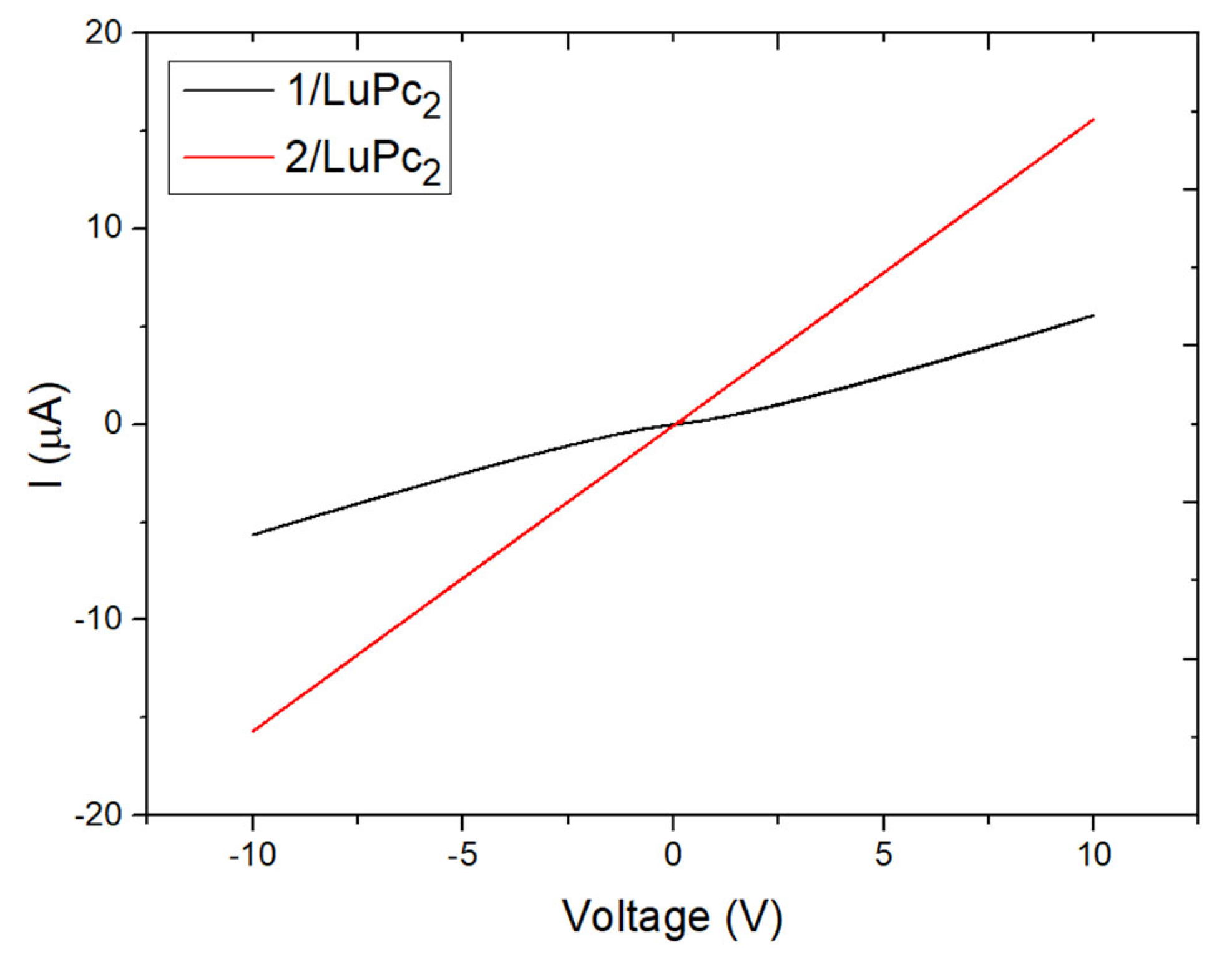
3.3.3. Electrical Characterization
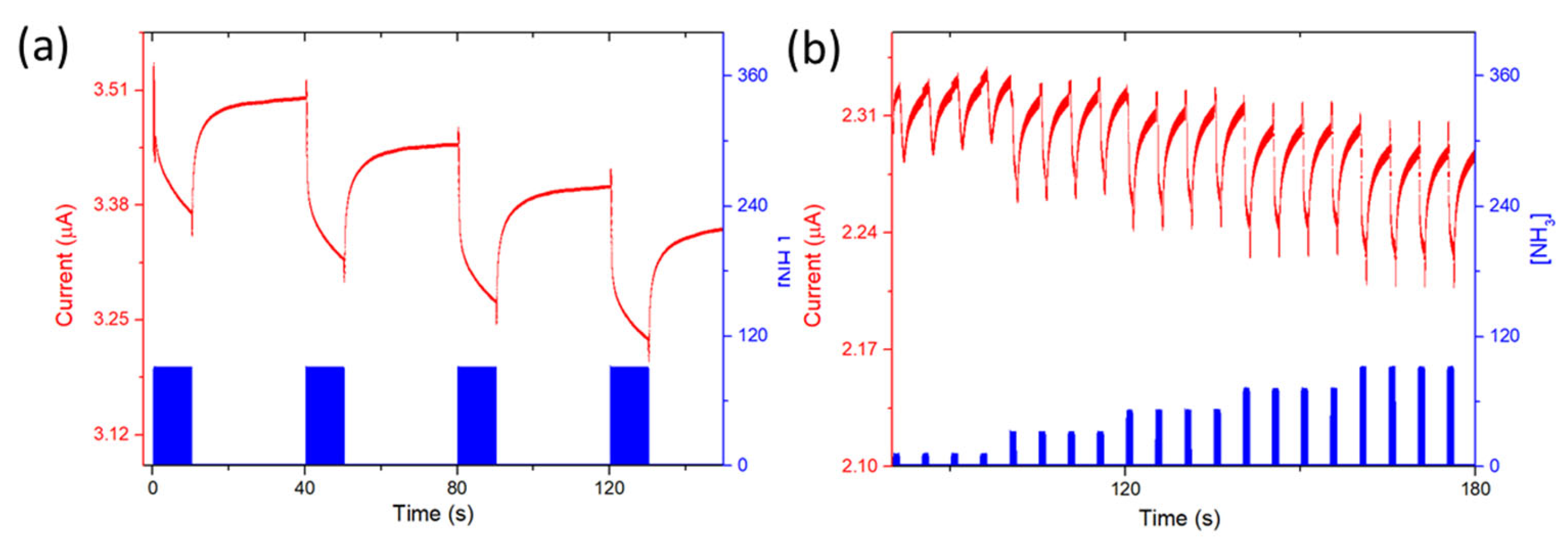
3.4. Ammonia-Sensing Properties
3.5. Humidity-Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Pauly, A. Molecular semiconductor-based gas sensors. In The Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, V., Eds.; American Scientific Publishers: Los Angeles, CA, USA, 2006; Volume 6, pp. 227–270. [Google Scholar]
- Paolesse, R.; Monti, D.; Nardis, S.; Di Natale, C. 54 Porphyrin-Based Chemical Sensors. In Handbook of Porphyrin Science; With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine; World Scientific Publishing Company: Singapore, 2011; Volume 12, pp. 121–225. [Google Scholar]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Parra, V.; Rei Vilar, M.; Battaglini, N.; Ferraria, A.M.; Botelho do Rego, A.M.; Boufi, S.; Rodríguez-Méndez, M.L.; Fonavs, E.; Muzikante, I.; Bouvet, M. New Hybrid Films Based on Cellulose and Hydroxygallium Phthalocyanine. Synergetic Effects in the Structure and Properties. Langmuir 2007, 23, 3712–3722. [Google Scholar]
- Di Natale, C.; Filippini, D.; Pennazza, G.; Santonico, M.; Paolesse, R.; Bellincontro, A.; Mencarelli, F.; D’Amico, A.; Lundström, I. Sorting of apricots with computer screen photoassisted spectral reflectance analysis and electronic nose. Sens. Actuators B Chem. 2006, 119, 70–77. [Google Scholar] [CrossRef]
- Cetó, X.; Apetrei, C.; del Valle, M.; Rodríguez-Méndez, M.L. Evaluation of red wines antioxidant capacity by means of a voltammetric e-tongue with an optimized sensor array. Electrochim. Acta 2014, 120, 180–186. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Barbe, J.-M.; Canard, G.; Brandès, S.; Guilard, R. Organic-inorganic hybrid sol-gel materials incorporating functionalized cobalt(III) corroles for the selective detection of CO. Angew. Chem. Int. Ed. 2005, 44, 3103–3106. [Google Scholar]
- Wang, Y.; Akhigbe, J.; Ding, Y.; Brückner, C.; Lei, Y. meso-Tritolylcorrole-functionalized single-walled carbon nanotube donor-acceptor nanocomposites for NO2 detection. Electroanalysis 2012, 24, 1348–1355. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. Corroles as anion chemosensors: Exploiting their fluorescence behaviour from solution to solid-supported devices. J. Mater. Chem. 2012, 22, 13811–13819. [Google Scholar] [CrossRef]
- Vanotti, M.; Poisson, S.; Soumann, V.; Quesneau, V.; Brandès, S.; Desbois, N.; Yang, J.; André, L.; Gros, C.P.; Blondeau-Patissier, V. Influence of interfering gases on a carbon monoxide differential sensor based on SAW devices functionalized with cobalt and copper corroles. Sens. Actuators B Chem. 2021, 332, 129507. [Google Scholar] [CrossRef]
- Di Natale, C.; Gros, C.P.; Paolesse, R. Corroles at work: A small macrocycle for great applications. Chem. Soc. Rev. 2022, 51, 1277–1335. [Google Scholar] [CrossRef]
- Johnson, A.W.; Kay, I.T. 306. Corroles. Part I. Synthesis. J. Chem. Soc. 1965, 1620–1629. [Google Scholar] [CrossRef]
- Paolesse, R.; Mini, S.; Sagone, F.; Boschi, T.; Jaquinod, L.; Nurco, D.J.; Smith, K.M. 5,10,15-Triphenylcorrole: A product from a modified Rothemund reaction. Chem. Commun. 1999, 14, 1307–1308. [Google Scholar]
- Gross, Z.; Galili, N.; Saltsman, I. The first direct synthesis of corroles from pyrrole. Angew. Chem. Int. Ed. Engl. 1999, 38, 1427–1429. [Google Scholar] [PubMed]
- Tang, J.; Chen, B.; Zhang, Y.; Lu, J.; Zhang, T.; Guo, Q.; Zhang, J. Synthesis and gas sensitivity properties of novel metallocorroles and functionalized graphene oxide. Funct. Mater. Let. 2019, 12, 1940001. [Google Scholar] [CrossRef]
- Parra, V.; Brunet, J.; Pauly, A.; Bouvet, M. Molecular semiconductor-doped insulator (MSDI) heterojunctions: An alternative transducer for gas chemosensing. Analyst 2009, 134, 1776–1778. [Google Scholar] [CrossRef]
- Mateos, M.; Meunier-Prest, R.; Heintz, O.; Herbst, F.; Suisse, J.-M.; Bouvet, M. Comprehensive Study of poly(2,3,5,6-tetrafluoroaniline): From electrosynthesis to heterojunctions and ammonia sensing. ACS Appl. Mater. Interfaces 2018, 10, 19974–19986. [Google Scholar] [PubMed]
- Kumar, A.; Alami Mejjati, N.; Meunier-Prest, R.; Krystianiak, A.; Heintz, O.; Lesniewska, E.; Devillers, C.H.; Bouvet, M. Tuning of interfacial charge transport in polyporphine/phthalocyanine heterojunctions by molecular geometry control for an efficient gas sensor. Chem. Eng. J. 2022, 429, 132453. [Google Scholar] [CrossRef]
- Di Zazzo, L.; Kumar, A.; Meunier-Prest, R.; Di Natale, C.; Paolesse, R.; Bouvet, M. Electrosynthesized copper polycorroles as versatile materials in double lateral heterojunctions. Chem. Eng. J. 2023, 458, 141465. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Meunier-Prest, R.; Kumar, A.; Bayo-Bangoura, M.; Bouvet, M. Modulating the electrical properties of organic heterojunction devices based on phthalocyanines for ambipolar sensors. ACS Sens. 2020, 5, 1849–1857. [Google Scholar] [CrossRef]
- Wannebroucq, A.; Gruntz, G.; Suisse, J.-M.; Nicolas, Y.; Meunier-Prest, R.; Mateos, M.; Toupance, T.; Bouvet, M. New n-type molecular semiconductor–doped insulator (MSDI) heterojunctions combining a triphenodioxazine (TPDO) and the lutetium bisphthalocyanine (LuPc2) for ammonia sensing. Sens. Actuators B Chem. 2018, 255, 1694–1700. [Google Scholar] [CrossRef]
- Sahin, Z.; Meunier-Prest, R.; Dumoulin, F.; Kumar, A.; Isci, U.; Bouvet, M. Tuning of organic heterojunction conductivity by the substituents’ electronic effects in phthalocyanines for ambipolar gas sensors. Sens. Actuators B Chem. 2021, 332, 129505. [Google Scholar]
- Feng, Q.; Li, X.; Wang, J.; Gaskov, A.M. Reduced graphene oxide (rGO) encapsulated Co3O4 composite nanofibers for highly selective ammonia sensors. Sens. Actuators B Chem. 2016, 222, 864–870. [Google Scholar] [CrossRef]
- Huang, X.L.; Hu, N.T.; Wang, Y.Y.; Zhang, Y.F. Ammonia gas sensor based on aniline reduced graphene oxide. Adv. Mater. Res. 2013, 669, 79–84. [Google Scholar]
- Bouvet, M.; Mateos, M.; Wannebroucq, A.; Navarrete, E.; Llobet, E. Tungsten oxide—Lutetium bisphthalocyanine n-p-n heterojunction: From nanomaterials to a new transducer for chemo-sensing. J. Mater. Chem. C 2019, 7, 6448–6455. [Google Scholar] [CrossRef]
- Bouvet, M. Radical phthalocyanines and intrinsic semiconduction. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2003; Volume 19, pp. 37–103. [Google Scholar]
- Bouvet, M.; Ouedraogo, S.; Meunier-Prest, R. Ambipolar materials for gas sensors. In Ambipolar Materials and Devices; Zhou, Y., Han, S.T., Eds.; Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar]
- Clarisse, C.; Riou, M.T. Synthesis and characterization of some lanthanide phthalocyanines. Inorg. Chim. Acta 1987, 130, 139–144. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Sagone, F.; Khoury, R.G. Synthesis and Functionalization of meso-Aryl-Substituted Corroles. J. Org. Chem. 2001, 66, 550–556. [Google Scholar]
- Stefanelli, M.; Mastroianni, M.; Nardis, S.; Licoccio, S.; Fronczek, F.R.; Smith, K.M.; Zhu, W.; Ou, Z.; Kadish, K.M.; Paolesse, R. Functionalization of corroles: The nitration reaction. Inorg. Chem. 2007, 46, 10791–10799. [Google Scholar] [CrossRef]
- Bouvet, M.; Xiong, H.; Parra, V. Molecular semiconductor-doped insulator (MSDI) heterojunctions: Oligothiophene/bisphtalocyanine (LuPc2) and perylene/bisphthalocyanine as new structures for gas sensing. Sens. Actuators B Chem. 2010, 145, 501–506. [Google Scholar]
- Ahmida, M.M.; Eichhorn, S.H. Measurements and prediction of electronic properties of discotic liquid crystalline triphenylenes and phthalocyanines. ECS Trans. 2010, 25, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Bouvet, M.; Sizun, T.; Gao, Y.; Plassard, C.; Lesniewska, E.; Jiang, J. Facile approaches to build ordered amphiphilic tris(phthalocyaninato) europium triple-decker complex thin films and their comparative performances in ozone sensing. Phys. Chem. Chem. Phys. 2010, 12, 12851–12861. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Parra, V.; Suisse, J.M. Molecular semiconductor-doped insulator (MSDI) heterojunctions as new transducers for chemical sensors. Eur. Phys. J. Appl. Phys. 2011, 56, 34103–34110. [Google Scholar] [CrossRef]
- Sharts, C.M.; Gorelik, V.S.; Agoltsov, A.M.; Zlobina, L.I.; Sharts, O.N. Detection of carbon-fluorine bonds in organofluorine compounds by Raman spectroscopy using a copper-vapor laser. Proc. SPIE-Int. Soc. Opt. Eng. 1999, 3537, 317. [Google Scholar]
- Menaa, F.; Menaa, B.; Sharts, O. Development of carbon-fluorine spectroscopy for pharmaceutical and biomedical applications. Faraday Discuss. 2011, 149, 269. [Google Scholar] [CrossRef]
- Wasbotten, I.H.; Wondimagegn, T.; Ghosh, A. Electronic Absorption, Resonance Raman, and electrochemical studies of planar and saddled copper(iii) meso-triarylcorroles. Highly substituent-sensitive Soret bands as a distinctive feature of high-valent transition metal corroles. J. Am. Chem. Soc. 2002, 124, 8104–8116. [Google Scholar] [CrossRef]
- Wannebroucq, A.; Ouedraogo, S.; Meunier-Prest, R.; Suisse, J.-M.; Bayo, M.; Bouvet, M. On the interest of ambipolar materials for gas sensing. Sens. Actuators B Chem. 2018, 258, 657–664. [Google Scholar] [CrossRef]


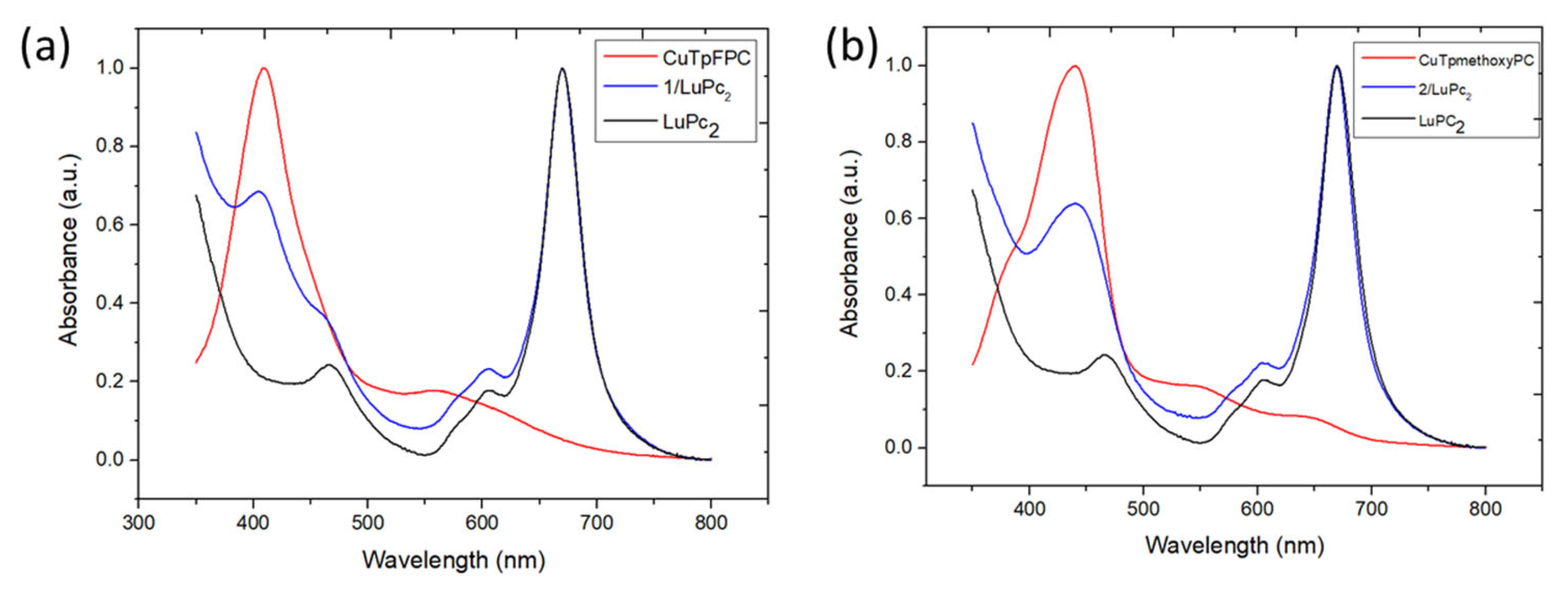







| Chemical | Soret Band and Other Bands (nm) | Q Band (nm) |
|---|---|---|
| LuPc2 | 460, 605 | 669 |
| CuTpFPC (1) in solution | 403 | 553 |
| CuTpFPC (1) on glass | 410 | 560 |
| 1/LuPc2 heterostructure | 409, 460, 604 | 669 |
| CuTp-methoxyPC (2) in solution | 432 | 541, 627 |
| CuTp-methoxyPC (2) on glass | 441 | 551, 647 |
| 2/LuPc2 heterostructure | 439, 605 | 669 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Zazzo, L.; Ganesh Moorthy, S.; Meunier-Prest, R.; Lesniewska, E.; Di Natale, C.; Paolesse, R.; Bouvet, M. Ammonia and Humidity Sensing by Phthalocyanine–Corrole Complex Heterostructure Devices. Sensors 2023, 23, 6773. https://doi.org/10.3390/s23156773
Di Zazzo L, Ganesh Moorthy S, Meunier-Prest R, Lesniewska E, Di Natale C, Paolesse R, Bouvet M. Ammonia and Humidity Sensing by Phthalocyanine–Corrole Complex Heterostructure Devices. Sensors. 2023; 23(15):6773. https://doi.org/10.3390/s23156773
Chicago/Turabian StyleDi Zazzo, Lorena, Sujithkumar Ganesh Moorthy, Rita Meunier-Prest, Eric Lesniewska, Corrado Di Natale, Roberto Paolesse, and Marcel Bouvet. 2023. "Ammonia and Humidity Sensing by Phthalocyanine–Corrole Complex Heterostructure Devices" Sensors 23, no. 15: 6773. https://doi.org/10.3390/s23156773
APA StyleDi Zazzo, L., Ganesh Moorthy, S., Meunier-Prest, R., Lesniewska, E., Di Natale, C., Paolesse, R., & Bouvet, M. (2023). Ammonia and Humidity Sensing by Phthalocyanine–Corrole Complex Heterostructure Devices. Sensors, 23(15), 6773. https://doi.org/10.3390/s23156773