Humidity Sensor Composed of Laser-Induced Graphene Electrode and Graphene Oxide for Monitoring Respiration and Skin Moisture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
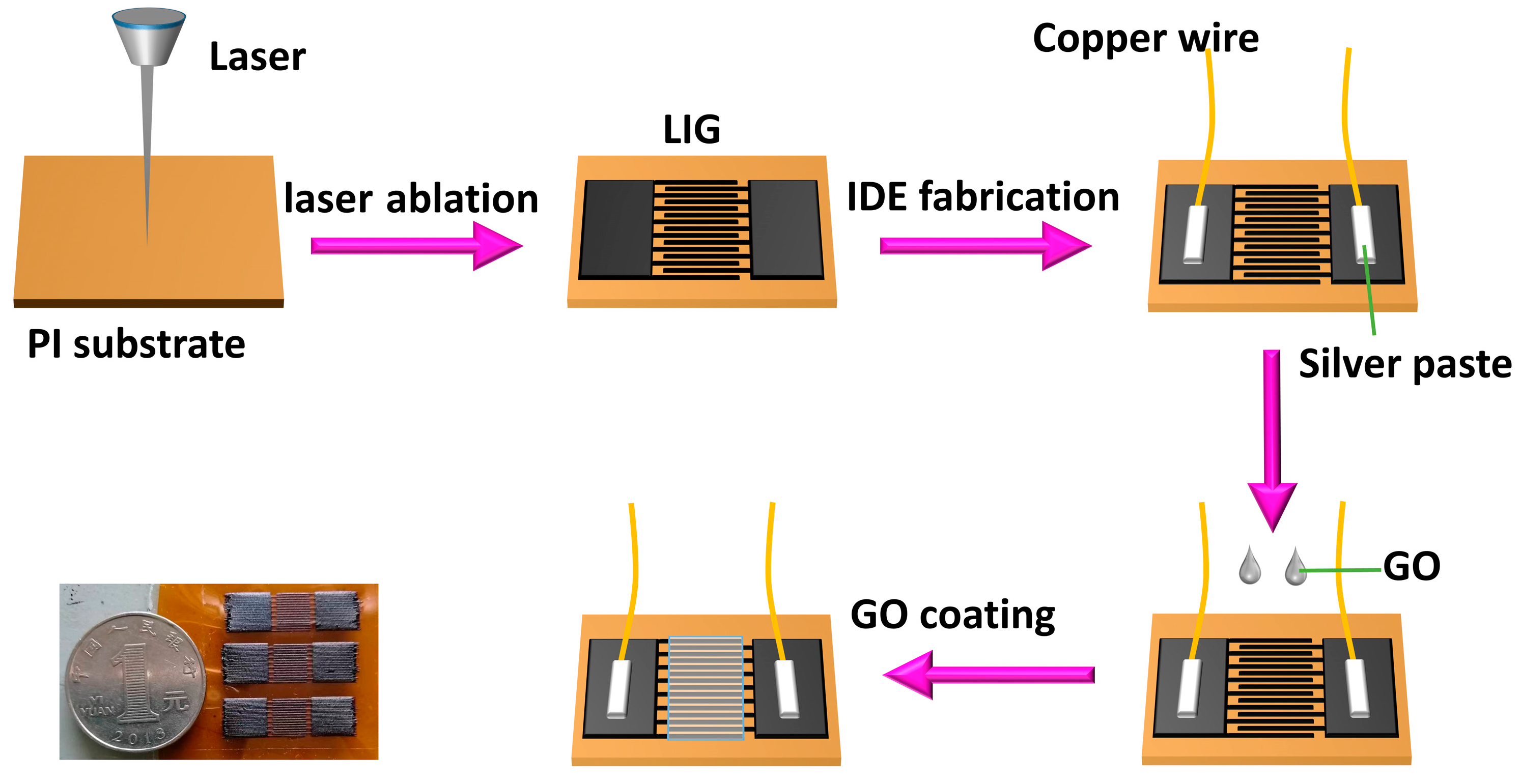
2.2. Fabrication of Humidity Sensor
2.3. Materials Characterizations
2.4. Humidity Sensing System
3. Results and Discussion
3.1. Morphology and Structure Analysis of LIG
3.2. Humidity Sensing Characteristics
3.2.1. Comparison of PI-Based Sensors with Different Electrode Gap Sizes
3.2.2. Comparison of GO-Based Sensors with Different Electrode Gap Sizes
3.2.3. Sensor Response Time and Stability
3.2.4. Comparison of Capacitive Humidity Sensors
3.3. Respiratory and Skin Humidity Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huo, Y.M.; Bu, M.M.; Ma, Z.T.; Sun, J.Y.; Yan, Y.H.; Xiu, K.H.; Wang, Z.Y.; Hu, N.; Li, Y.F. Flexible, non-contact and multifunctional humidity sensors based on two-dimensional phytic acid doped co-metal organic frameworks nanosheets. J. Colloid Interface Sci. 2022, 607, 2010–2018. [Google Scholar] [CrossRef]
- Tai, H.L.; Duan, Z.H.; Wang, Y.; Wang, S.; Jiang, Y.D. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- He, J.; Xiao, P.; Shi, J.W.; Liang, Y.; Lu, W.; Chen, Y.S.; Wang, W.Q.; Theato, P.; Kuo, S.W.; Chen, T. High Performance Humidity Fluctuation Sensor for Wearable Devices via a Bioinspired Atomic-Precise Tunable Graphene-Polymer Heterogeneous Sensing Junction. Chem. Mat. 2018, 30, 4343–4354. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Fei, T.; Zhang, T. Design strategy for ultrafast-response humidity sensors based on gel polymer electrolytes and application for detecting respiration. Sens. Actuators B Chem. 2020, 304, 127270. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhao, Z.Z.; Zeng, X.W.; Fu, X.L.; Hu, Y. Expandable microsphere-based triboelectric nanogenerators as ultrasensitive pressure sensors for respiratory and pulse monitoring. Nano Energy 2019, 59, 295–301. [Google Scholar] [CrossRef]
- Luo, Y.; Pei, Y.; Feng, X.; Zhang, H.; Lu, B.; Wang, L. Silk fibroin based transparent and wearable humidity sensor for ultra-sensitive respiration monitoring. Mater. Lett. 2020, 260, 126945. [Google Scholar] [CrossRef]
- Zhu, C.C.; Tao, L.Q.; Wang, Y.; Zheng, K.; Yu, J.B.; Xiandong, L.; Chen, X.P.; Huang, Y.X. Graphene oxide humidity sensor with laser-induced graphene porous electrodes. Sens. Actuator B Chem. 2020, 325, 128790. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Capacitive type humidity sensor based on PANI decorated Cu-ZnS porous microspheres. Talanta 2020, 219, 121361. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, N.; Xu, D.; Tang, L.; Sheng, B. Flexible humidity sensors composed with electrodes of laser induced graphene and sputtered sensitive films derived from poly(ether-ether-ketone). Sens. Actuators B Chem. 2023, 375, 132846. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Xu, Z.Y.; Yang, Z.M.; Song, X.S. High-performance flexible self-powered tin disulfide nanoflowers/reduced graphene oxide nanohybrid-based humidity sensor driven by triboelectric nanogenerator. Nano Energy 2020, 67, 104251. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, H.; Ward, J.E.; Liu, X.; Yin, B.; Fu, T.; Chen, J.; Lovley, D.R.; Yao, J. Power generation from ambient humidity using protein nanowires. Nature 2020, 578, 550–554. [Google Scholar] [CrossRef]
- Gong, L.; Wang, X.; Zhang, D.; Ma, X.; Yu, S. Flexible wearable humidity sensor based on cerium oxide/graphitic carbon nitride nanocomposite self-powered by motion-driven alternator and its application for human physiological detection. J. Mater. Chem. A 2021, 9, 5619–5629. [Google Scholar] [CrossRef]
- Zhao, Q.; Duan, Z.; Wu, Y.; Liu, B.; Yuan, Z.; Jiang, Y.; Tai, H. Facile primary battery-based humidity sensor for multifunctional application. Sens. Actuators B Chem. 2022, 370, 132369. [Google Scholar] [CrossRef]
- Shao, M.; Sun, H.N.; Zhang, R.; Li, L.P.; Liu, Y.G.; Qiao, X.G. In-fiber humidity sensor based on Black Phosphorus-Polyvinyl alcohol. Opt. Fiber Technol. 2022, 68, 102782. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, R.; Song, X.; Wang, D.; Zhang, H.; Xia, H.; Ma, Y.; Gao, Y. Humidity sensing properties and respiratory behavior detection based on chitosan-halloysite nanotubes film coated QCM sensor combined with support vector machine. Sens. Actuators B Chem. 2023, 374, 132824. [Google Scholar] [CrossRef]
- Han, Y.C.; Kong, X.Y.; Wu, W.; Li, J.S.; Yang, X.; Guo, Y.J.; Fu, Y.Q.; Torun, H.; Xiang, X.; Tang, Y.L.; et al. Environment-friendly surface acoustic wave humidity sensor with sodium alginate sensing layer. Micro Nano Eng. 2022, 15, 100127. [Google Scholar] [CrossRef]
- Beniwal, A.; Ganguly, P.; Aliyana, A.K.; Khandelwal, G.; Dahiya, R. Screen-printed graphene-carbon ink based disposable humidity sensor with wireless communication. Sens. Actuators B Chem. 2023, 374, 132731. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, L.N. Humidity sensors based on TiO2 nanoparticles/polypyrrole composite thin films. Sens. Actuator B Chem. 2007, 123, 501–507. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Yuan, Z.; Li, S.; Liang, J.; Jiang, Y.; Tai, H. High performance humidity sensor based on 3D mesoporous Co3O4 hollow polyhedron for multifunctional applications. Appl. Surf. Sci. 2022, 585, 152698. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Yuan, Z.; Zhang, Y.; Li, X.; Wu, Y.; Jiang, Y.; Tai, H. Novel application of attapulgite on high performance and low-cost humidity sensors. Sens. Actuators B Chem. 2020, 305, 127534. [Google Scholar] [CrossRef]
- Hassan, M.; Afify, A.S.; Tulliani, J.M. Synthesis of ZnO Nanoparticles onto Sepiolite Needles and Determination of Their Sensitivity toward Humidity, NO2 and H2. J. Mater. Sci. Technol. 2016, 32, 573–582. [Google Scholar] [CrossRef]
- Khan, M.U.; Abbas, Y.; Abunahla, H.; Rezeq, M.d.; Alazzam, A.; Alamoodi, N.; Mohammad, B. Biocompatible humidity sensor using paper cellulose fiber/GO matrix for human health and environment monitoring. Sens. Actuators B Chem. 2023, 393, 134188. [Google Scholar] [CrossRef]
- Wu, Z.J.; Liu, W.Q.; Shi, J.; Han, B.S.; Li, D.T.; Xu, X.B.; Chen, W.H. Renewable and fast response humidity sensors based on multiple construction of water graftable molecules highly sensitive surface. Surf. Interfaces 2022, 31, 102035. [Google Scholar] [CrossRef]
- Han, M.; Shen, W. Nacre-inspired cellulose nanofiber/MXene flexible composite film with mechanical robustness for humidity sensing. Carbohydr. Polym. 2022, 298, 120109. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakamura, T.; Tsuchiya, T. Flexible humidity sensors composed of graphite-like carbon micro-pinecone arrays. RSC Adv. 2016, 6, 95342–95348. [Google Scholar] [CrossRef]
- Pang, Y.; Jian, J.M.; Tu, T.; Yang, Z.; Ling, J.; Li, Y.X.; Wang, X.F.; Qiao, Y.C.; Tian, H.; Yang, Y.; et al. Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 2018, 116, 123–129. [Google Scholar] [CrossRef]
- Li, B.L.; Tian, Q.; Su, H.X.; Wang, X.W.; Wang, T.E.; Zhang, D.Z. High sensitivity portable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuator B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Li, J.Z.; Zhang, J.Q.; Sun, H.; Yang, Y.X.; Ye, Y.L.; Cui, J.T.; He, W.M.; Yong, X.; Xie, Y. An optical fiber sensor based on carboxymethyl cellulose/carbon nanotubes composite film for simultaneous measurement of relative humidity and temperature. Opt. Commun. 2020, 467, 125740. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.D.; Yu, X.; Ding, X.; Yu, X.L.; Chen, X.P. Fast response humidity sensor based on graphene oxide films supported by TiO2 nanorods. Diam. Relat. Mat. 2020, 109, 108031. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhai, H.; Chen, X.; Liu, Y.L.; Wang, M.; Liu, Z.C.; Umar, M.; Ji, C.Y.; Chen, Z.D.; Jin, L.; et al. Coolmax/graphene-oxide functionalized textile humidity sensor with ultrafast response for human activities monitoring. Chem. Eng. J. 2021, 412, 128639. [Google Scholar] [CrossRef]
- Li, X.; Feng, W.D.; Zhang, X.X.; Lin, S.Y.; Chen, Y.Q.; Chen, C.W.; Chen, S.J.; Wang, W.; Zhang, Y.N. Facile fabrication of laser-scribed-graphene humidity sensors by a commercial DVD drive. Sens. Actuator B Chem. 2020, 321, 128483. [Google Scholar] [CrossRef]
- Le, X.H.; Liu, Y.H.; Peng, L.; Pang, J.T.; Xu, Z.; Gao, C.; Xie, J. Surface acoustic wave humidity sensors based on uniform and thickness controllable graphene oxide thin films formed by surface tension. Microsyst. Nanoeng. 2019, 5, 10. [Google Scholar] [CrossRef]
- Li, X.Y.; Ni, L.; Chen, N.; Liu, J.L.; Li, W.J.; Xian, Y. Enhanced sensitivity of humidity sensor using Nafion/graphene oxide quantum dot nanocomposite. Measurement 2021, 181, 109566. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Li, W.; Gou, C.; Zheng, M.; Zhang, Y.; Chen, Z.; Hong, Y. High-sensitive humidity sensor based on MoS2/graphene oxide quantum dot nanocomposite. Mater. Chem. Phys. 2022, 287, 126146. [Google Scholar] [CrossRef]
- Nassar, J.M.; Khan, S.M.; Villalva, D.R.; Nour, M.M.; Almuslem, A.S.; Hussain, M.M. Compliant plant wearables for localized microclimate and plant growth monitoring. npj Flex. Electron. 2018, 2, 24. [Google Scholar] [CrossRef]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd-WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.M.; Wu, Z.X.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon Nanocoil-Based Fast-Response and Flexible Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Lamberti, A.; Clerici, F.; Fontana, M.; Scaltrito, L. A Highly Stretchable Supercapacitor Using Laser-Induced Graphene Electrodes onto Elastomeric Substrate. Adv. Energy Mater. 2016, 6, 1600050. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.L.; Be’er, A.; Oren, Y.; Tour, J.M.; Arnusch, C.J. Laser-Induced Graphene Layers and Electrodes Prevents Microbial Fouling and Exerts Antimicrobial Action. ACS Appl. Mater. Interfaces 2017, 9, 18238–18247. [Google Scholar] [CrossRef]
- Ran, M.; Jia, L.S.; Cheng, C.G.; Wu, Q.L. Temperature-variable Raman scattering study on micromechanical properties of the carbon fiber reinforced polyimide composite film. New Carbon Mater. 2019, 34, 105–109. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Loir, A.S.; Barnier, V.; Garrelie, F.; Donnet, C. Raman study of the substrate influence on graphene synthesis using a solid carbon source via rapid thermal annealing. J. Raman Spectrosc. 2019, 50, 1630–1641. [Google Scholar] [CrossRef]
- Bi, H.C.; Yin, K.B.; Xie, X.; Ji, J.; Wan, S.; Sun, L.T.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef]
- Shen, D.D.; Liu, Y.; Yu, M.; Kong, F.Y.; Xin, B.J.; Liu, Y. Bioinspired flexible and highly responsive PVDF-based humidity sensors for respiratory monitoring. Polymer 2022, 254, 125103. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Liu, J.; Moon, K.-s.; Lu, L.; Lin, Z.; Yuan, W.; Shen, C.; Zang, X.; Lin, L.; et al. Laser-induced and KOH-activated 3D graphene: A flexible activated electrode fabricated via direct laser writing for in-plane micro-supercapacitors. Chem. Eng. J. 2020, 393, 124672. [Google Scholar] [CrossRef]
- Seeneevassen, S.; Leong, A.; Kashan, M.A.M.; Swamy, V.; Ramakrishnan, N. Effect of effluent gas composition on characteristics of graphene oxide film based relative humidity sensor. Measurement 2022, 195, 111156. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Mu, Y.; Jin, P.; Zheng, L.; Wang, C.; Hou, Y.; Liu, W.; Si, L.; Liu, Z. An enhanced MEMS-based polyimide capacitive-type relative-humidity sensor with halloysite nanotube as a modifier. Microchem. J. 2023, 191, 108934. [Google Scholar] [CrossRef]
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-step and large-scale fabrication of flexible and wearable humidity sensor based on laser-induced graphene for real-time tracking of plant transpiration at bio-interface. Biosens. Bioelectron. 2020, 165, 112360. [Google Scholar] [CrossRef]
- Qiang, T.; Wang, C.; Liu, M.-Q.; Adhikari, K.K.; Liang, J.-G.; Wang, L.; Li, Y.; Wu, Y.-M.; Yang, G.-H.; Meng, F.-Y.; et al. High-Performance porous MIM-type capacitive humidity sensor realized via inductive coupled plasma and reactive-Ion etching. Sens. Actuators B Chem. 2018, 258, 704–714. [Google Scholar] [CrossRef]
- Dwiputra, M.A.; Fadhila, F.; Imawan, C.; Fauzia, V. The enhanced performance of capacitive-type humidity sensors based on ZnO nanorods/WS2 nanosheets heterostructure. Sens. Actuators B Chem. 2020, 310, 127810. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B Chem. 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Niu, H.; Yue, W.; Li, Y.; Yin, F.; Gao, S.; Zhang, C.; Kan, H.; Yao, Z.; Jiang, C.; Wang, C. Ultrafast-response/recovery capacitive humidity sensor based on arc-shaped hollow structure with nanocone arrays for human physiological signals monitoring. Sens. Actuators B Chem. 2021, 334, 129637. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Ziaie, B. Comparison of Direct and Indirect Laser Ablation of Metallized Paper for Inexpensive Paper-Based Sensors. ACS Appl. Mater. Interfaces 2018, 10, 36332–36341. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Smolander, M. Paper as Active Layer in Inkjet-Printed Capacitive Humidity Sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Yang, Z. Facile fabrication of graphene oxide/Nafion/indium oxide for humidity sensing with highly sensitive capacitance response. Sens. Actuators B Chem. 2019, 292, 187–195. [Google Scholar] [CrossRef]









| Gap Size (Electrode Area) | GO (30 μL) | GO (60 μL) | GO (90 μL) | GO (120 μL) | Max Response |
|---|---|---|---|---|---|
| 50 μm(A 1 = 32.34 mm2) | 0.93 μL/mm2 (1934 pF/RH) | 1.86 μL/mm2 (2228 pF/RH) | 2.79 μL/mm2 (1594 pF/RH) | 3.72μL/mm2 (1696 pF/RH) | 1.86 μL/mm2 |
| 150 μm(A = 41.34 mm2) | 0.73 μL/mm2 (2398 pF/RH) | 1.45 μL/mm2 (3862 pF/RH) | 2.2 μL/mm2 (2385 pF/RH) | 2.9 μL/mm2 (3087 pF/RH) | 1.45 μL/mm2 |
| 350 μm(A = 59.34 mm2) | 0.5 μL/mm2 (256 pF/RH) | 1.01 μL/mm2 (2730 pF/RH) | 1.51 μL/mm2 (3748 pF/RH) | 2.02 μL/mm2 (3656 pF/RH) | 1.51 μL/mm2 |
| Material | Structure | Frequency (Hz) | Range (%RH) | Sensitivity (pF/RH) | Response/Recovery Time (s) | Ref. |
|---|---|---|---|---|---|---|
| HNTs-NH2/PI | MIM a | - | 10–90 | 0.87 | 12/8 | [49] |
| In2O3/GO | IDE b | 100 | 11–97 | 1061.6 | 15/2.5 | [29] |
| LIG/GO | IDE | 50 | 10–90 | 3215 | 15.8/- | [50] |
| PMDA/ODA/TiO2 | MIM | 1000 | 10–90 | 1.24 | 25/25 | [51] |
| ZnO NR/WS2 | Electrode | 1000 | 18–85 | 0.107 | 74.5/25.6 | [52] |
| GO/PDDA | IDE | 104 | 11–97 | 1552.3 | -/- | [53] |
| P(VDF-TrFE) | Arc-shaped hollow | 106 | 20–90 | ~0.009 | 3.7/3.4 | [54] |
| PCFGOM | paper cellulose | 1000 | 10–90 | 0.74 | 1.3/0.8 | [24] |
| Paper by laser ablation | IDE | 1000 | 0–90 | 2 | 266/126 | [55] |
| Ag colloidal ink | IDE | 105 | 30–85 | 2 | 250/175 | [56] |
| GO/Nafion/In2O3 | IDE | 100 | 11–97 | 3080 | -/- | [57] |
| LIG/GO | IDE | 100 | 10–90 | 3862 | 58/15 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, X.; Huang, J.; Shi, W. Humidity Sensor Composed of Laser-Induced Graphene Electrode and Graphene Oxide for Monitoring Respiration and Skin Moisture. Sensors 2023, 23, 6784. https://doi.org/10.3390/s23156784
Fei X, Huang J, Shi W. Humidity Sensor Composed of Laser-Induced Graphene Electrode and Graphene Oxide for Monitoring Respiration and Skin Moisture. Sensors. 2023; 23(15):6784. https://doi.org/10.3390/s23156784
Chicago/Turabian StyleFei, Xianxiang, Junyi Huang, and Wenqing Shi. 2023. "Humidity Sensor Composed of Laser-Induced Graphene Electrode and Graphene Oxide for Monitoring Respiration and Skin Moisture" Sensors 23, no. 15: 6784. https://doi.org/10.3390/s23156784
APA StyleFei, X., Huang, J., & Shi, W. (2023). Humidity Sensor Composed of Laser-Induced Graphene Electrode and Graphene Oxide for Monitoring Respiration and Skin Moisture. Sensors, 23(15), 6784. https://doi.org/10.3390/s23156784






