Monitoring the Opening of Rapid Palatal Expansion (RPE) in a 3D-Printed Skull Model Using Fiber Optic F–P Sensors
Abstract
:1. Introduction
2. Materials and Methods
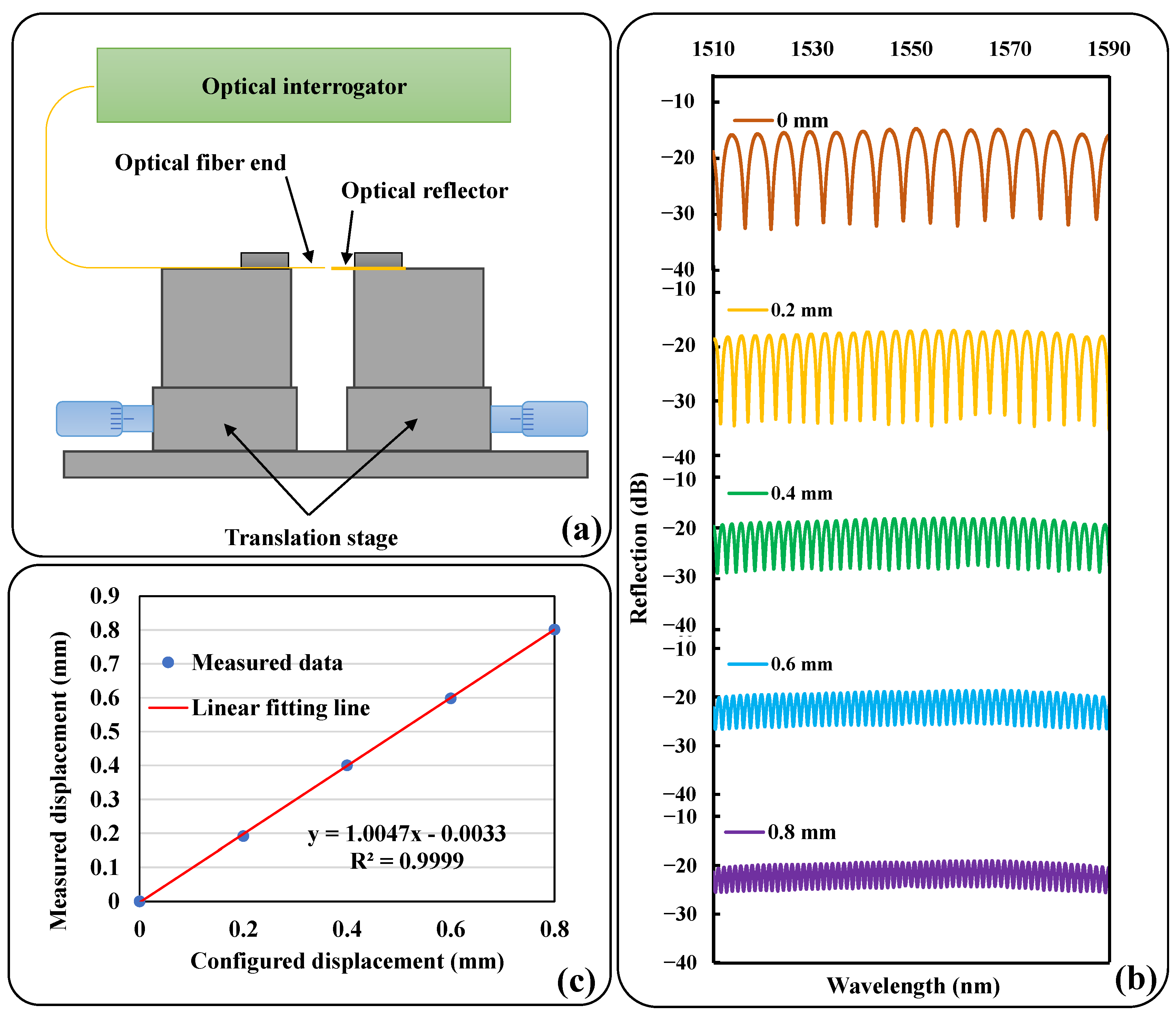
2.1. Sensor Concepts and Principles
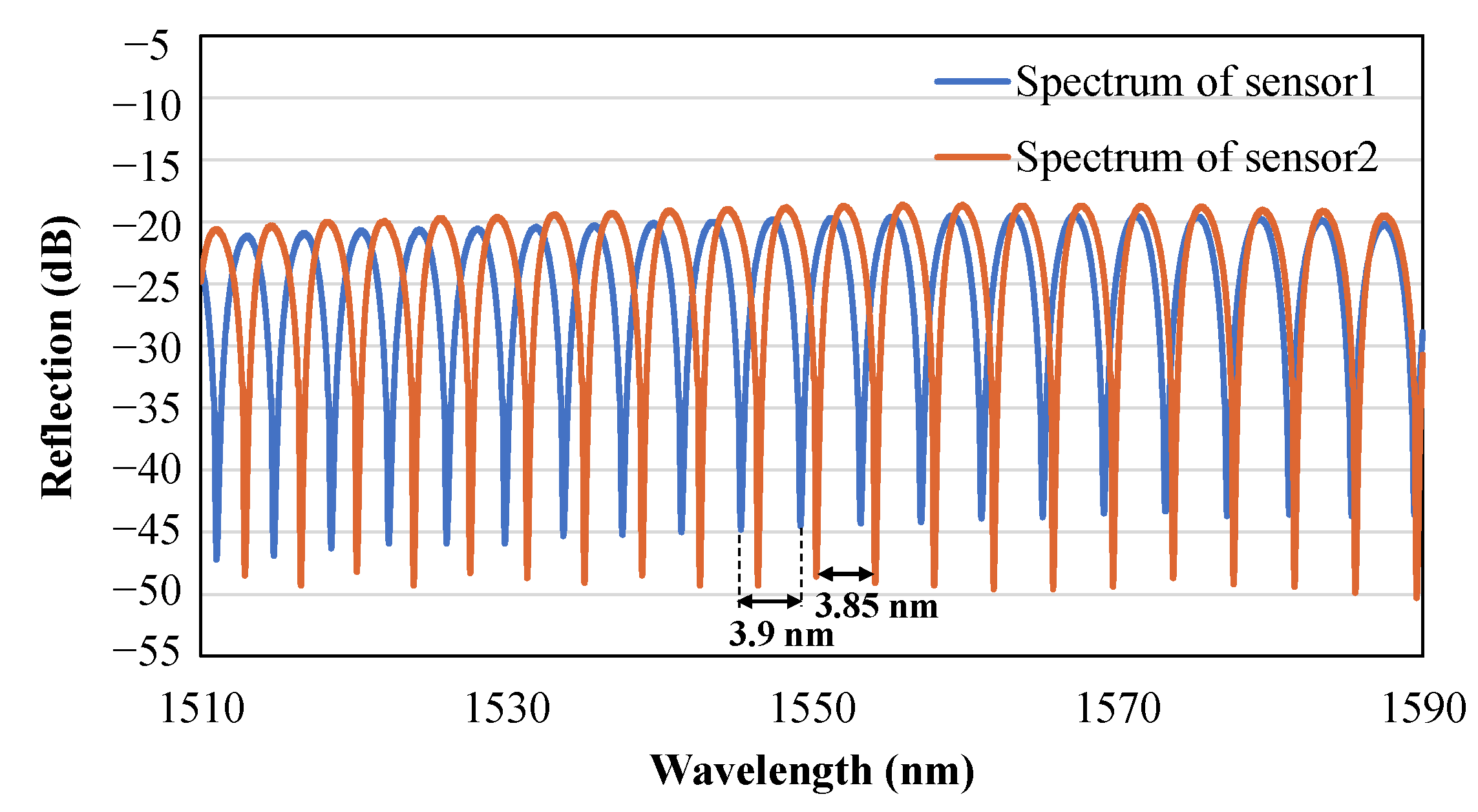
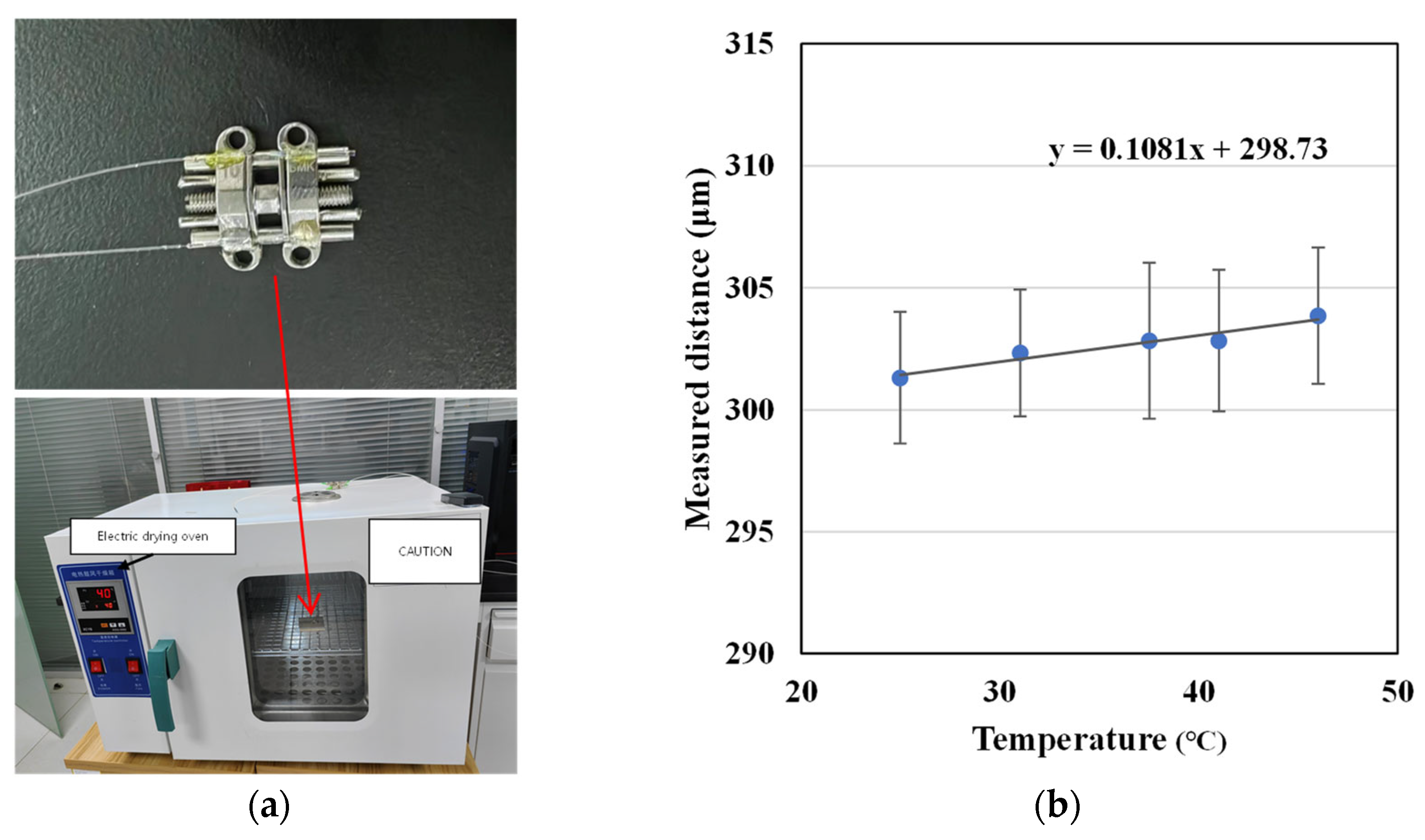
2.2. Preliminary Test
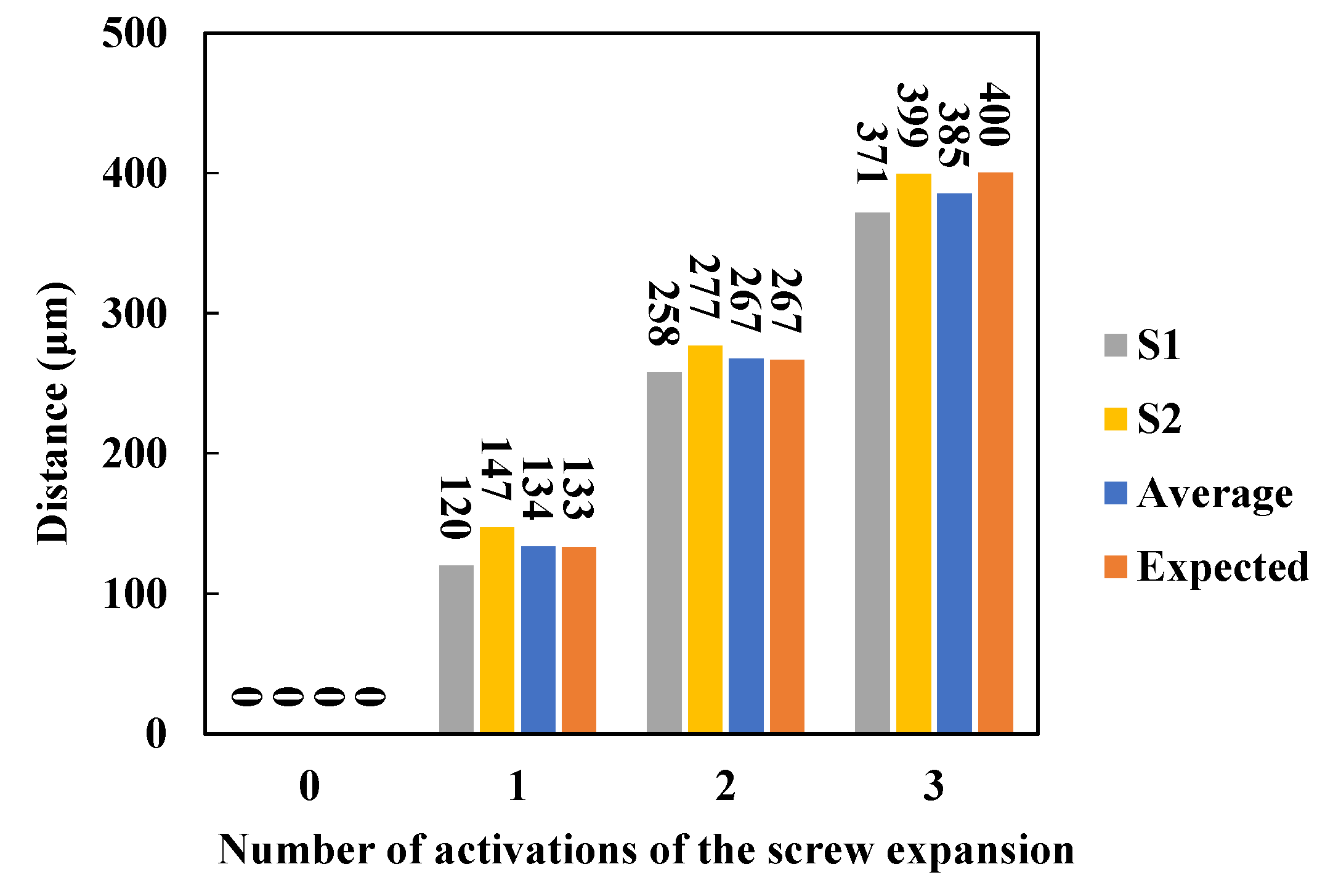
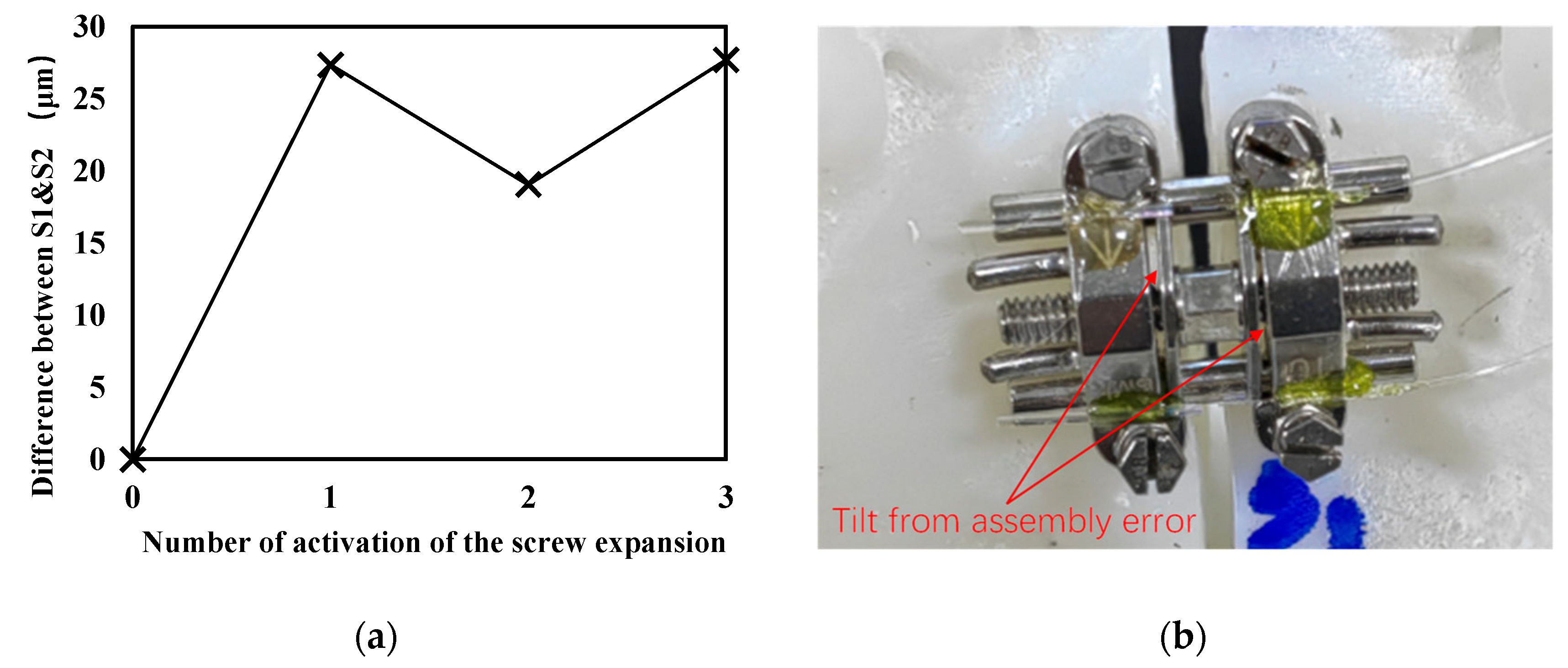
2.3. Simulation System Based on 3D-Printed Model
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simões, J.A. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: A review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Fakih, E.; Osman, N.; Adikan, F. The use of fiber bragg grating sensors in biomechanics and rehabilitation applications: The state-of-the-art and ongoing research topics. Sensors 2012, 12, 12890–12926. [Google Scholar] [CrossRef] [PubMed]
- Zaltieri, M.; Presti, D.; Bravi, M.; Caponero, M.; Sterzi, S.; Schena, E.; Massaroni, C. Assessment of a Multi-Sensor FBG-Based Wearable System in Sitting Postures Recognition and Respiratory Rate Evaluation of Office Workers. IEEE Trans. Biomed. Eng. 2023, 70, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, A.; Karam, L.Z.; Pegorini, V.; Renzo, A.B.D.; Pitta, C.S.R.; Cardoso, R.; Assmann, T.S.; Kalinowski, H.J.; Silva, J.C.C.D. Optical Fiber Bragg Grating Strain Sensor for Bone Stress Analysis in Bovine During Masticatory Movements. IEEE Sens. J. 2017, 17, 2385–2392. [Google Scholar] [CrossRef]
- Vakiel, C.R.; Dennison; Shekarforoush, M.; Scott, M.; Hart, D.A.; Shrive, N.G. Measuring the Internal Stress in Ovine Meniscus During Simulated In Vivo Gait Kinematics: A Novel Method Using Fibre Optic Technology. Ann. Biomed. Eng. 2021, 49, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Romanyk, D.L.; Guan, R.; Major, P.W.; Dennison, C.R. Repeatability of strain magnitude and strain rate measurements in the periodontal ligament using fibre Bragg gratings: An ex vivo study in a swine model. J. Biomech. 2017, 54, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.P.G.D.O.; Costa, M.M.M.; Karam, L.Z.; Gomes, O.M.M.; Kalinowski, H.J. Dentistry Applications of Fiber Bragg Gratings: Irradiation Protocols for Bulk Fill Flow Dental Composites. J. Light. Technol. 2019, 37, 4881–4887. [Google Scholar] [CrossRef]
- Houg, K.P.; Camarillo, A.M.; Doschak, M.R.; Major, P.W.; Popowics, T.; Dennison, C.R.; Romanyk, D. Strain Measurement within an Intact Swine Periodontal Ligament. J. Dent. Res. 2022, 101, 1474–1480. [Google Scholar] [CrossRef]
- Coimbra, W.; Oliveira, P.; Marques, C.; Leal-Junior, A. Chirped Fiber Bragg Grating Sensors for Force Intensity and Location Assessment in Occlusal Splints: A Proof-of-Concept. IEEE Trans. Biomed. Eng. 2023, 70, 1189–1195. [Google Scholar] [CrossRef]
- Park, J.J.; Park, Y.C.; Lee, K.J.; Cha, J.Y.; Tahk, J.H.; Choi, Y.J. Skeletal and dentoalveolar changes after miniscrew assisted rapid palatal expansion in young adults: A cone-beam computed tomography study. Korean J. Orthod. 2017, 47, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, M.; Akin, M. Comparison of root resorption after bone-borne and tooth-borne rapid maxillary expansion evaluated with the use of microtomography. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ventura, V.; Botelho, J.; Machado, V.; Mascarenhas, P.; Pereira, F.D.; Mendes, J.J.; Delgado, A.S.; Pereira, P.M. Miniscrew-Assisted Rapid Palatal Expansion (MARPE): An Umbrella Review. J. Clin. Med. 2022, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Shi, K.K.; Cha, J.Y.; Leee, Y.C.P.J. Nonsurgical miniscrew-assistedrapid maxillary expansion results in acceptable stability in young adults. Angle Orthod. 2016, 86, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, C.; Tang, B.; Hua, F.; He, H.; Ngan, P. Skeletal and dentoalveolar changes in the transverse dimension using microimplant-assisted rapid palatal expansion (MARPE) appliances. Semin. Orthod. 2019, 25, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, A.; Theodorou, C.I.; Berge, S.J.; Schols, J.; Xi, T. Efficacy of Miniscrew-Assisted Rapid Palatal Expansion (MARPE) in late adolescents and adults: A systematic review and meta-analysis. Eur. J. Orthod. 2021, 43, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Alcin, R.; Malkoc, S. Does mini-implant-supported rapid maxillary expansion cause less root resorption than traditional approaches? A micro-computed tomography study. Korean J. Orthod. 2021, 51, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhuang, L.; Zhang, N.; Bian, Y.; Li, S. Comparison of skeletal maxillary transverse deficiency treated by microimplant-assisted rapid palatal expansion and tooth-borne expansion during the post-pubertal growth spurt stage. Angle Orthod. 2021, 91, 36–45. [Google Scholar] [CrossRef]
- Krusi, T.M.; Eliades; Papageorgiou, S.N. Are there benefits from using bone-borne maxillary expansion instead of tooth-borne maxillary expansion? A systematic review with meta-analysis. Prog. Orthod. 2019, 20, 1–9. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Ayub, P.; Angelieri, F.; Murata, W.H.; Suzuki, S.S.; Ravelli, D.B.; Santos-Pinto, A. Evaluation of factors related to the success of miniscrew-assisted rapid palatal expansion. Angle Orthod. 2021, 91, 187–194. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, D.Y.; Jung, S.K. Influence of changing various parameters in miniscrew-assisted rapid palatal expansion: A three-dimensional finite element analysis. Korean J. Orthod. 2019, 49, 150–160. [Google Scholar] [CrossRef]
- Lee, R.J.; Moon, W.; Hong, C. Effects of monocortical and bicortical mini-implant anchorage on bone-borne palatal expansion using finite element analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Shyagali, T.; Kambalyal, P.; Doshi, Y.R.J. Comparison and evaluation of stresses generated by rapid maxillary expansion and the implant-supported rapid maxillary expansion on the craniofacial structures using finite element method of stress analysis. Prog. Orthod. 2017, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.B.; Rino-Neto, J.; Iared, W.; Pasqua, B.P.M.; Nascimento, F.D. Stress distribution and displacement of three different types of micro-implant assisted rapid maxillary expansion (MARME): A three-dimensional finite element study. Prog. Orthod. 2021, 22, 1–201. [Google Scholar]
- Coimbra, W.; Oliveira, P.; Theodosiou, A.; Marques, C.; Kalli, K.; Leal-Junior, A. Strain Measurement in Hyrax Appliances Using FBG Sensors in a 3D-Printed Human Maxillary Model. IEEE Photonic. Tech. Lett. 2022, 34, 811–814. [Google Scholar] [CrossRef]
- Coimbra, W.; Campos, V.; Oliveira, P.L.E.; Frizera, A.; Sant, E.F.; Araújo, M.T.S.; Andrade, R.; Leal-Junior, A. Fibre Bragg grating sensors for sutural expansion assessment in rapid palatal expanders: An ex-vivo validation. IET Optoelectron. 2020, 14, 337–342. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, Z.; Jiang, S.; Ni, J.; Zhao, Q.; Wang, C. Ultrahigh-Resolution and Large-Dynamic-Range Temperature Sensor Based on Fiber-Optic EFPI Cavity. IEEE Sens. J. 2023, 23, 6857–6863. [Google Scholar] [CrossRef]
- Song, K.T.; Park, J.H.; Moon, W.; Chae, J.M.; Kang, K.H. Three-dimensional changes of the zygomaticomaxillary complex after mini-implant assisted rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Elkenawy, I.; Fijany, L.; Colak, O.; Paredes, N.A.; Gargoum, A.; Abedini, S.; Cantarella, D.; Dominguez-Mompell, R.; Sfogliano, L.; Moon, W. An assessment of the magnitude, parallelism, and asymmetry of micro-implant-assisted rapid maxillary expansion in non-growing patients. Prog. Orthod. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Cho, A.R.; Park, J.H.; Moon, W.; Chae, J.M.; Kang, K.H. Short-term effects of microimplant-assisted rapid palatal expansion on the circummaxillary sutures in skeletally mature patients: A cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, e187–e197. [Google Scholar] [CrossRef]
- Liao, Y.C.; Ho, K.H.; Wang, C.W.; Wang, K.L.; Hsieh, S.C.; Chang, H.M. Skeletal and dental changes after microimplant-assisted rapid palatal expansion (MARPE)—A Cephalometric and Cone-Beam Computed Tomography (CBCT) study. Clin. Investig. Orthod. 2022, 81, 84–92. [Google Scholar] [CrossRef]
- Chang, C.; Chen, M.; Chang, C.; Chang, H. Investigation of the role of midpalatal and circummaxillary sutures in bone-anchored rapid maxillary expansion using a verified finite-element model. Am. J. Orthod. Dentofac. 2023, 163, 198–209. [Google Scholar] [CrossRef]




| Study | Methodology | Expansion Pattern | Angle of Opening |
|---|---|---|---|
| Jia et al., 2021 [17] | CBCT * | Type V | 2.89° ** |
| Song et al., 2019 [27] | CBCT * | Parallel | 0.19° ** |
| Elkenawy et al., 2020 [28] | CBCT * | Parallel | 0.24° ** |
| Cho et al., 2022 [29] | CBCT * | Parallel | 0.60° ** |
| Liao et al., 2022 [30] | CBCT * | Parallel | 0.8° ** |
| Lee, et al., 2017 [21] | Finite element analysis | Type V | 3.2° ** |
| Chang et al., 2023 [31] | CBCT * and Finite element analysis | Parallel | 0.57° ** |
| This study | 3D-printed model and sensors | Type V | 1.78° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, S.; Zhang, F.; Duan, Z.; Wang, Y. Monitoring the Opening of Rapid Palatal Expansion (RPE) in a 3D-Printed Skull Model Using Fiber Optic F–P Sensors. Sensors 2023, 23, 7168. https://doi.org/10.3390/s23167168
Zhao Z, Zhang S, Zhang F, Duan Z, Wang Y. Monitoring the Opening of Rapid Palatal Expansion (RPE) in a 3D-Printed Skull Model Using Fiber Optic F–P Sensors. Sensors. 2023; 23(16):7168. https://doi.org/10.3390/s23167168
Chicago/Turabian StyleZhao, Zhen, Shijie Zhang, Faxiang Zhang, Zhenhui Duan, and Yingying Wang. 2023. "Monitoring the Opening of Rapid Palatal Expansion (RPE) in a 3D-Printed Skull Model Using Fiber Optic F–P Sensors" Sensors 23, no. 16: 7168. https://doi.org/10.3390/s23167168






