Links between Neuroanatomy and Neurophysiology with Turning Performance in People with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
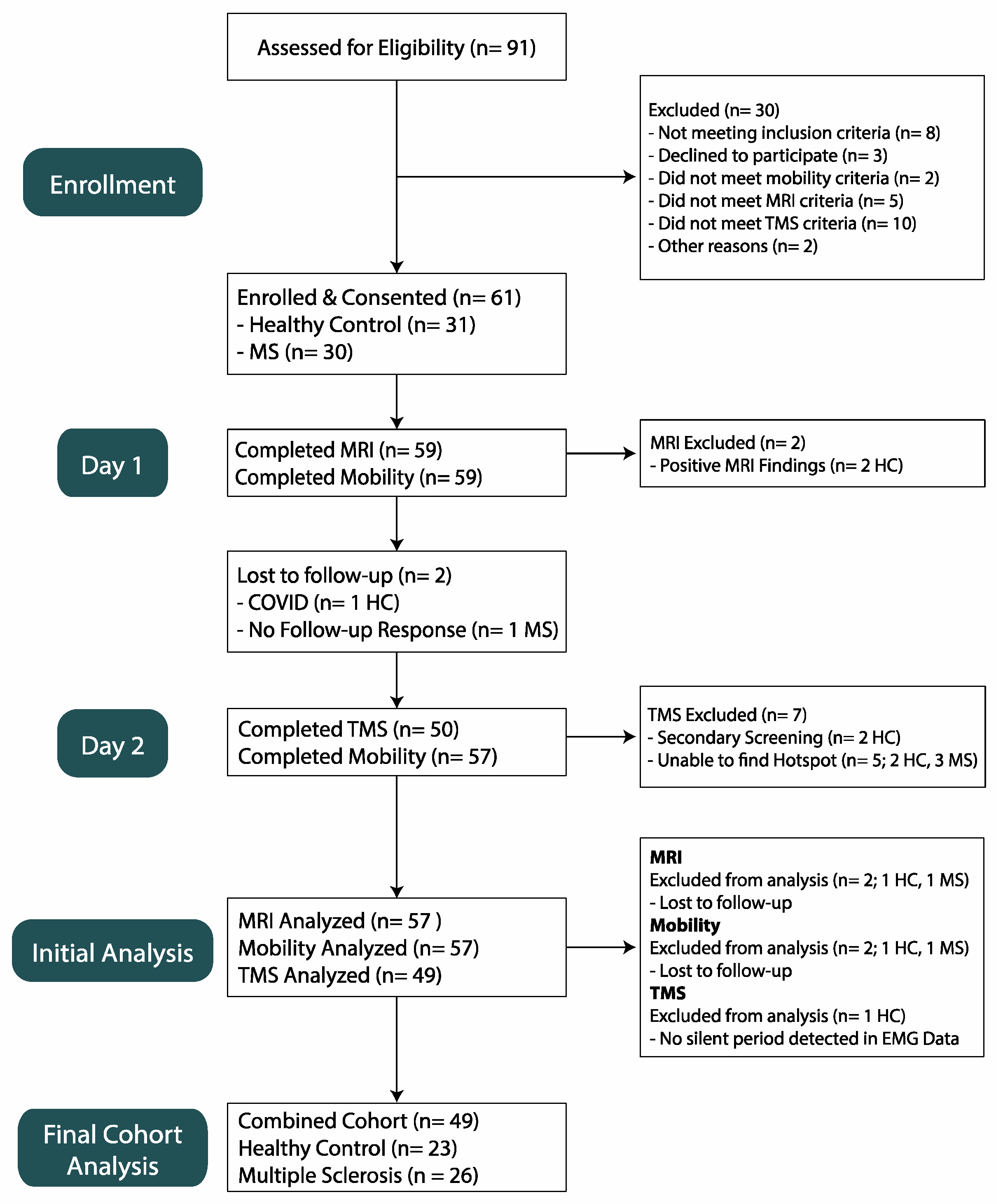
2.1. Population
2.2. Turning Acquisition
2.3. Turning Processing
2.4. MRI Acquisition
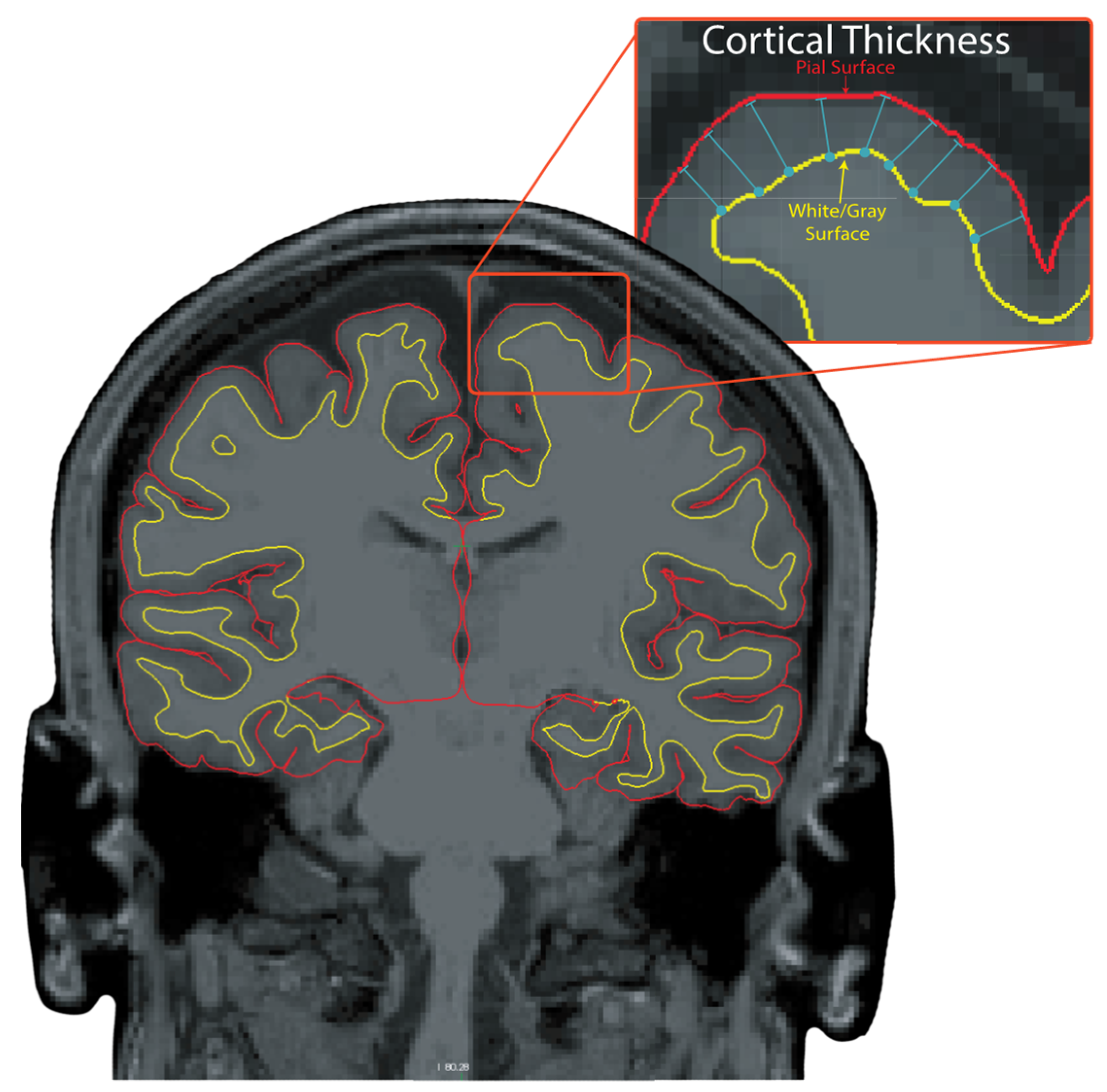
2.5. MRI Processing
2.6. Muscle Strength Acquisition
2.7. TMS Acquisition
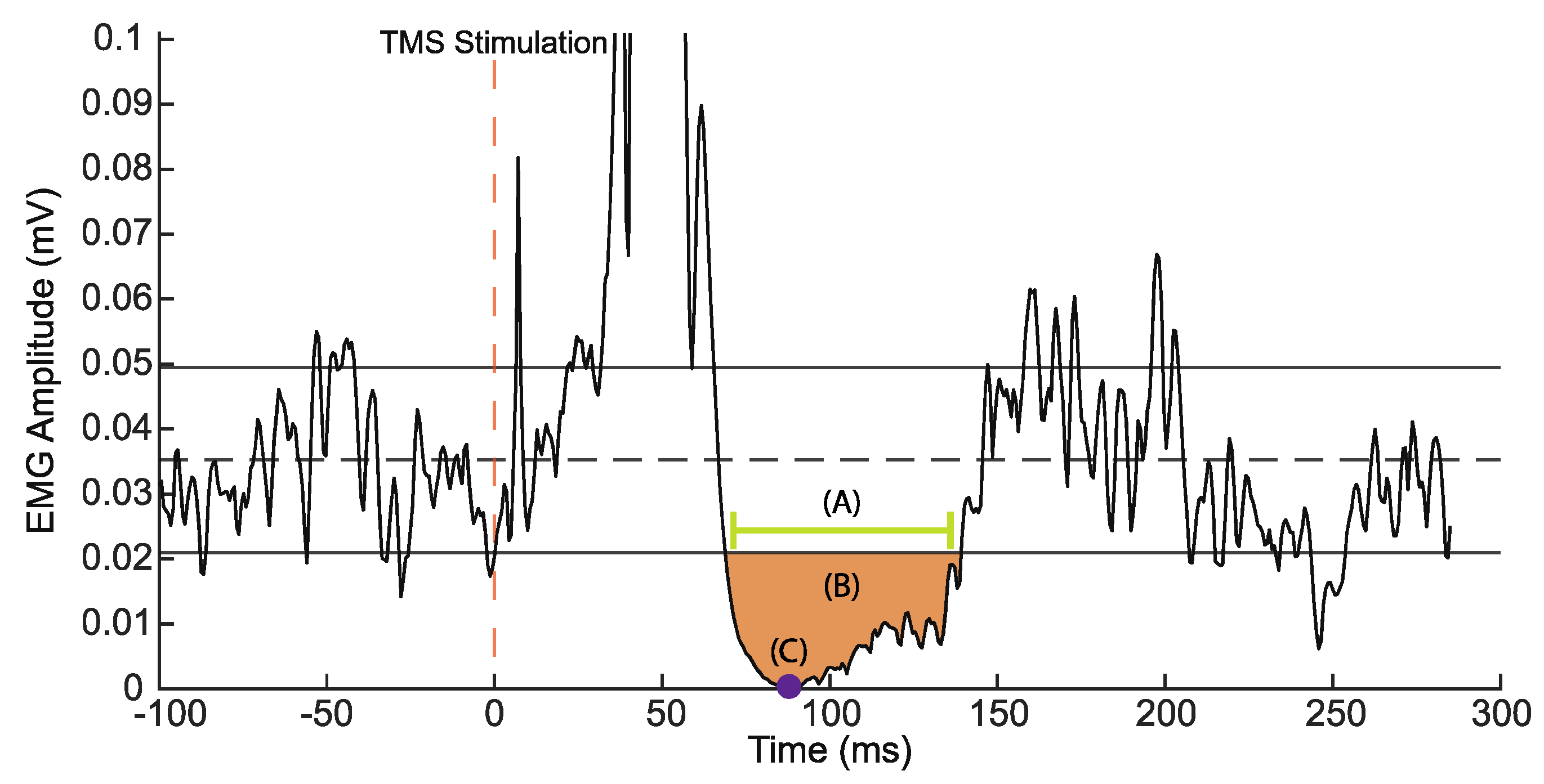
2.8. TMS Processing
2.9. Statistical Analysis
3. Results
3.1. Participants
3.2. Turning Performance
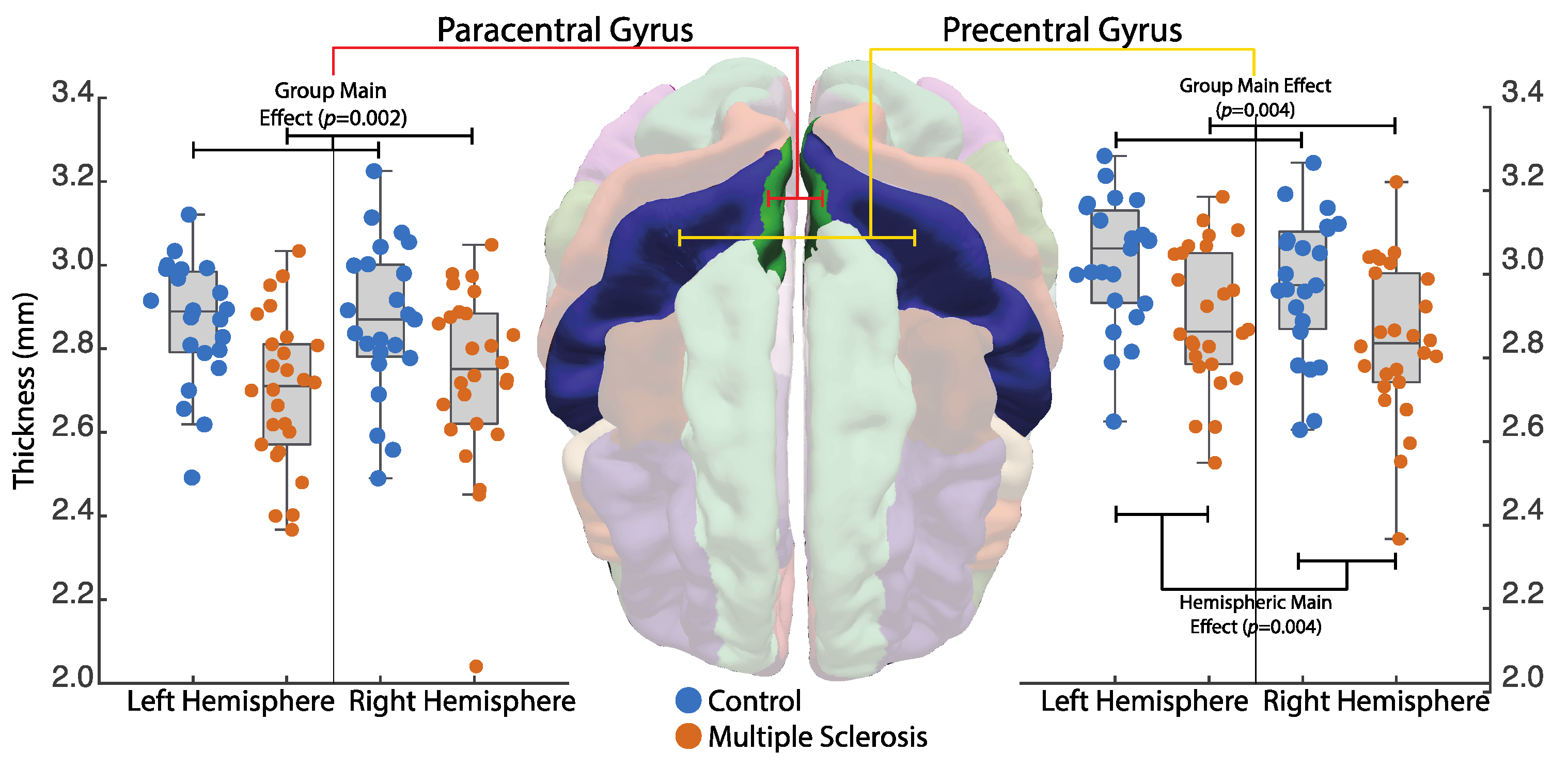
3.3. Motor Cortex Thickness
3.4. TMS
3.5. Associations
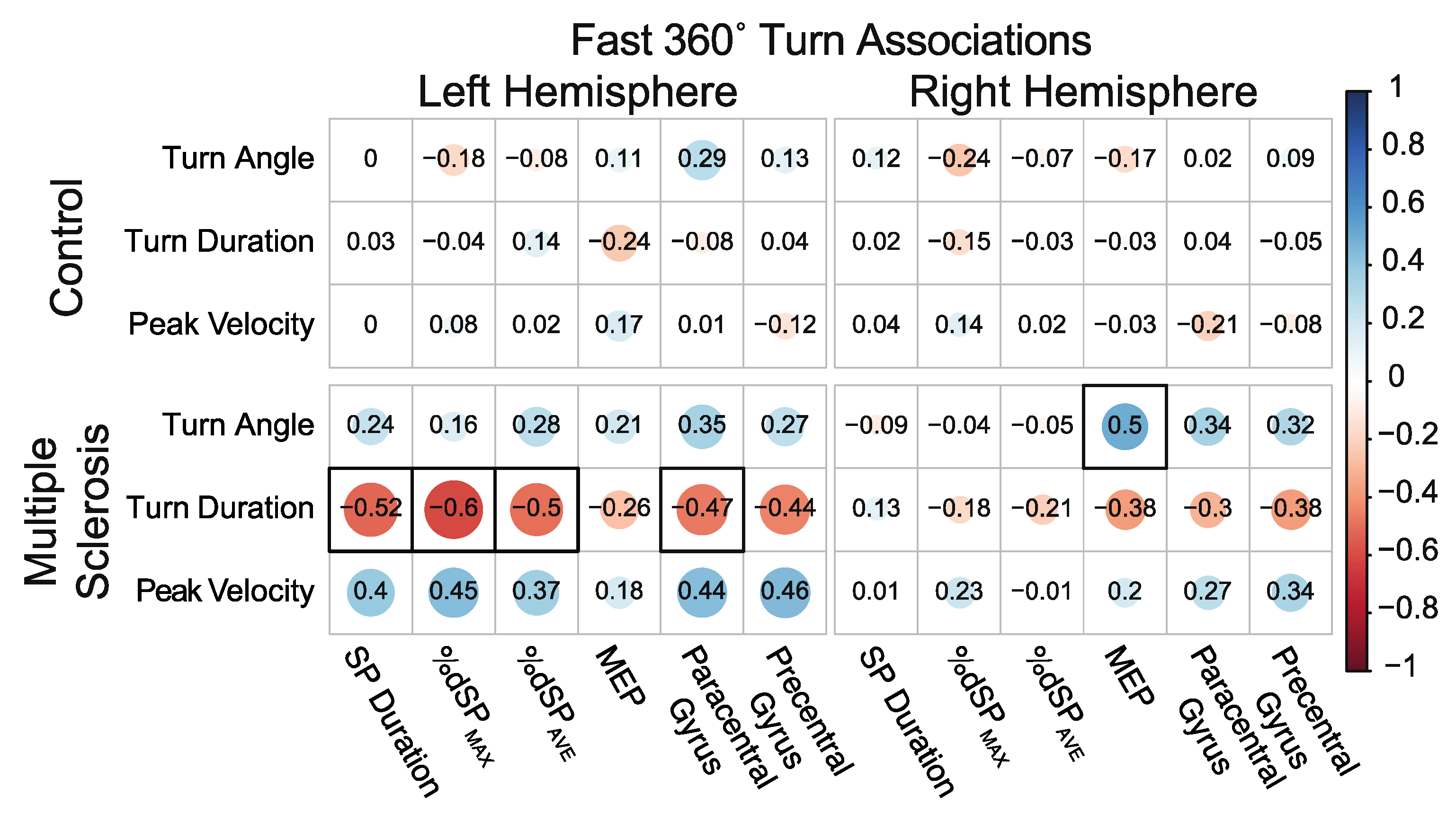
3.5.1. Associations between 360° In-Place Fast Turns and Neurophysiology and Neuroanatomical Structure
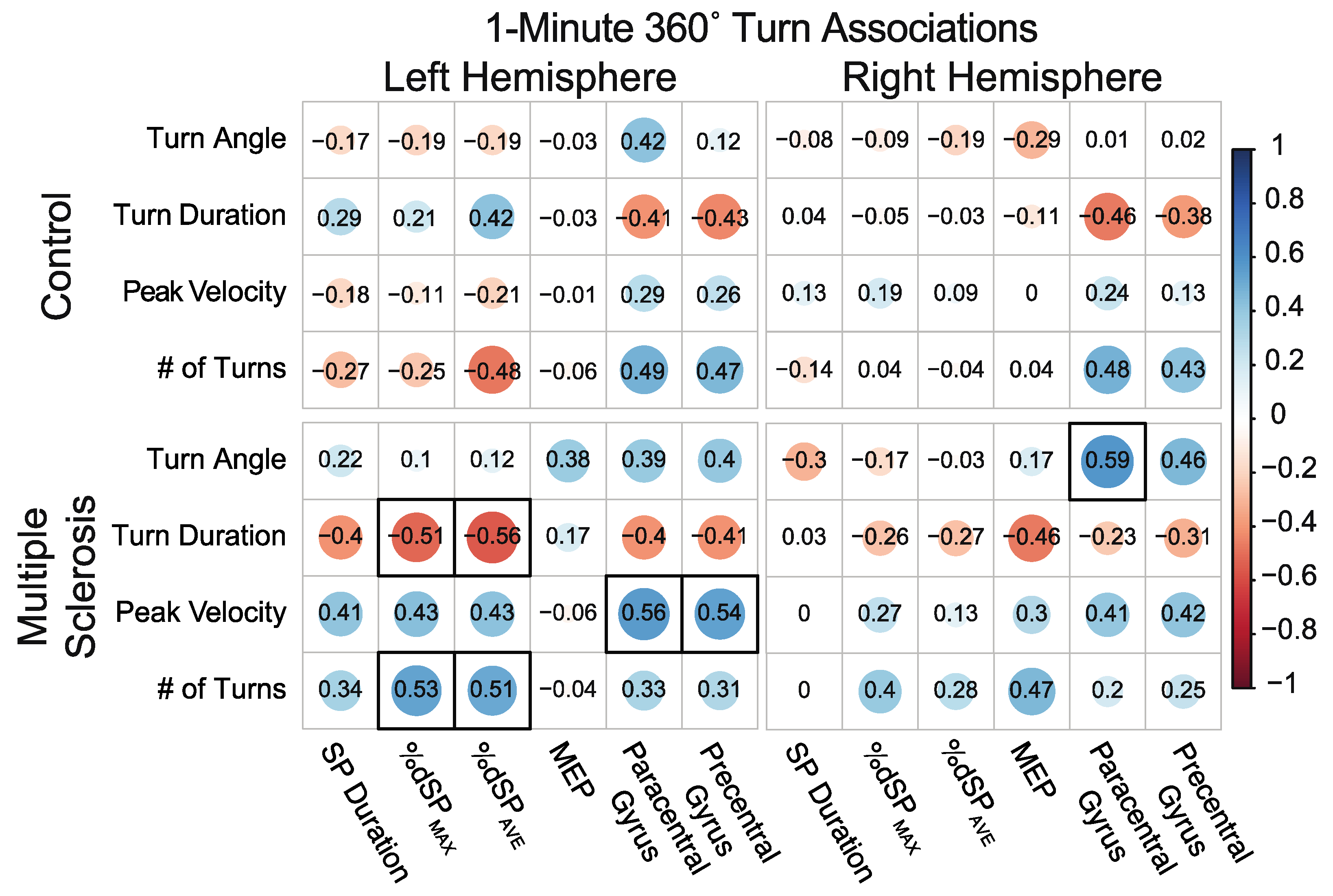
3.5.2. Associations between 360° Self-Selected Pace In-Place 1-min Continuous Turns and Neurophysiology and Neuroanatomical Structure
3.5.3. Associations between 180° Self-Selected Pace Turns While Walking and Neurophysiology and Neuroanatomical Structure
4. Discussion
4.1. Turning
4.2. MRI
4.3. TMS
4.4. Associations
4.4.1. Neuroanatomical Associations
4.4.2. Neurophysiologic Associations
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Mori, F.; Buttari, F.; Marfia, G.A.; Sancesario, A.; Centonze, D.; Iezzi, E. Neurophysiology of synaptic functioning in multiple sclerosis. Clin. Neurophysiol. 2017, 128, 1148–1157. [Google Scholar] [CrossRef]
- Bendfeldt, K.; Kuster, P.; Traud, S.; Egger, H.; Winklhofer, S.; Mueller-Lenke, N.; Naegelin, Y.; Gass, A.; Kappos, L.; Matthews, P.M.; et al. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis—A longitudinal voxel-based morphometry study. Neuroimage 2009, 45, 60–67. [Google Scholar] [CrossRef]
- Bergsland, N.; Horakova, D.; Dwyer, M.G.; Uher, T.; Vaneckova, M.; Tyblova, M.; Seidl, Z.; Krasensky, J.; Havrdova, E.; Zivadinov, R. Gray matter atrophy patterns in multiple sclerosis: A 10-year source-based morphometry study. Neuroimage Clin. 2018, 17, 444–451. [Google Scholar] [CrossRef]
- Boroojerdi, B.; Hungs, M.; Mull, M.; Topper, R.; Noth, J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Trapp, B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011, 93, 1–12. [Google Scholar] [CrossRef]
- Miller, D.H.; Barkhof, F.; Frank, J.A.; Parker, G.J.; Thompson, A.J. Measurement of atrophy in multiple sclerosis: Pathological basis, methodological aspects and clinical relevance. Brain 2002, 125, 1676–1695. [Google Scholar] [CrossRef] [PubMed]
- Chard, D.; Miller, D. Grey matter pathology in clinically early multiple sclerosis: Evidence from magnetic resonance imaging. J. Neurol. Sci. 2009, 282, 5–11. [Google Scholar] [CrossRef]
- Chard, D.T.; Griffin, C.M.; Parker, G.J.; Kapoor, R.; Thompson, A.J.; Miller, D.H. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 2002, 125, 327–337. [Google Scholar] [CrossRef]
- Chard, D.T.; Griffin, C.M.; Rashid, W.; Davies, G.R.; Altmann, D.R.; Kapoor, R.; Barker, G.J.; Thompson, A.J.; Miller, D.H. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult. Scler. 2004, 10, 387–391. [Google Scholar] [CrossRef]
- Dalton, C.M.; Chard, D.T.; Davies, G.R.; Miszkiel, K.A.; Altmann, D.R.; Fernando, K.; Plant, G.T.; Thompson, A.J.; Miller, D.H. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 2004, 127, 1101–1107. [Google Scholar] [CrossRef]
- Calabrese, M.; Atzori, M.; Bernardi, V.; Morra, A.; Romualdi, C.; Rinaldi, L.; McAuliffe, M.J.; Barachino, L.; Perini, P.; Fischl, B.; et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J. Neurol. 2007, 254, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, N.; Matthews, P.M.; Filippi, M.; Agosta, F.; De Luca, M.; Bartolozzi, M.L.; Guidi, L.; Ghezzi, A.; Montanari, E.; Cifelli, A.; et al. Evidence of early cortical atrophy in MS: Relevance to white matter changes and disability. Neurology 2003, 60, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Sailer, M.; Fischl, B.; Salat, D.; Tempelmann, C.; Schonfeld, M.A.; Busa, E.; Bodammer, N.; Heinze, H.J.; Dale, A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 2003, 126, 1734–1744. [Google Scholar] [CrossRef]
- Sahota, P.; Prabhakar, S.; Lal, V.; Khurana, D.; Das, C.P.; Singh, P. Transcranial magnetic stimulation: Role in the evaluation of disability in multiple sclerosis. Neurol. India 2005, 53, 197–201, discussion 201. [Google Scholar] [CrossRef]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef]
- Chaves, A.R.; Devasahayam, A.J.; Riemenschneider, M.; Pretty, R.W.; Ploughman, M. Walking Training Enhances Corticospinal Excitability in Progressive Multiple Sclerosis-A Pilot Study. Front. Neurol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Obrenovitch, T.P.; Urenjak, J.; Zilkha, E.; Jay, T.M. Excitotoxicity in neurological disorders--the glutamate paradox. Int. J. Dev. Neurosci. 2000, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef]
- Ayache, S.S.; Creange, A.; Farhat, W.H.; Zouari, H.G.; Lesage, C.; Palm, U.; Abdellaoui, M.; Lefaucheur, J.P. Cortical excitability changes over time in progressive multiple sclerosis. Funct. Neurol. 2015, 30, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Creange, A.; Farhat, W.H.; Zouari, H.G.; Mylius, V.; Ahdab, R.; Abdellaoui, M.; Lefaucheur, J.P. Relapses in multiple sclerosis: Effects of high-dose steroids on cortical excitability. Eur. J. Neurol. 2014, 21, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.R.; Wallack, E.M.; Kelly, L.P.; Pretty, R.W.; Wiseman, H.D.; Chen, A.; Moore, C.S.; Stefanelli, M.; Ploughman, M. Asymmetry of Brain Excitability: A New Biomarker that Predicts Objective and Subjective Symptoms in Multiple Sclerosis. Behav. Brain Res. 2019, 359, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Illarramendi, A.; Domercq, M.; Perez-Cerda, F.; Ravid, R.; Matute, C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol. Dis. 2006, 21, 154–164. [Google Scholar] [CrossRef]
- Cruz-Martinez, A.; Gonzalez-Orodea, J.I.; Lopez Pajares, R.; Arpa, J. Disability in multiple sclerosis. The role of transcranial magnetic stimulation. Electromyogr. Clin. Neurophysiol. 2000, 40, 441–447. [Google Scholar]
- Gagliardo, A.; Galli, F.; Grippo, A.; Amantini, A.; Martinelli, C.; Amato, M.P.; Borsini, W. Motor evoked potentials in multiple sclerosis patients without walking limitation: Amplitude vs. conduction time abnormalities. J. Neurol. 2007, 254, 220–227. [Google Scholar] [CrossRef]
- Jorgensen, L.M.; Nielsen, J.E.; Ravnborg, M. MEP recruitment curves in multiple sclerosis and hereditary spastic paraplegia. J. Neurol. Sci. 2005, 237, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Wohlfarth, K.; Rollnik, J.D.; Dengler, R. Walking and fatigue in multiple sclerosis: The role of the corticospinal system. Muscle Nerve 1998, 21, 1068–1070. [Google Scholar] [CrossRef]
- Jones, S.M.; Streletz, L.J.; Raab, V.E.; Knobler, R.L.; Lublin, F.D. Lower extremity motor evoked potentials in multiple sclerosis. Arch. Neurol. 1991, 48, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Kukowski, B. Duration, configuration and amplitude of the motor response evoked by magnetic brain stimulation in patients with multiple sclerosis. Electromyogr. Clin. Neurophysiol. 1993, 33, 295–297. [Google Scholar]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Mott, D.D.; Lewis, D.V. The Pharmacology and Function of Central GabaB Receptors. In International Review of Neurobiology; Bradley, R.J., Harris, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 36, pp. 97–223. [Google Scholar]
- Swanson, C.W.; Fling, B.W. Associations between Turning Characteristics and Corticospinal Inhibition in Young and Older Adults. Neuroscience 2020, 425, 59–67. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Mingers, D.; Heesen, C.; Baumer, T.; Weiller, C. Motor cortex excitability and fatigue in multiple sclerosis: A transcranial magnetic stimulation study. Mult. Scler. 2005, 11, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Drew, T.; Marigold, D.S. Taking the next step: Cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Ferreira-Pinto, M.J.; Ruder, L.; Capelli, P.; Arber, S. Connecting Circuits for Supraspinal Control of Locomotion. Neuron 2018, 100, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Musienko, P.E.; Zelenin, P.V. Nervous mechanisms of locomotion in different directions. Curr. Opin. Physiol. 2019, 8, 7–13. [Google Scholar] [CrossRef]
- Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Stern, Y.; Habeck, C.G. Distinct cortical thickness patterns link disparate cerebral cortex regions to select mobility domains. Sci. Rep. 2021, 11, 6600. [Google Scholar] [CrossRef]
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous monitoring of turning in patients with movement disability. Sensors 2014, 14, 356–369. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Lindroth, H.; Nair, V.A.; Stanfield, C.; Casey, C.; Mohanty, R.; Wayer, D.; Rowley, P.; Brown, R.; Prabhakaran, V.; Sanders, R.D. Examining the identification of age-related atrophy between T1 and T1 + T2-FLAIR cortical thickness measurements. Sci. Rep. 2019, 9, 11288. [Google Scholar] [CrossRef]
- Fischl, B.; van der Kouwe, A.; Destrieux, C.; Halgren, E.; Segonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-induced silent periods: A review of methods and call for consistency. J. Neurosci. Methods 2020, 346, 108950. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef]
- Fling, B.W.; Seidler, R.D. Task-dependent effects of interhemispheric inhibition on motor control. Behav. Brain Res. 2012, 226, 211–217. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Adusumilli, G.; Lancia, S.; Levasseur, V.A.; Amblee, V.; Orchard, M.; Wagner, J.M.; Naismith, R.T. Turning is an important marker of balance confidence and walking limitation in persons with multiple sclerosis. PLoS ONE 2018, 13, e0198178. [Google Scholar] [CrossRef] [PubMed]
- Soke, F.; Guclu-Gunduz, A.; Ozkul, C.; Cekim, K.; Irkec, C.; Gonenli Kocer, B. Reliability and validity of the timed 360 degrees turn test in people with multiple sclerosis. Physiother. Theory Pract. 2021, 37, 736–747. [Google Scholar] [CrossRef]
- Spain, R.I.; St George, R.J.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Porta, M.; Coghe, G.; Corona, F.; Pilloni, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Are static and functional balance abilities related in individuals with Multiple Sclerosis? Mult. Scler. Relat. Disord. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Spain, R.I.; Nutt, J.G.; El-Gohary, M.; Curtze, C.; Horak, F.B. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J. Neurol. 2020, 267, 1188–1196. [Google Scholar] [CrossRef]
- Peterson, D.S.; Huisinga, J.M.; Spain, R.I.; Horak, F.B. Characterization of Compensatory Stepping in People With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Steenwijk, M.D.; Geurts, J.J.; Daams, M.; Tijms, B.M.; Wink, A.M.; Balk, L.J.; Tewarie, P.K.; Uitdehaag, B.M.; Barkhof, F.; Vrenken, H.; et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2016, 139, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Narayana, P.A.; Govindarajan, K.A.; Goel, P.; Datta, S.; Lincoln, J.A.; Cofield, S.S.; Cutter, G.R.; Lublin, F.D.; Wolinsky, J.S.; Houston, M.R.I.A.C.a.; et al. Regional cortical thickness in relapsing remitting multiple sclerosis: A multi-center study. Neuroimage Clin. 2012, 2, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, N.; Lagana, M.M.; Tavazzi, E.; Caffini, M.; Tortorella, P.; Baglio, F.; Baselli, G.; Rovaris, M. Corticospinal tract integrity is related to primary motor cortex thinning in relapsing-remitting multiple sclerosis. Mult. Scler. 2015, 21, 1771–1780. [Google Scholar] [CrossRef]
- Reddy, H.; Narayanan, S.; Woolrich, M.; Mitsumori, T.; Lapierre, Y.; Arnold, D.L.; Matthews, P.M. Functional brain reorganization for hand movement in patients with multiple sclerosis: Defining distinct effects of injury and disability. Brain 2002, 125, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Nantes, J.C.; Zhong, J.; Holmes, S.A.; Whatley, B.; Narayanan, S.; Lapierre, Y.; Arnold, D.L.; Koski, L. Intracortical inhibition abnormality during the remission phase of multiple sclerosis is related to upper limb dexterity and lesions. Clin. Neurophysiol. 2016, 127, 1503–1511. [Google Scholar] [CrossRef]
- Lansley, J.; Mataix-Cols, D.; Grau, M.; Radua, J.; Sastre-Garriga, J. Localized grey matter atrophy in multiple sclerosis: A meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci. Biobehav. Rev. 2013, 37, 819–830. [Google Scholar] [CrossRef]
- Snow, N.J.; Wadden, K.P.; Chaves, A.R.; Ploughman, M. Transcranial Magnetic Stimulation as a Potential Biomarker in Multiple Sclerosis: A Systematic Review with Recommendations for Future Research. Neural Plast. 2019, 2019, 6430596. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Gharabaghi, A. Neuromuscular Plasticity: Disentangling Stable and Variable Motor Maps in the Human Sensorimotor Cortex. Neural Plast. 2016, 2016, 7365609. [Google Scholar] [CrossRef] [PubMed]
- Bungert, A.; Antunes, A.; Espenhahn, S.; Thielscher, A. Where does TMS Stimulate the Motor Cortex? Combining Electrophysiological Measurements and Realistic Field Estimates to Reveal the Affected Cortex Position. Cereb. Cortex 2017, 27, 5083–5094. [Google Scholar] [CrossRef]
- Kloppel, S.; Baumer, T.; Kroeger, J.; Koch, M.A.; Buchel, C.; Munchau, A.; Siebner, H.R. The cortical motor threshold reflects microstructural properties of cerebral white matter. Neuroimage 2008, 40, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Lamy, J.C. Does Resting Motor Threshold Predict Motor Hand Recovery After Stroke? Front. Neurol. 2018, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U. Cortical threshold and excitability measurements. In Clinical Neurophysiology of Motor Neuron Diseases; Handbook of Clinical Neurophysiology; Eisen, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 4, pp. 317–335. [Google Scholar]
- Levy, L.M.; Ziemann, U.; Chen, R.; Cohen, L.G. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann. Neurol. 2002, 52, 755–761. [Google Scholar] [CrossRef]
- Zoupi, L.; Booker, S.A.; Eigel, D.; Werner, C.; Kind, P.C.; Spires-Jones, T.L.; Newland, B.; Williams, A.C. Selective vulnerability of inhibitory networks in multiple sclerosis. Acta Neuropathol. 2021, 141, 415–429. [Google Scholar] [CrossRef]
- Vucic, S.; Burke, T.; Lenton, K.; Ramanathan, S.; Gomes, L.; Yannikas, C.; Kiernan, M.C. Cortical dysfunction underlies disability in multiple sclerosis. Mult. Scler. 2012, 18, 425–432. [Google Scholar] [CrossRef]
- Santarnecchi, E.; Rossi, S.; Bartalini, S.; Cincotta, M.; Giovannelli, F.; Tatti, E.; Ulivelli, M. Neurophysiological Correlates of Central Fatigue in Healthy Subjects and Multiple Sclerosis Patients before and after Treatment with Amantadine. Neural Plast. 2015, 2015, 616242. [Google Scholar] [CrossRef]
- Cawley, N.; Solanky, B.S.; Muhlert, N.; Tur, C.; Edden, R.A.; Wheeler-Kingshott, C.A.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 2015, 138, 2584–2595. [Google Scholar] [CrossRef]
- Tataroglu, C.; Genc, A.; Idiman, E.; Cakmur, R.; Idiman, F. Cortical silent period and motor evoked potentials in patients with multiple sclerosis. Clin. Neurol. Neurosurg. 2003, 105, 105–110. [Google Scholar] [CrossRef]
- Neva, J.L.; Lakhani, B.; Brown, K.E.; Wadden, K.P.; Mang, C.S.; Ledwell, N.H.; Borich, M.R.; Vavasour, I.M.; Laule, C.; Traboulsee, A.L.; et al. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behav. Brain Res. 2016, 297, 187–195. [Google Scholar] [CrossRef]
- Oliviero, A.; Profice, P.; Tonali, P.A.; Pilato, F.; Saturno, E.; Dileone, M.; Ranieri, F.; Di Lazzaro, V. Effects of aging on motor cortex excitability. Neurosci. Res. 2006, 55, 74–77. [Google Scholar] [CrossRef]
- Sale, M.V.; Semmler, J.G. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J. Appl. Physiol. 2005, 99, 1483–1493. [Google Scholar] [CrossRef]
- Jung, P.; Ziemann, U. Differences of the ipsilateral silent period in small hand muscles. Muscle Nerve 2006, 34, 431–436. [Google Scholar] [CrossRef]
- Fling, B.W.; Seidler, R.D. Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cereb. Cortex 2012, 22, 2643–2652. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Dubuc, T.; Boufadel, D.F.; Fisher, B.E. Measuring ipsilateral silent period: Effects of muscle contraction levels and quantification methods. Brain Res. 2017, 1674, 77–83. [Google Scholar] [CrossRef]
- Lin, Y.L.; Cunningham, D.A.; Plow, E.B. Reply to “On the issue of measuring interhemispheric inhibition in unilateral stroke”. Clin. Neurophysiol. 2021, 132, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.A.; Knutson, J.S.; Sankarasubramanian, V.; Potter-Baker, K.A.; Machado, A.G.; Plow, E.B. Bilateral contralaterally controlled functional electrical stimulation reveals new insights into the interhemispheric competition model in chronic stroke. Neurorehabilit. Neural Repair 2019, 33, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.K.; Newham, D.J. Reliability of Transcallosal Inhibition in Healthy Adults. Front. Hum. Neurosci. 2016, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Sankarasubramanian, V.; Machado, A.G.; Conforto, A.B.; Potter-Baker, K.A.; Cunningham, D.A.; Varnerin, N.M.; Wang, X.; Sakaie, K.; Plow, E.B. Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin. Neurophysiol. 2017, 128, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.W.; Dutta, G.G.; Schlueter, H.; Cameron, M.H.; Horak, F.B. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front. Hum. Neurosci. 2014, 8, 814. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R. The neural basis of inhibition in cognitive control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef]
- Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. The effects of brain lateralization on motor control and adaptation. J. Mot. Behav. 2012, 44, 455–469. [Google Scholar] [CrossRef]
- Benedict, R.H.; Holtzer, R.; Motl, R.W.; Foley, F.W.; Kaur, S.; Hojnacki, D.; Weinstock-Guttman, B. Upper and lower extremity motor function and cognitive impairment in multiple sclerosis. J. Int. Neuropsychol. Soc. 2011, 17, 643–653. [Google Scholar] [CrossRef]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Battaglini, M.; Bartolozzi, M.L.; Stromillo, M.L.; Guidi, L.; Siracusa, G.; Sorbi, S.; et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch. Neurol. 2007, 64, 1157–1161. [Google Scholar] [CrossRef]
- Chen, J.T.; Narayanan, S.; Collins, D.L.; Smith, S.M.; Matthews, P.M.; Arnold, D.L. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. Neuroimage 2004, 23, 1168–1175. [Google Scholar] [CrossRef]
- Roosendaal, S.D.; Bendfeldt, K.; Vrenken, H.; Polman, C.H.; Borgwardt, S.; Radue, E.W.; Kappos, L.; Pelletier, D.; Hauser, S.L.; Matthews, P.M.; et al. Grey matter volume in a large cohort of MS patients: Relation to MRI parameters and disability. Mult. Scler. 2011, 17, 1098–1106. [Google Scholar] [CrossRef]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Prinster, A.; Quarantelli, M.; Lanzillo, R.; Orefice, G.; Vacca, G.; Carotenuto, B.; Alfano, B.; Brunetti, A.; Morra, V.B.; Salvatore, M. A voxel-based morphometry study of disease severity correlates in relapsing-- remitting multiple sclerosis. Mult. Scler. 2010, 16, 45–54. [Google Scholar] [CrossRef]
- Lorefice, L.; Coghe, G.; Fenu, G.; Porta, M.; Pilloni, G.; Frau, J.; Corona, F.; Sechi, V.; Barracciu, M.A.; Marrosu, M.G.; et al. ‘Timed up and go’ and brain atrophy: A preliminary MRI study to assess functional mobility performance in multiple sclerosis. J. Neurol. 2017, 264, 2201–2204. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Amadi, U.; Gudberg, C.A.; Ilie, A.S.; Sampaio-Baptista, C.; O’Shea, J.; Woolrich, M.; Smith, S.M.; Filippini, N. Local GABA concentration is related to network-level resting functional connectivity. Elife 2014, 3, e01465. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.W.; Richmond, S.B.; Sharp, B.E.; Fling, B.W. Middle-age people with multiple sclerosis demonstrate similar mobility characteristics to neurotypical older adults. Mult. Scler. Relat. Disord. 2021, 51, 102924. [Google Scholar] [CrossRef]
- Clark, D.J. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front. Hum. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Drew, T.; Prentice, S.; Schepens, B. Cortical and brainstem control of locomotion. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2004; Volume 143, pp. 251–261. [Google Scholar]
- Haaland, K.Y.; Harrington, D.L.; Knight, R.T. Neural representations of skilled movement. Brain 2000, 123 Pt 11, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.T.; Diedrichsen, J.; Verstynen, T.; Duque, J.; Ivry, R.B. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc. Natl. Acad. Sci. USA 2010, 107, 17751–17756. [Google Scholar] [CrossRef]
- Castiello, U.; Paine, M. Effects of left parietal injury on covert orienting of attention. J. Neurol. Neurosurg. Psychiatry 2002, 72, 73–76. [Google Scholar] [CrossRef]
- Russo, M.; Crupi, D.; Naro, A.; Avanzino, L.; Buccafusca, M.; Dattola, V.; Terranova, C.; Sottile, F.; Rizzo, V.; Ghilardi, M.F.; et al. Fatigue in patients with multiple sclerosis: From movement preparation to motor execution. J. Neurol. Sci. 2015, 351, 52–57. [Google Scholar] [CrossRef]
- Abidi, Z.; Faeghi, F.; Mardanshahi, Z.; Mortazavi, H. Assessment of the diagnostic accuracy of double inversion recovery sequence compared with FLAIR and T2W_TSE in detection of cerebral multiple sclerosis lesions. Electron. Physician 2017, 9, 4162–4170. [Google Scholar] [CrossRef]

| Variable | Healthy Control | Multiple Sclerosis | p-Value |
|---|---|---|---|
| Sex, female/male | 15/8 | 19/7 | |
| Age (years), mean ± SD | 46.76 ± 15.93 | 48.19 ± 11.95 | 0.72 |
| Height (cm), mean ± SD | 169.85 ± 7.59 | 165.73 ± 7.32 | 0.06 |
| Weight (kg), mean ± SD | 71.89 ± 13.20 | 68.39 ± 9.35 | 0.29 |
| BMI, mean ± SD | 24.79 ± 3.35 | 24.99 ± 3.84 | 0.84 |
| Disease duration (years), mean ± SD | - | 11.69 ± 10.72 | |
| EDSS, median [range] | - | 3.5 [0–4] |
| Turn Variables | Healthy Control | Multiple Sclerosis | p-Value |
|---|---|---|---|
| 360° Fast Turn | |||
| Turn Duration (s), mean ± SD | 1.85 ± 0.30 | 2.44 ± 0.69 | <0.0001 |
| Turn Velocity (°/s), mean ± SD | 368.61 ± 65.98 | 293.30 ± 80.36 | <0.0001 |
| Turn Angle (°), mean ± SD | 384.60 ± 11.72 | 378.57 ± 15.36 | 0.05 |
| 1 Min 360° Turns | |||
| Turn Duration (s), mean ± SD | 2.87 ± 0.46 | 3.36 ± 0.90 | 0.01 |
| Turn Velocity (°/s), mean ± SD | 232.42 ± 38.43 | 203.21 ± 50.25 | 0.01 |
| Turn Angle (°), mean ± SD | 391.05 ± 15.25 | 377.53 ± 25.77 | 0.01 |
| Turns Completed (#), mean ± SD | 19.43 ± 3.26 | 16.96 ± 4.40 | 0.02 |
| 180° Turns | |||
| Turn Duration (s), mean ± SD | 2.04 ± 0.31 | 2.18 ± 0.37 | 0.12 |
| Turn Velocity (°/s), mean ± SD | 212.14 ± 29.38 | 206.16 ± 38.40 | 0.43 |
| Turn Angle (°), mean ± SD | 184.44 ± 7.93 | 183.48 ± 7.91 | 0.75 |
| Steps in Turn (#), mean ± SD | 3.57 ± 0.67 | 3.96 ± 0.67 | 0.06 |
| TMS Variable | Hemisphere | Healthy Control | Multiple Sclerosis |
|---|---|---|---|
| Resting Motor Threshold (%MSO), mean ± SD | Left | 68.96 ± 8.09 | 66.77 ± 7.42 |
| Right | 69.09 ± 7.68 | 67.50 ± 7.91 | |
| MEP Amp (mV), mean ± SD | Left | 16.89 ± 7.38 | 18.41 ± 8.58 |
| Right | 17.16 ± 6.39 | 14.91 ± 6.27 | |
| Silent Period Duration (ms), mean ± SD | Left | 115.66 ± 46.24 | 108.26 ± 50.04 |
| Right | 117.83 ± 42.54 | 123.94 ± 67.88 | |
| dSPAVE (%), mean ± SD | Left | 76.89 ± 7.27 | 70.29 ± 9.63 |
| Right | 75.13 ± 7.81 | 71.09 ± 7.84 | |
| dSPMAX (%), mean ± SD | Left | 95.98 ± 2.87 | 92.89 ± 5.32 |
| Right | 95.35 ± 3.10 | 93.02 ± 4.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swanson, C.W.; Fling, B.W. Links between Neuroanatomy and Neurophysiology with Turning Performance in People with Multiple Sclerosis. Sensors 2023, 23, 7629. https://doi.org/10.3390/s23177629
Swanson CW, Fling BW. Links between Neuroanatomy and Neurophysiology with Turning Performance in People with Multiple Sclerosis. Sensors. 2023; 23(17):7629. https://doi.org/10.3390/s23177629
Chicago/Turabian StyleSwanson, Clayton W., and Brett W. Fling. 2023. "Links between Neuroanatomy and Neurophysiology with Turning Performance in People with Multiple Sclerosis" Sensors 23, no. 17: 7629. https://doi.org/10.3390/s23177629
APA StyleSwanson, C. W., & Fling, B. W. (2023). Links between Neuroanatomy and Neurophysiology with Turning Performance in People with Multiple Sclerosis. Sensors, 23(17), 7629. https://doi.org/10.3390/s23177629








