The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review
Abstract
:1. Introduction
- It outlines the application of wearable sensors and machine learning technology in rehabilitation training;
- It specifically analyzes the sensor type, sensor location, and feature extraction applied in the recovery process of different diseases;
- It evaluates the type and accuracy of machine learning algorithms applied in different rehabilitation exercises;
- It discusses the limitations, trends, and directions of sensors and machine learning algorithms in rehabilitation applications.
2. Methods
2.1. Search Method
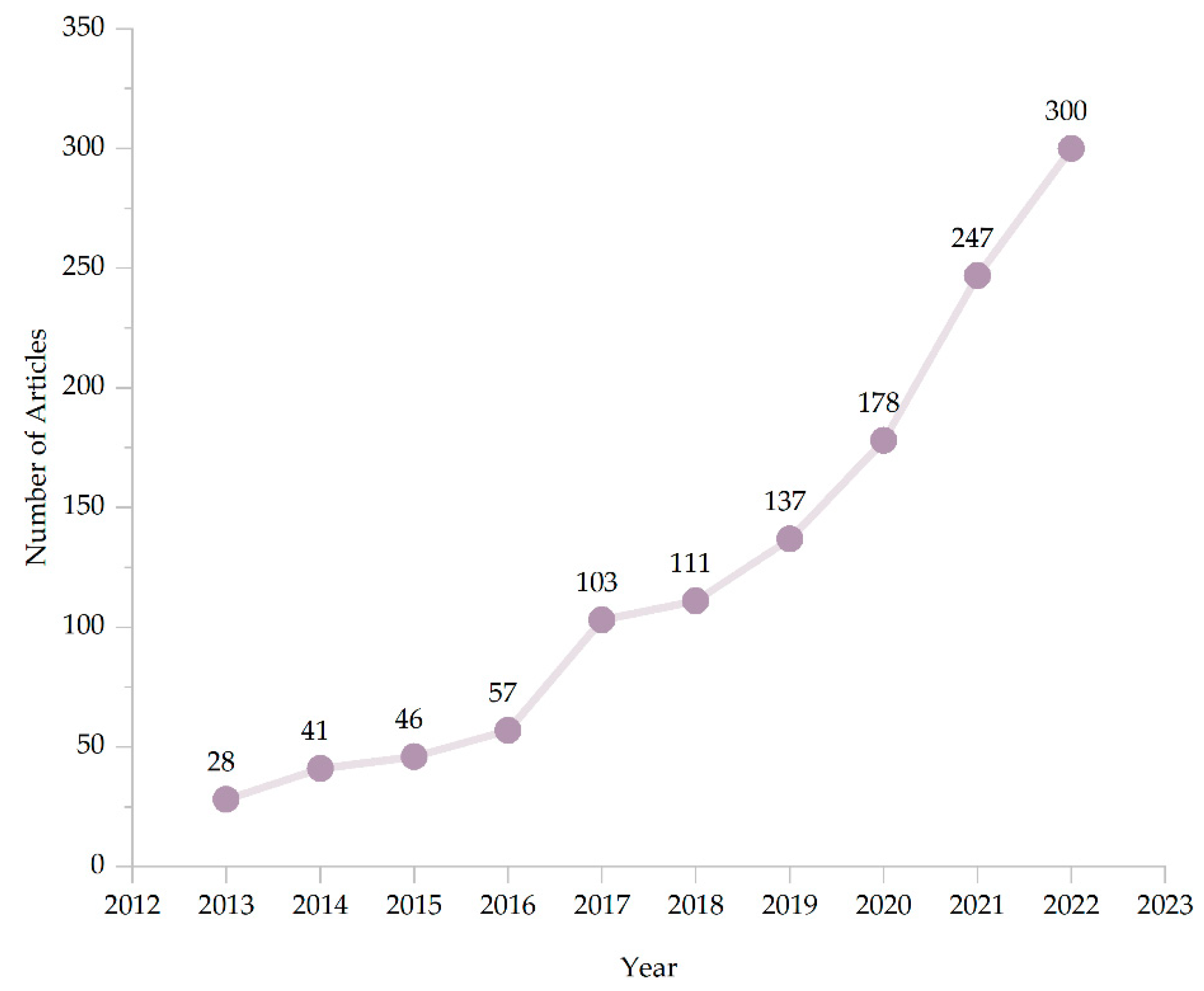
2.2. Document Retrieval
2.3. Screening Criteria
- The paper should include research conducted on wearable sensors, machine learning, and disease rehabilitation;
- The paper should provide a detailed analysis of the performance characteristics, where the sensor is worn, and the accuracy of the wearable sensor;
- The paper should elaborate on the application of the machine learning algorithm involved in data processing;
- The paper should include research conducted on the treatment and rehabilitation of one or more diseases.
- Exclude all review papers, review articles, and papers that lack specific research results;
- If the research exists in both academic journals and conference papers, select the former;
- Exclude papers that briefly mention wearable sensors or machine learning or disease recovery.
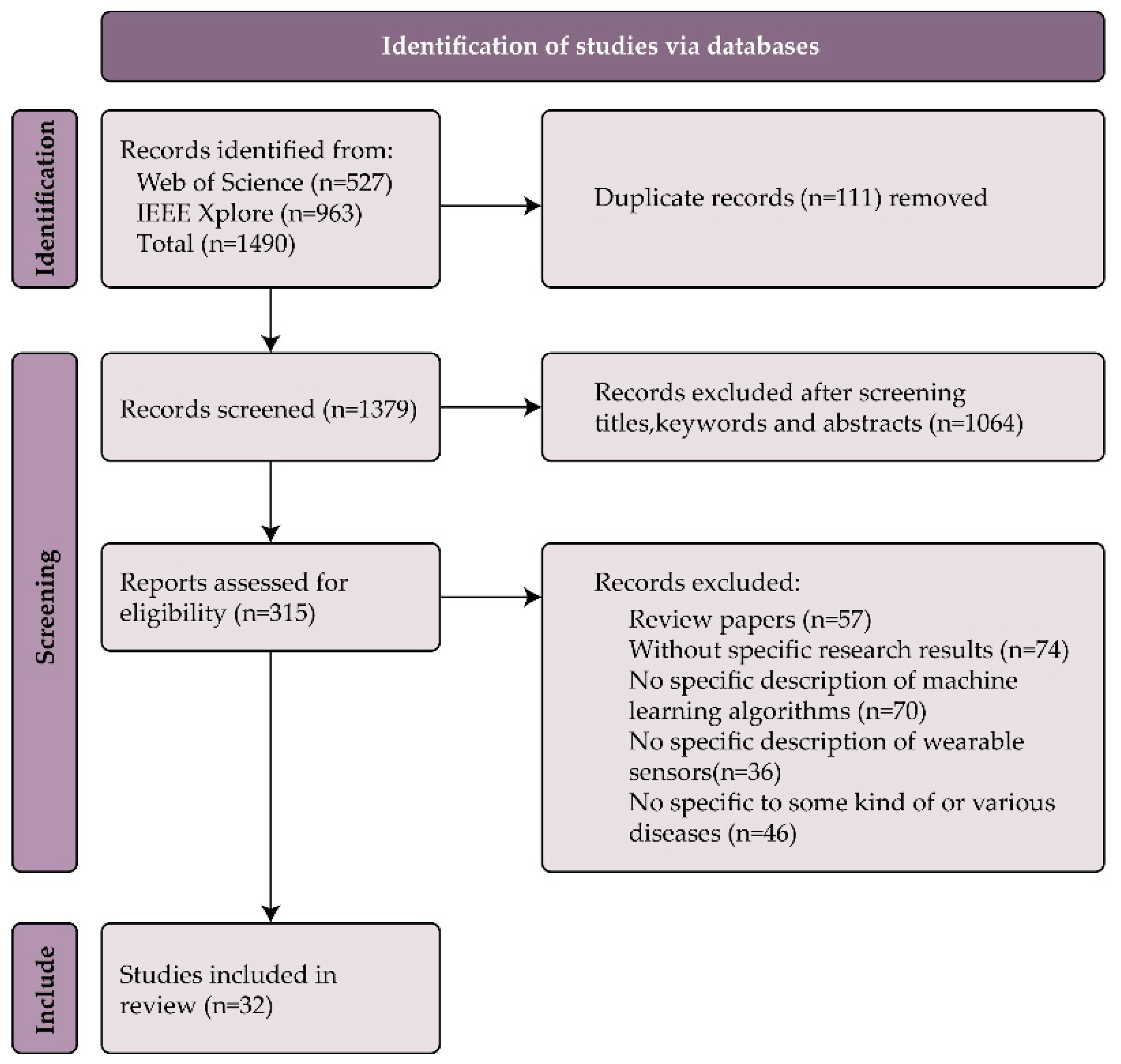
2.4. Article Screening Process
3. Results
3.1. Wearable Sensors
3.2. Wearable Sensor Type
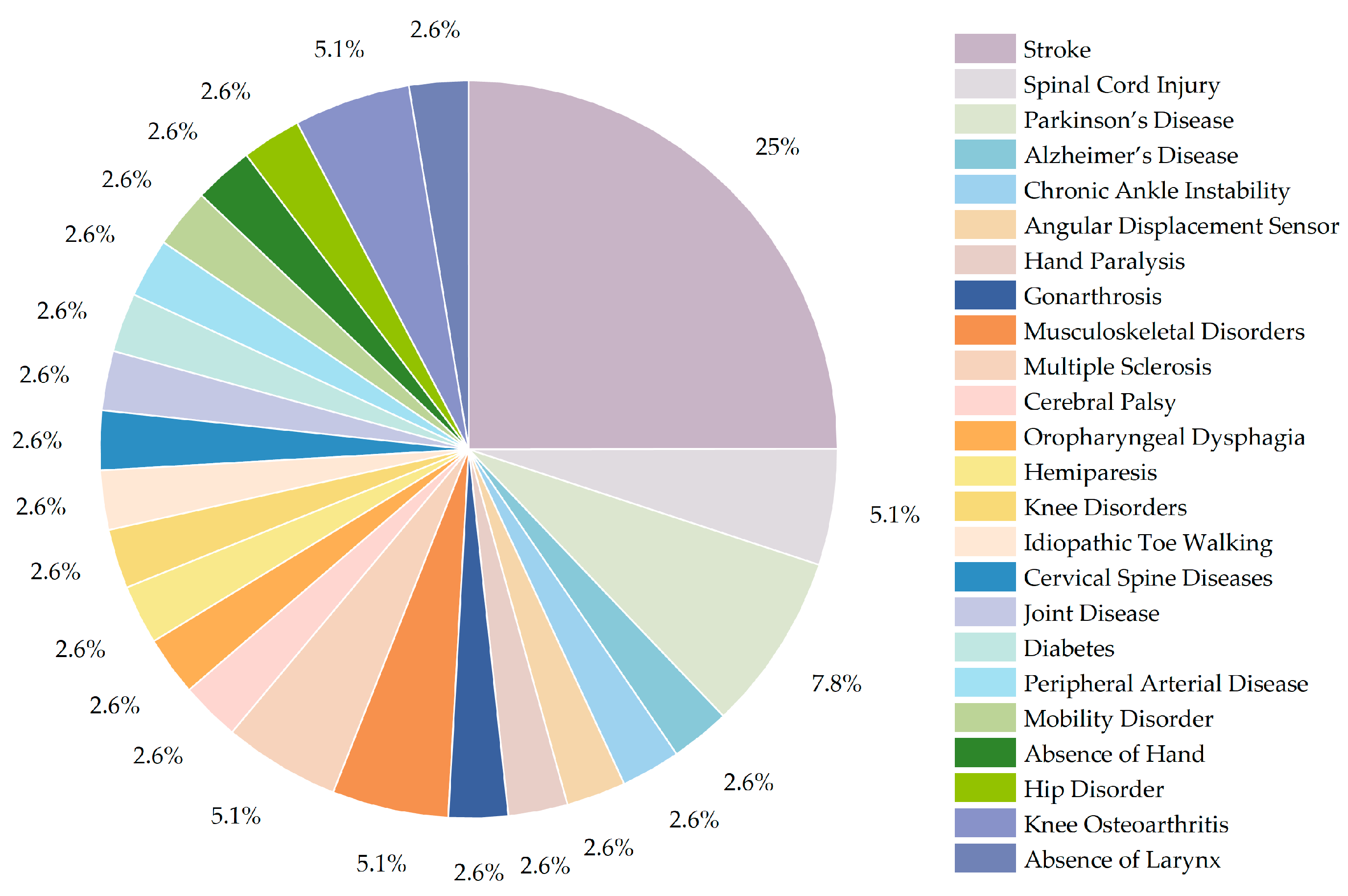
3.3. Disease Types
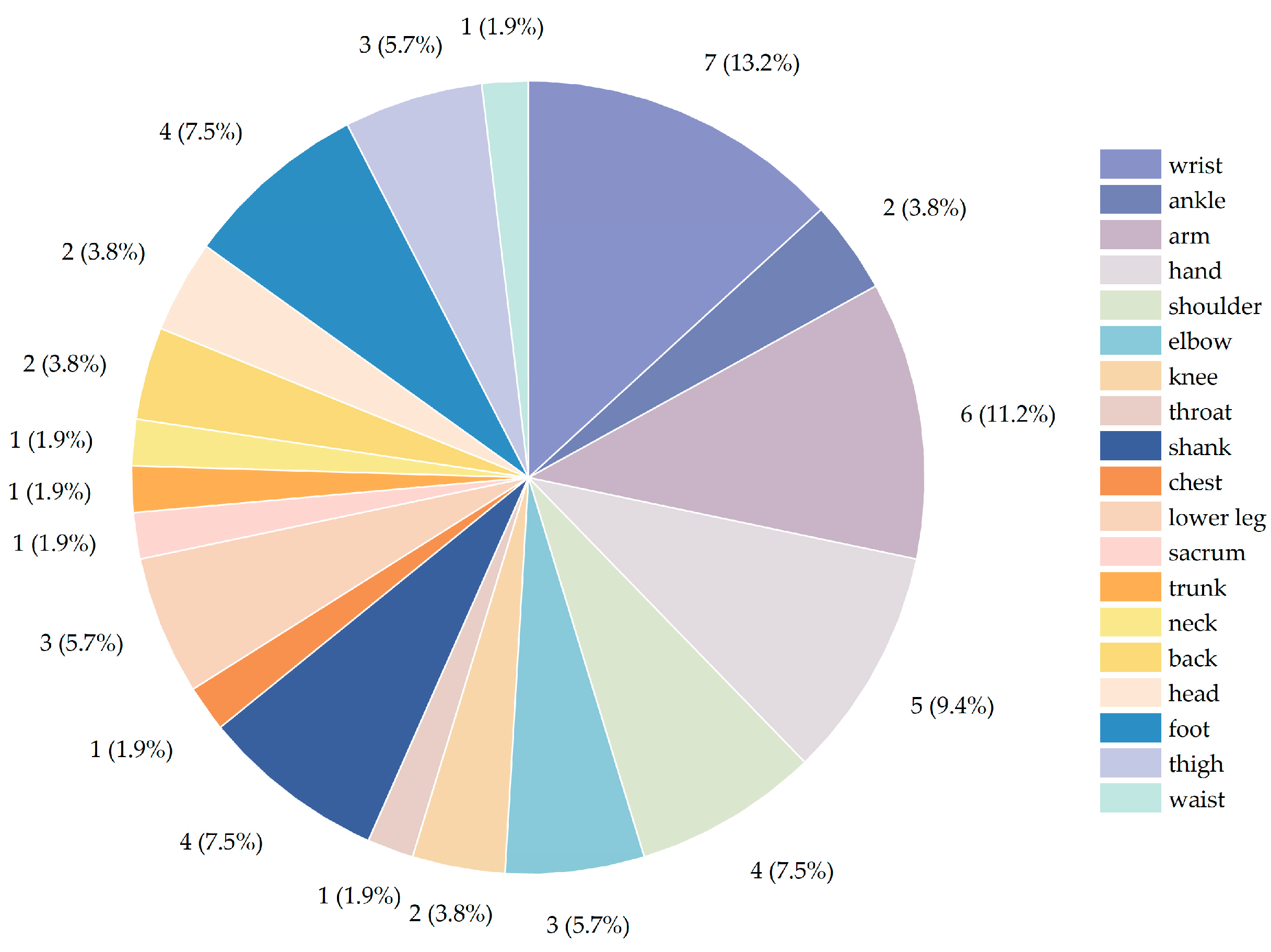
3.4. Sensor Location
3.5. Rehabilitation Exercise
3.6. Feature Extraction
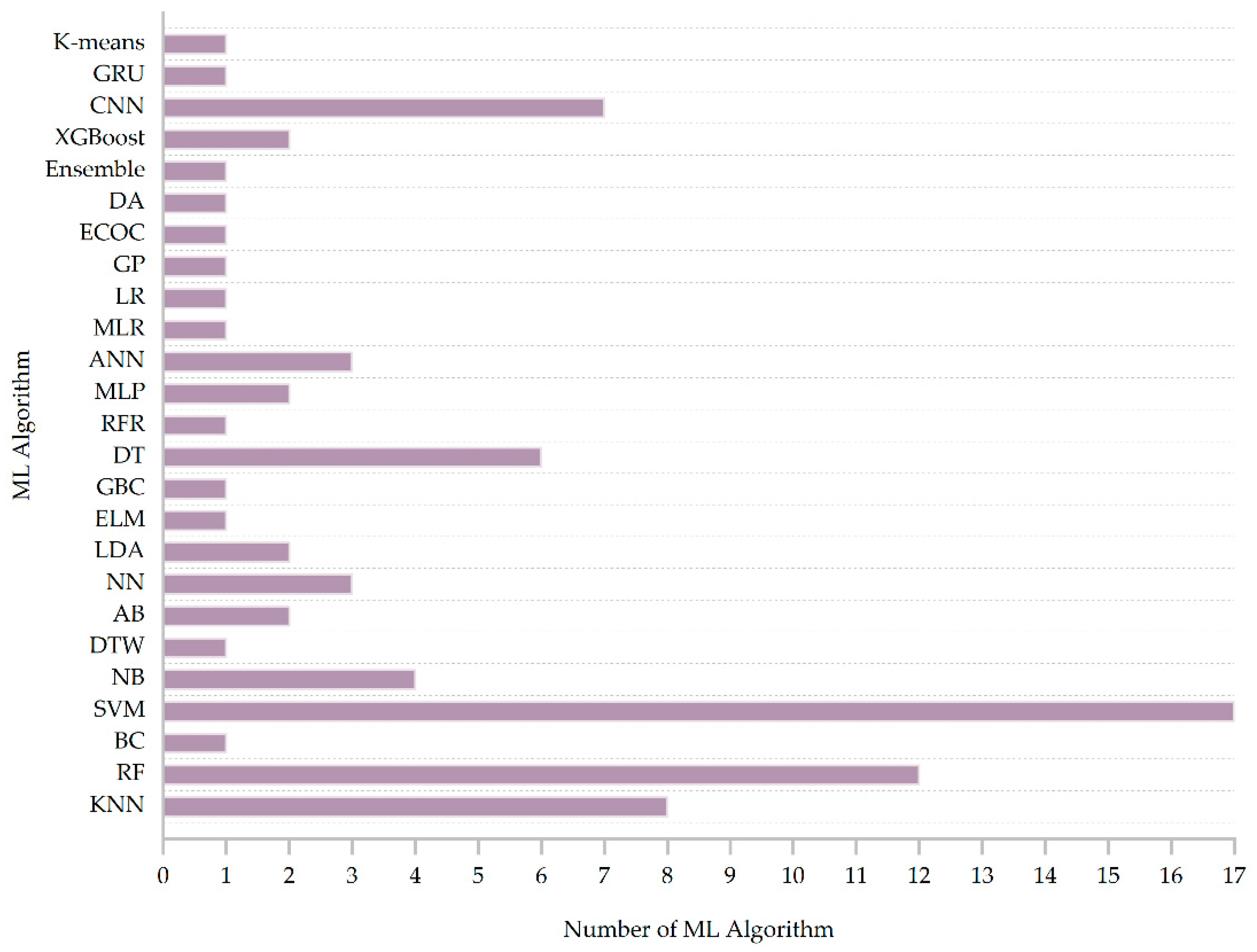
3.7. Machine Learning Methods
4. Discussion
4.1. Wearable Sensor Type Selection
4.2. Application Analysis of Machine Learning Algorithms
4.3. Rehabilitation
4.4. Propositions for Future Studies
4.4.1. Participants
4.4.2. Multiple Sensors and Special Patients
4.4.3. Robot-Assisted Rehabilitation System
4.4.4. Sensor Durability
4.4.5. Virtual Reality
4.4.6. Machine Learning Optimization and Deep Learning Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, S.; Zhang, J. Sensor-Based Exercise Rehabilitation Robot Training Method. J. Sens. 2023, 2023, 7881084. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, H.; Jiang, N.; Wang, Z.; Liu, L.; An, Y.; Zhao, H.; Miao, X.; Liu, R.; Fortino, G. Multi-Sensor Information Fusion Based on Machine Learning for Real Applications in Human Activity Recognition: State-of-the-Art and Research Challenges. Inf. Fusion 2022, 80, 241–265. [Google Scholar] [CrossRef]
- Semwal, V.B.; Gupta, A.; Lalwani, P. An Optimized Hybrid Deep Learning Model Using Ensemble Learning Approach for Human Walking Activities Recognition. J. Supercomput. 2021, 77, 12256–12279. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Vargas, L.; Hu, X.; Zhu, Y. A Novel Finger Kinematic Tracking Method Based on Skin-Like Wearable Strain Sensors. IEEE Sens. J. 2018, 18, 3010–3015. [Google Scholar] [CrossRef]
- Mainali, S.; Darsie, M.E.; Smetana, K.S. Machine Learning in Action: Stroke Diagnosis and Outcome Prediction. Front. Neurol. 2021, 12, 734345. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. The Role of Artificial Intelligence in Future Rehabilitation Services: A Systematic Literature Review. IEEE Access 2023, 11, 11024–11043. [Google Scholar] [CrossRef]
- Liao, Y.; Vakanski, A.; Xian, M.; Paul, D.; Baker, R. A Review of Computational Approaches for Evaluation of Rehabilitation Exercises. Comput. Biol. Med. 2020, 119, 103687. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Hua, Z.; Zhang, J.; Guo, P.; Hao, D.; Gao, Y.; Huang, J. Recent Advancements in Flexible and Wearable Sensors for Biomedical and Healthcare Applications. J. Phys. D Appl. Phys. 2022, 55, 134001. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent Developments in Sensors for Wearable Device Applications. Anal. Bioanal. Chem. 2021, 413, 6037–6057. [Google Scholar] [CrossRef]
- Park, Y.-G.; Lee, S.; Park, J.-U. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors 2019, 19, 4353. [Google Scholar] [CrossRef] [PubMed]
- Stack, E.; Agarwal, V.; King, R.; Burnett, M.; Tahavori, F.; Janko, B.; Harwin, W.; Ashburn, A.; Kunkel, D. Identifying Balance Impairments in People with Parkinson’s Disease Using Video and Wearable Sensors. Gait Posture 2018, 62, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Esquivel, K.M.; Gillespie, J.; Condell, J.; Davies, R.; Karim, S.; Nevala, E.; Alamäki, A.; Jalovaara, J.; Barton, J.; et al. Feasibility of Sensor Technology for Balance Assessment in Home Rehabilitation Settings. Sensors 2021, 21, 4438. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, A.; Fujiyama, H.; Machida, M. A Wireless Multi-Layered EMG/MMG/NIRS Sensor for Muscular Activity Evaluation. Sensors 2023, 23, 1539. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Mohd Zahid, M.S.; Ul Hassan, S.; Hasbullah, S.; Mandala, S. Advances of ECG Sensors from Hardware, Software and Format Interoperability Perspectives. Electronics 2021, 10, 105. [Google Scholar] [CrossRef]
- Dan, J.; Foged, M.T.; Vandendriessche, B.; Van Paesschen, W.; Bertrand, A. Sensor Selection and Miniaturization Limits for Detection of Interictal Epileptiform Discharges with Wearable EEG. J. Neural Eng. 2023, 20, 016045. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vargas, P.; Flor, O.; Salvador-Acosta, B.; Suárez-Carreño, F.; Santórum, M.; Solorzano, S.; Salvador-Ullauri, L. Inertial Sensors for Hip Arthroplasty Rehabilitation: A Scoping Review. Sensors 2023, 23, 5048. [Google Scholar] [CrossRef]
- Gill, W.A.; Howard, I.; Mazhar, I.; McKee, K. A Review of MEMS Vibrating Gyroscopes and Their Reliability Issues in Harsh Environments. Sensors 2022, 22, 7405. [Google Scholar] [CrossRef]
- Javed, A.R.; Sarwar, M.U.; Khan, S.; Iwendi, C.; Mittal, M.; Kumar, N. Analyzing the Effectiveness and Contribution of Each Axis of Tri-Axial Accelerometer Sensor for Accurate Activity Recognition. Sensors 2020, 20, 2216. [Google Scholar] [CrossRef]
- Li, W.; Lu, W.; Sha, X.; Xing, H.; Lou, J.; Sun, H.; Zhao, Y. Wearable Gait Recognition Systems Based on MEMS Pressure and Inertial Sensors: A Review. IEEE Sens. J. 2022, 22, 1092–1104. [Google Scholar] [CrossRef]
- Sun, S.; Cao, Z.; Zhu, H.; Zhao, J. A Survey of Optimization Methods from a Machine Learning Perspective. IEEE Trans. Cybern. 2020, 50, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Doupe, P.; Faghmous, J.; Basu, S. Machine Learning for Health Services Researchers. Value Health 2019, 22, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Abualigah, L.; Shambour, Q.; Abu-Hashem, M.A.; Shambour, M.K.Y.; Alsalibi, A.I.; Gandomi, A.H. Machine Learning in Medical Applications: A Review of State-of-the-Art Methods. Comput. Biol. Med. 2022, 145, 105458. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial Intelligence (AI) and Internet of Medical Things (IoMT) Assisted Biomedical Systems for Intelligent Healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Rahman, S.; Zhou, J.; Kang, J.J. A Comprehensive Review on Machine Learning in Healthcare Industry: Classification, Restrictions, Opportunities and Challenges. Sensors 2023, 23, 4178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, W.; Zhang, J. A Secure Clinical Diagnosis with Privacy-Preserving Multiclass Support Vector Machine in Clouds. IEEE Syst. J. 2022, 16, 67–78. [Google Scholar] [CrossRef]
- Zhang, Z. Big Data Analysis with Artificial Intelligence Technology Based on Machine Learning Algorithm. IFS 2020, 39, 6733–6740. [Google Scholar] [CrossRef]
- Chen, K.-H.; Su, C.; Hakert, C.; Buschjäger, S.; Lee, C.-L.; Lee, J.-K.; Morik, K.; Chen, J.-J. Efficient Realization of Decision Trees for Real-Time Inference. ACM Trans. Embed. Comput. Syst. 2022, 21, 1–26. [Google Scholar] [CrossRef]
- Nanfack, G.; Temple, P.; Frénay, B. Constraint Enforcement on Decision Trees: A Survey. ACM Comput. Surv. 2022, 54, 1–36. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Zhang, S.; Liang, J. Extreme Random Forest Method for Machine Fault Classification. Meas. Sci. Technol. 2021, 32, 114006. [Google Scholar] [CrossRef]
- Schonlau, M.; Zou, R.Y. The Random Forest Algorithm for Statistical Learning. Stata J. 2020, 20, 3–29. [Google Scholar] [CrossRef]
- Tang, K.; Luo, R.; Zhang, S. An Artificial Neural Network Algorithm for the Evaluation of Postoperative Rehabilitation of Patients. J. Healthc. Eng. 2021, 2021, 3959844. [Google Scholar] [CrossRef] [PubMed]
- Teslyuk, V.; Kazarian, A.; Kryvinska, N.; Tsmots, I. Optimal Artificial Neural Network Type Selection Method for Usage in Smart House Systems. Sensors 2020, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.M.; Lim, J.H. A Clinical Perspective on Bespoke Sensing Mechanisms for Remote Monitoring and Rehabilitation of Neurological Diseases: Scoping Review. Sensors 2023, 23, 536. [Google Scholar] [CrossRef]
- Topham, L.K.; Khan, W.; Al-Jumeily, D.; Hussain, A. Human Body Pose Estimation for Gait Identification: A Comprehensive Survey of Datasets and Models. ACM Comput. Surv. 2023, 55, 120. [Google Scholar] [CrossRef]
- Newaz, N.T.; Hanada, E. The Methods of Fall Detection: A Literature Review. Sensors 2023, 23, 5212. [Google Scholar] [CrossRef]
- Bhoir, A.A.; Mishra, T.A.; Narayan, J.; Dwivedy, S.K. Machine Learning Algorithms in Human Gait Analysis. In Encyclopedia of Data Science and Machine Learning; IGI Global: Hershey, PA, USA, 2023; pp. 922–937. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, A.; Gu, Y.; Zhao, L.; Shim, V.; Fernandez, J. Recent Machine Learning Progress in Lower Limb Running Biomechanics with Wearable Technology: A Systematic Review. Front. Neurorobotics 2022, 16, 913052. [Google Scholar] [CrossRef]
- Jourdan, T.; Debs, N.; Frindel, C. The Contribution of Machine Learning in the Validation of Commercial Wearable Sensors for Gait Monitoring in Patients: A Systematic Review. Sensors 2021, 21, 4808. [Google Scholar] [CrossRef]
- Usmani, S.; Saboor, A.; Haris, M.; Khan, M.A.; Park, H. Latest Research Trends in Fall Detection and Prevention Using Machine Learning: A Systematic Review. Sensors 2021, 21, 5134. [Google Scholar] [CrossRef]
- Boukhennoufa, I.; Zhai, X.; Utti, V.; Jackson, J.; McDonald-Maier, K.D. Wearable Sensors and Machine Learning in Post-Stroke Rehabilitation Assessment: A Systematic Review. Biomed. Signal Process. Control 2022, 71, 103197. [Google Scholar] [CrossRef]
- Chen, X.; Gong, L.; Wei, L.; Yeh, S.-C.; Da Xu, L.; Zheng, L.; Zou, Z. A Wearable Hand Rehabilitation System with Soft Gloves. IEEE Trans. Ind. Inf. 2021, 17, 943–952. [Google Scholar] [CrossRef]
- Facciorusso, S.; Spina, S.; Reebye, R.; Turolla, A.; Calabrò, R.S.; Fiore, P.; Santamato, A. Sensor-Based Rehabilitation in Neurological Diseases: A Bibliometric Analysis of Research Trends. Brain Sci. 2023, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Jia, S.; Gao, H.; Xue, Z.; Meng, X. Recent Advances in Multifunctional Wearable Sensors and Systems: Design, Fabrication, and Applications. Biosensors 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, S.; Guo, Z.; Pirbhulal, S.; Wu, W.; Feng, J.; Dan, G. A Comparative Study of Motion Recognition Methods for Efficacy Assessment of Upper Limb Function. Adapt. Control Signal 2019, 33, 1248–1256. [Google Scholar] [CrossRef]
- Amiri, A.M.; Shoaib, N.; Hiremath, S.V. A Framework to Enhance Assistive Technology Based Mobility Tracking in Individuals with Spinal Cord Injury. In Proceedings of the 2017 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Montreal, QC, Canada, 14–16 November 2017; pp. 467–471. [Google Scholar] [CrossRef]
- Huo, W.; Angeles, P.; Tai, Y.F.; Pavese, N.; Wilson, S.; Hu, M.T.; Vaidyanathan, R. A Heterogeneous Sensing Suite for Multisymptom Quantification of Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1397–1406. [Google Scholar] [CrossRef]
- Xu, P.; Xia, D.; Zheng, B.; Huang, L.; Xie, L. A Novel Compensatory Motion Detection Method Using Multiple Signals and Machine Learning. IEEE Sens. J. 2022, 22, 17162–17172. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.; Guo, L.; Wang, J. A Remote Quantitative Fugl-Meyer Assessment Framework for Stroke Patients Based on Wearable Sensor Networks. Comput. Methods Programs Biomed. 2016, 128, 100–110. [Google Scholar] [CrossRef]
- Guo, R.; Cheng, X.; Hou, Z.-C.; Ma, J.-Z.; Zheng, W.-Q.; Wu, X.-M.; Jiang, D.; Pan, Y.; Ren, T.-L. A Shoe-Integrated Sensor System for Long- Term Center of Pressure Evaluation. IEEE Sens. J. 2021, 21, 27037–27044. [Google Scholar] [CrossRef]
- Wood, D.S.; Jensen, K.; Crane, A.; Lee, H.; Dennis, H.; Gladwell, J.; Shurtz, A.; Fullwood, D.T.; Seeley, M.K.; Mitchell, U.H.; et al. Accurate Prediction of Knee Angles during Open-Chain Rehabilitation Exercises Using a Wearable Array of Nanocomposite Stretch Sensors. Sensors 2022, 22, 2499. [Google Scholar] [CrossRef]
- Bavan, L.; Surmacz, K.; Beard, D.; Mellon, S.; Rees, J. Adherence Monitoring of Rehabilitation Exercise with Inertial Sensors: A Clinical Validation Study. Gait Posture 2019, 70, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, G.; Lee, S.-A.; Nam, Y. Analysis of Machine Learning-Based Assessment for Elbow Spasticity Using Inertial Sensors. Sensors 2020, 20, 1622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chee, P.-S.; Lim, E.-H.; Tan, C.-H. Artificial Intelligence-Assisted Throat Sensor Using Ionic Polymer–Metal Composite (IPMC) Material. Polymers 2021, 13, 3041. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.H.; Zambrana, C.; Idelsohn-Zielonka, S.; Claramunt-Molet, M.; Ugartemendia-Etxarri, A.; Rovini, E.; Moschetti, A.; Molleja, C.; Martin, C.; Salleras, E.O.; et al. Assessment of Purposeful Movements for Post-Stroke Patients in Activites of Daily Living with Wearable Sensor Device. In Proceedings of the 2019 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Siena, Italy, 9–11 July 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Zhang, N.; Zhang, X.; Zhang, B.; Wang, S.; Liu, T.; Yi, J. Automatic Assessments of Parkinsonian Gait with Wearable Sensors for Human Assistive Systems. Sensors 2023, 23, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Huang, B.; Argent, R.; Caulfield, B.; Kechadi, T. Automatic Classification of Knee Rehabilitation Exercises Using a Single Inertial Sensor: A Case Study. In Proceedings of the 2018 IEEE 15th International conference on wearable and implantable Body Sensor Networks (BSN), Chicago, IL, USA, 19–22 May 2018; pp. 21–24. [Google Scholar] [CrossRef]
- Lueken, M.J.; Misgeld, B.J.E.; Leonhardt, S. Classification of Spasticity Affected EMG-Signals. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Soangra, R.; Wen, Y.; Yang, H.; Grant-Beuttler, M. Classifying Toe Walking Gait Patterns Among Children Diagnosed With Idiopathic Toe Walking Using Wearable Sensors and Machine Learning Algorithms. IEEE Access 2022, 10, 77054–77067. [Google Scholar] [CrossRef]
- Singhvi, S.; Ren, H. Comparative Study of Motion Recognition with Temporal Modelling of Electromyography for Thumb and Index Finger Movements Aiming for Wearable Robotic Finger Exercises. In Proceedings of the 2018 3rd International Conference on Advanced Robotics and Mechatronics (ICARM), Singapore, 18–20 July 2018; pp. 509–514. [Google Scholar] [CrossRef]
- Salinas, S.A.; Elgalhud, M.A.T.A.; Tambakis, L.; Salunke, S.V.; Patel, K.; Ghenniwa, H.; Ouda, A.; McIsaac, K.; Grolinger, K.; Trejos, A.L. Comparison of Machine Learning Techniques for Activities of Daily Living Classification with Electromyographic Data. In Proceedings of the 2022 International Conference on Rehabilitation Robotics (ICORR), Rotterdam, The Netherlands, 25–29 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- An, S.; Pu, X.; Zhou, S.; Wu, Y.; Li, G.; Xing, P.; Zhang, Y.; Hu, C. Deep Learning Enabled Neck Motion Detection Using a Triboelectric Nanogenerator. ACS Nano 2022, 16, 9359–9367. [Google Scholar] [CrossRef]
- Zhu, Z.-A.; Lu, Y.-C.; You, C.-H.; Chiang, C.-K. Deep Learning for Sensor-Based Rehabilitation Exercise Recognition and Evaluation. Sensors 2019, 19, 887. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, Y.; Lee, K.-S.; Park, H.-S. Development and Clinical Evaluation of a Web-Based Upper Limb Home Rehabilitation System Using a Smartwatch and Machine Learning Model for Chronic Stroke Survivors: Prospective Comparative Study. JMIR Mhealth Uhealth 2020, 8, e17216. [Google Scholar] [CrossRef]
- Chen, H.-C.; Sunardi; Liau, B.-Y.; Lin, C.-Y.; Akbari, V.B.H.; Lung, C.-W.; Jan, Y.-K. Estimation of Various Walking Intensities Based on Wearable Plantar Pressure Sensors Using Artificial Neural Networks. Sensors 2021, 21, 6513. [Google Scholar] [CrossRef]
- Yen, C.-T.; Liao, J.-X.; Huang, Y.-K. Feature Fusion of a Deep-Learning Algorithm into Wearable Sensor Devices for Human Activity Recognition. Sensors 2021, 21, 8294. [Google Scholar] [CrossRef]
- Maheen, A.; Bin Shahzad, M.; Asif, M.U.; Ahmad, S.F.; Zafar, S.; Maqbool, H.F.; Usman, M.; Arshad, H. Human Hand Gesture Recognition System Using Body Sensor Network. In Proceedings of the 2021 International Conference on Robotics and Automation in Industry (ICRAI), Xi’an, China, 30 May–5 June 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Zhang, R.; Pan, Z.; Chen, Y.; Xie, L.; Luo, J.; Zhu, Y. Interpretable Evaluation for the Brunnstrom Recovery Stage of the Lower Limb Based on Wearable Sensors. Front. Neuroinform. 2022, 16, 1006494. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arrunategui, J.P.; Eng, J.J.; Hodgson, A.J. Monitoring Arm Movements Post-Stroke for Applications in Rehabilitation and Home Settings. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, J.C.; Moghaddamnia, S.; Penner, M.; Peissig, J. Monitoring the Rehabilitation Progress Using a DCNN and Kinematic Data for Digital Healthcare. In Proceedings of the 2020 28th European Signal Processing Conference (EUSIPCO), Amsterdam, The Netherlands, 18–21 January 2021; pp. 1333–1337. [Google Scholar] [CrossRef]
- Burns, M.K.; Pei, D.; Vinjamuri, R. Myoelectric Control of a Soft Hand Exoskeleton Using Kinematic Synergies. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Liu, K.-C.; Chan, C.-T. Online Segmentation with Multi-Layer SVM for Knee Osteoarthritis Rehabilitation Monitoring. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 55–60. [Google Scholar] [CrossRef]
- Rameau, A. Pilot Study for a Novel and Personalized Voice Restoration Device for Patients with Laryngectomy. Head Neck 2020, 42, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Cranny, A.; Gupta, N.; Maharatna, K.; Achner, J.; Klemke, J.; Jöbges, M.; Ortmann, S. Recognizing Upper Limb Movements with Wrist Worn Inertial Sensors Using K-Means Clustering Classification. Hum. Mov. Sci. 2015, 40, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Papi, E.; Spulber, I.; Kotti, M.; Georgiou, P.; McGregor, A.H. Smart Sensing System for Combined Activity Classification and Estimation of Knee Range of Motion. IEEE Sens. J. 2015, 15, 5535–5544. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.; Chen, Y.; Li, M. A Lightweight and Accurate Localization Algorithm Using Multiple Inertial Measurement Units. IEEE Robot. Autom. Lett. 2020, 5, 1508–1515. [Google Scholar] [CrossRef]
- Regterschot, G.R.H.; Selles, R.W.; Ribbers, G.M.; Bussmann, J.B.J. Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients. Sensors 2021, 21, 4353. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; OuYang, X.; Chan, C.C.; Ruan, S. Wearable Physiological Monitoring System Based on Electrocardiography and Electromyography for Upper Limb Rehabilitation Training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef]
- Lim, S.; D’Souza, C. Measuring Effects of Two-Handed Side and Anterior Load Carriage on Thoracic-Pelvic Coordination Using Wearable Gyroscopes. Sensors 2020, 20, 5206. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Yu, L.; Yeung, E.H.K.; Luo, J.; Tsui, K.-L.; Zhao, Y. A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults. Sensors 2022, 22, 6752. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Shen, C.; Feng, X.; Zhu, Q.; Shorfuzzaman, M.; Lv, Z. Upper Limb Rehabilitation System for Stroke Survivors Based on Multi-Modal Sensors and Machine Learning. IEEE Access 2021, 9, 30283–30291. [Google Scholar] [CrossRef]
- Gomes, M.A.S.; Kovaleski, J.L.; Pagani, R.N.; Da Silva, V.L. Machine Learning Applied to Healthcare: A Conceptual Review. J. Med. Eng. Technol. 2022, 46, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial Intelligence in Disease Diagnosis: A Systematic Literature Review, Synthesizing Framework and Future Research Agenda. J. Ambient. Intell. Hum. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fan, E.; Wang, P. Comparative Analysis of Image Classification Algorithms Based on Traditional Machine Learning and Deep Learning. Pattern Recognit. Lett. 2021, 141, 61–67. [Google Scholar] [CrossRef]
- saacs-Itua, A.; Wong, S. Stroke Rehabilitation and Recovery. Br. J. Hosp. Med. 2021, 82, 1–7. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, A.E.-P.; Durairaj, P.; Tan, K.K.; Verawaty, V. Development of a Compensation-Aware Virtual Rehabilitation System for Upper Extremity Rehabilitation in Community-Dwelling Older Adults with Stroke. J. Neuroeng. Rehabil. 2023, 20, 56. [Google Scholar] [CrossRef]
- Pérennou, D.; Dai, S.; Gastaldi, R.; Fraix, V.; Leroux, N.; Clarac, E.; Davoine, P.; Piscicelli, C.; Krack, P. Retropulsion with Tilted Postural Vertical Causing Backward Falls in an Individual with Parkinson’s Disease: Improvement by Specific Rehabilitation. Ann. Phys. Rehabil. Med. 2023, 66, 101728. [Google Scholar] [CrossRef]
- Brognara, L.; Palumbo, P.; Grimm, B.; Palmerini, L. Assessing Gait in Parkinson’s Disease Using Wearable Motion Sensors: A Systematic Review. Diseases 2019, 7, 18. [Google Scholar] [CrossRef]
- Roy, A.; Chakraborty, S. Support Vector Machine in Structural Reliability Analysis: A Review. Reliab. Eng. Syst. Saf. 2023, 233, 109126. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Yang, W.; Peng, S.; Zhou, J. A Survey of Convolutional Neural Networks: Analysis, Applications, and Prospects. IEEE Trans. Neural Netw. Learning Syst. 2022, 33, 6999–7019. [Google Scholar] [CrossRef]
- Xuefang, L.; Guihua, W.; Fengru, M. The Effect of Early Cognitive Training and Rehabilitation for Patients with Cognitive Dysfunction in Stroke. Int. J. Methods Psych. Res. 2021, 30, e1882. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.; Coelho, J.; Rogado, P.; Correia, R.; Castro, C.; Fernandes, J.B. Barriers to Gait Training among Stroke Survivors: An Integrative Review. JFMK 2022, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Li, Y.; Liu, H.; Wang, I.; Ren, Y. Influencing Factors Analysis of Rehabilitation for Patients with Spinal Cord Injury. Intell. Autom. Soft Comput. 2022, 34, 455–466. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, C.-M.; Chen, P.-C.; See, A.R.; Chen, S.-C. Pressure-Sensor-Based Gait Analysis for Disabled People. Sens. Mater. 2022, 34, 225. [Google Scholar] [CrossRef]
- Pan, Z.; Gao, H.; Chen, Y.; Xie, Z.; Xie, L. Evaluation of Hemiplegic Gait Based on Plantar Pressure and Inertial Sensors. IEEE Sens. J. 2023, 23, 12008–12017. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhao, J.; Zang, X.; Guan, Y. Research on Theory and a Performance Analysis of an Innovative Rehabilitation Robot. Sensors 2022, 22, 3929. [Google Scholar] [CrossRef]
- Di Tocco, J.; Carnevale, A.; Presti, D.L.; Bravi, M.; Bressi, F.; Miccinilli, S.; Sterzi, S.; Longo, U.G.; Denaro, V.; Schena, E.; et al. Wearable Device Based on a Flexible Conductive Textile for Knee Joint Movements Monitoring. IEEE Sens. J. 2021, 21, 26655–26664. [Google Scholar] [CrossRef]
- Franco, T.; Sestrem, L.; Henriques, P.R.; Alves, P.; Varanda Pereira, M.J.; Brandão, D.; Leitão, P.; Silva, A. Motion Sensors for Knee Angle Recognition in Muscle Rehabilitation Solutions. Sensors 2022, 22, 7605. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Yang, T.; Tian, G.; Xiong, D.; Xiao, X.; Zhang, H.; Sun, Y.; Ao, Y.; Huang, J.; et al. Body-Area Sensor Network Featuring Micropyramids for Sports Healthcare. Nano Res. 2023, 16, 1330–1337. [Google Scholar] [CrossRef]
- Proffitt, R.; Ma, M.; Skubic, M. Novel Clinically-Relevant Assessment of Upper Extremity Movement Using Depth Sensors. Top. Stroke Rehabil. 2023, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Postolache, O.; Hemanth, D.J.; Alexandre, R.; Gupta, D.; Geman, O.; Khanna, A. Remote Monitoring of Physical Rehabilitation of Stroke Patients Using IoT and Virtual Reality. IEEE J. Select. Areas Commun. 2021, 39, 562–573. [Google Scholar] [CrossRef]
- Ramasamy, P.; Calderon-Sastre, E.; Renganathan, G.; Das, S.; Kurita, Y. Soft Actuators-Based Skill Training Wearables: A Review on the Interaction Modes, Feedback Types, VR Scenarios, Sensors Utilization and Applications. Robomech J. 2023, 10, 1. [Google Scholar] [CrossRef]
- Shafee, A.; Awaad, T.A. Privacy Attacks against Deep Learning Models and Their Countermeasures. J. Syst. Archit. 2021, 114, 101940. [Google Scholar] [CrossRef]
- Qian, Y. School of Science, Hubei University of Technology, Wuhan 430068, China Exploration of Machine Algorithms Based on Deep Learning Model and Feature Extraction. MBE 2021, 18, 7602–7618. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ullah, Z.; Gwak, J. MRI-Based Brain Tumor Classification Using Ensemble of Deep Features and Machine Learning Classifiers. Sensors 2021, 21, 2222. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, D.; Yao, L.; Guo, B.; Yu, Z.; Liu, Y. Deep Learning for Sensor-Based Human Activity Recognition: Overview, Challenges, and Opportunities. ACM Comput. Surv. 2022, 54, 1–40. [Google Scholar] [CrossRef]



| Academic Library | Search String |
|---|---|
| Web of Science | ((TS = (wearable OR wearable sensor OR wearable device OR wearable sensing device OR accelerometer)) AND TS = (machine learning OR intelligent system OR deep learning OR SVM OR support vector machines OR random forest algorithms OR neural network algorithms OR multilayer perceptron OR artificial neural networks OR ANN)) AND TS = (rehabilitation OR recovery OR rehabilitation training) |
| IEEE Xplore | (“All Metadata”: wearable OR “All Metadata”: wearable sensor OR “All Metadata”: wearable device OR “All Metadata”: wearable sensing device OR “All Metadata”: accelerometer) AND (“All Metadata”: machine learning OR “All Metadata”: intelligent system OR “All Metadata”: deep learning OR “All Metadata”: SVM OR “All Metadata”: support vector machines OR “All Metadata”: random forest algorithms OR “All Metadata”: neural network algorithms OR “All Metadata”: multi-layer perceptron OR “All Metadata”: artificial neural networks OR “All Metadata”: ANN) AND (“All Metadata”: rehabilitation OR “All Metadata”: recovery OR “All Metadata”: rehabilitation training) |
| References | Wearable Sensors Type | Participants | Sensor Location | Feature | Sampling Rate | Exercise | Disease Type | Methods |
|---|---|---|---|---|---|---|---|---|
| [46] | Nine-axis sensor (non-invasive) | 10 post-stroke hemiplegic-simulated subjects Male: 7 Female: 3 | Wrist | Mean value/standard deviation/root square mean value of motion tasks | 100 Hz | Hand to lumbar spine/shoulder flexion 90 degrees/forearm pronation | Post-stroke hemiplegia | Upper-limb evaluation method in the Fugl–Meyer scale |
| [47] | Accelerometer (non-invasive) | Two individuals without spinal cord injuries | Wrist/ankle | Time domain feature: mean/mean absolute deviation/peaks; frequency domain features: total power between a band of frequencies/energy/entropy | 32 Hz | Wheelchair propulsion/walking/walking using crutches | Spinal cord injury | Framework that uses a combination of machine learning models and wearable sensors to capture and track assistive technology-based mobility and function in individuals with SCI |
| [48] | MMG/IMU/force sensor (non-invasive) | 23 Parkinson’s disease patients Male: 12 Female: 11 10 healthy subjects Male: 8 Female: 2 | Upper arm/forearm/wrist/hand | Rigidity features: mean and standard deviation of the calculated torque/standard deviation of the joint angle and angular velocities, etc. Bradykinesia features: root mean square of pronation/supination motion speeds, etc. Tremor features: means and standard deviations of processed rates-of-turn and accelerations | 100 Hz | Pronation supination movements | Parkinson’s disease | Establish a new PDD model and evaluate it using Unified Parkinson’s Disease Rating Scale scores |
| [49] | Force sensor/angular displacement sensor/sEMG (non-invasive) | Fifteen healthy, right-handed male subjects aged between 22 and 30 years old | Hinge mechanism/trapezius muscle | RMS of the force sensor/RMS of the angular displacement sensor | - | Side-to-side reaching/back and forth/ up and down | Stroke | By combining force, angular displacement, and electromyographic signals with torso constraints as the main body, automatic detection of compensated motion is achieved |
| [50] | Accelerometer/flex sensor (non-invasive) | 24 stroke patients Male: 16 Female: 8 | Shoulder/elbow/wrist/fingers | AMP /MEAN /RMS/JERK/ApEn | 20 Hz | Shoulder anteflexion/shoulder extension/forearm pronation and supination/lumbar touch/wrist flexion and extension/lateral pinch/finger touch | Stroke | A novel remote quantitative Fugl- Meyer evaluation (FMA) framework that maps sensor data to clinical FMA scores |
| [51] | Pressure sensor (non-invasive) | 13 young participants Male: 7 Female: 6 | Plantar | Means and standard deviations of all the pressure data | 100 Hz | Standing/walking/siting | Alzheimer’s disease/Parkinson’s disease/chronic ankle instability | Long-term center of pressure monitoring system in a smart-shoe form |
| [42] | Force sensor/flex sensor (non-invasive) | 8 subjects with normal hand motor functions Male: 5 Female: 3 | Knuckle/fingertips/palm | MAV/ RMS/ WL/VAR /standard deviation | 200 Hz | Finger flexion | Hand paralysis | Hand rehabilitation system that supports both mirror therapy and task-oriented therapy |
| [52] | Piezoresistive sensor (non-invasive) | 18 healthy subjects Male: 9 Female: 9 | Knee | - | 18.75 Hz | Open-chain knee flexion | Gonarthrosis | Instrumented knee sleeve and modeled using an adaptive enhanced RFR model |
| [53] | Accelerometer/gyroscope/magnetometer (non-invasive) | 20 patients Male: 8 Female: 12 | Shoulder | Time domain features: mean/root mean square/standard deviation, etc. Frequency domain features: maximum frequency component/mean frequency component/energy spectral density, etc. | 100 Hz (accelerometer) 100 Hz (gyroscope) 25 Hz (magnetometer) | Shoulder abduction/shoulder flexion/wall slide/wall press/shoulder rotation | Musculoskeletal disorders | Using a single inertial sensor and supervised machine learning technology to identify and classify shoulder rehabilitation activities |
| [54] | Accelerometer/gyroscope/magnetometer (non-invasive) | 48 patients Male: 26 Female: 22 | Dorsal side of the elbow | Root mean square/mean/standard deviation/energy/spectral energy/absolute difference/variance/SMA/SV | 256 Hz | Elbow flexion and extension movements | Stroke/multiple sclerosis/cerebral palsy/spinal cord injury | Machine learning algorithms and inertia signals collected during passive stretching are used to grade spasms |
| [55] | IPMC sensor (non-invasive) | - | Throat | Raw voltage data | - | Cough/hum/nod/swallow | Oropharyngeal dysphagia | Self-powered IPMC sensor that can distinguish between the different pressures exerted by throat movements |
| [56] | IMU (non-invasive) | 10 healthy and 12 post-stroke volunteers | Fingertip/hand | Mean value of movement intensity/smoothness of MI/average acceleration and rotation energy, etc. | 100 Hz | Arm movements | Hemiparesis | IMUs used to recognize the purposeful and non-purposeful movements in ADLs for identifying and promoting the use of the impaired limb during daily life in people affected by stroke |
| [57] | IMU (non-invasive) | 25 PD patients and 28 healthy subjects | Ankle/shank | SL/GD/PSP/MH/RL/RSZ/RSY/RSX/MPV/MVV/MSV/MHD | 100 Hz | Walk | Parkinson’s disease | Novel method for automatic assessment of the gait task in UPDRS based on only two shank-mounted IMUs and 12 m straight walking test |
| [58] | IMU/accelerometer/gyroscope (non-invasive) | 44 clinical and 10 healthy subjects | Shin | Mean/median/standard deviation/variance, etc. | 102.4 Hz | Heel slide/seated knee extension/inner range quadriceps/straight leg raise | Knee disorders | System that provides patients with automatic feedback on knee rehabilitation exercises |
| [59] | EMG (non-invasive) | 4 healthy male subjects | Lower leg | EMG data | - | Walk | Stroke/multiple sclerosis | New approach for spastic detection in hemiplegia-affected EMG data using the IPANEMA BSN in combination with SVM |
| [60] | Accelerometer /gyroscope (noninvasive) | 36 pediatric patients | Trunk/sacrum/shank | Mean frequency/the first 5 DFT coefficients/the first 5 maxima of DFT coefficients and their corresponding frequencies | 75 Hz | Walk | Idiopathic toe walking | Using wearable sensors and ML, real-time step detection can be combined with assistive devices for intervention and motor rehabilitation purposes |
| [61] | IMU/EMG (non-invasive) | - | Arm | Mean absolute value/standard deviation/variance/root mean square/waveform length/zero crossing/integrated EMG | 50 Hz (IMU) 200 Hz (EMG) | Thumb and index finger movements | Stroke | Pattern recognition of thumb and index finger gestures using EMG signal recording obtained from Myo armband |
| [62] | EMG (non-invasive) | 22 subjects | Forearm | Mean/variance of EMG/MAV, etc. | 1000 Hz | Hand movement | Musculoskeletal disorders or injuries | An off-line classification approach for the 26 upper-limb ADLs included in the KIN-MUS UJI dataset |
| [63] | Triboelectric sensor (non-invasive) | - | Neck | - | - | Neck movement | Cervical spine diseases | A neck motion detector comprising a self-powered triboelectric sensor set and a deep learning module to recognize neck motion |
| [64] | Accelerometer (non-invasive) | 49 healthy volunteers | Shoulders/back/elbows/forehead | - | 32 Hz | Place hands behind the head with ten fingers crossed/push the elbows back to the body/stretch both hands up with ten fingers crossed/bend over to the left/right | Joint disease | Multi-path convolutional neural network (MP-CNN) based on sensor data for rehabilitation training recognition |
| [65] | IMU/accelerometer/gyroscope (non-invasive) | 17 participants in the HBR group and 6 participants in the control group | Wrist | - | 10 Hz | Bilateral shoulder flexion with both hands interlocked/wall push /move the scapula /towel slide | Chronic stroke | Home-based rehabilitation (HBR) system that identifies and records the type and frequency of rehabilitation exercises performed by the user |
| [66] | Pressure sensor (non-invasive) | 12 healthy subjects | Foot | - | 300 Hz | Walk | Diabetes/peripheral arterial disease | Method based on the artificial neural network to classify walking speed and walking time by using plantar pressure images |
| [67] | Accelerometer /gyroscope (non-invasive) | 21 healthy male volunteers | Waist | - | 50 Hz | Walk/walk upstairs/walk downstairs/sit/stand/lay | Mobility disorder | Device consisting of a single-board computer (SBC) and a six-axis sensor that recognizes activities through deep learning algorithm |
| [68] | EMG/muscle sensor (non-invasive) | 5 healthy male subjects. | Arm | - | - | Hand open/hand close/pinch/pointing finger | Stroke/absence of hand | Method for controlling a 3D prosthetic hand using electromyographic data of basic gestures and manipulating the prosthetic hand using classified data |
| [69] | IMU/pressure sensor (non-invasive) | 20 hemiplegic patients and 10 healthy individuals | Bilateral feet/bilateral calves/bilateral thighs/waist | Gait line/regional pressure/gait phase/acceleration/step length/joint angle | 200 Hz | Walk | Stroke | Method for interpretable BRS-L evaluation of lower extremity motion data and plantar pressure data collected using IMUs and pressure sensors |
| [70] | IMU (non-invasive) | 12 stroke patients Male: 7 Female: 5 | Wrist | Mean of the signal/variance of the signal/ RMS, etc. | 20 Hz | Arm movement | Stroke | Arm rehabilitation monitor system using an IMU sensor placed on a single wrist to acquire arm motion information and process the data using a machine learning classifier |
| [71] | IMU (non-invasive) | 12 patients with hip unilateral arthroplasty | Foot/lower leg/upper leg/lower back | - | 60 Hz | Walk | Hip disorder | Method used to monitor the progress of rehabilitation using kinematic data obtained from a wearable sensor system and a deep convolutional neural network |
| [72] | EMG (non-invasive) | 5 healthy subjects Male: 4 Female: 1 | Hand/arm | - | 13.33 Hz | Wrist/elbow/shoulder flexions | Stroke | Flexible cable-driven full-hand exoskeleton to aid the rehabilitation of stroke patients |
| [73] | IMU (non-invasive) | 10 subjects Male: 5 Female: 5 | Chest/thigh (close to the knee)/shank (close to ankle) of the working leg | Angle of shank for SAE and QSM/angle of thigh for SLR | - | Short-arc exercise (SAE)/straight leg raise (SLR)/quadriceps strengthening mini-squats (QSM) | Knee osteoarthritis | Online segmentation method for knee OA rehabilitation monitoring that can provide real-time feedback to patients and physical therapists |
| [74] | sEMG (non-invasive) | Laryngectomee volunteer | Articulatory muscles on hemiface | Vector of all 0 values, except for 1 in elements where the target sEMG feature is represented | 250 Hz | Specific facial expressions/palpating face | Absence of larynx | Method used for applying the machine learning algorithm to electromyographic signals of joint muscles to identify silent speech in patients undergoing a total laryngectomy |
| [75] | Accelerometer/gyroscope/magnetometer (non-invasive) | 4 healthy subjects and 4 stroke patients | Wrist/arm | Standard deviation/ RMS/ information entropy, etc. | 50 Hz | Extension and flexion of the forearm/rotation of the forearm about the elbow/rotation of the wrist about long axis of forearm | Stroke | Method using data collected from a wristband, a wireless three-axis accelerometer, and a three-axis rate gyroscope combined with partial k-means clustering to identify basic movements of the upper body in everyday life |
| [76] | IMU (non-invasive) | 12 healthy subjects with no reported knee pain | Right knee | MDF/power of the spectrum/peak frequency/maximum spectral amplitude/output range of the signal in the time domain | 122 Hz | Walk/run both indoors and outdoors/ travel up and down the stairs | Knee osteoarthritis | Sensor system capable of monitoring knee motion and classifying aspects of daily living activities to aid in the rehabilitation of patients with knee OA |
| References | ML Algorithm | Accuracy | Description | Limitation |
|---|---|---|---|---|
| [46] | KNN/RF/BC/SVM | 84.95% (KNN) 88.12% (RF) 85.05% (BC) 97.79% (SVM) | The five-fold cross-validation method was used to divide the feature data and action labels into five groups, four groups were used to train, and the remaining group was used to validate the accuracy. | It can recognize upper-limb movements. It cannot identify lower-limb movements. |
| [47] | SVM/RF/NB/DTW | 87.4% to 97.6% | Classification accuracy was assessed using multiple assessments, including 10-fold-stratified cross-validation and 50% cross-validation (50% for training, 50% for testing). | Lack of evaluation of a high number of individuals with varying degrees of spinal cord injuries. |
| [48] | KNN/AB/NN/RF | 85.1% (KNN(K = 1)) 83.0% (AB) 81.9% (NN) 73.6% (KNN(K = 3)) 72.4% (RF) | A voting classification model was established by combining three basic classifiers, and a soft voting algorithm was used to select the final UPDRS score. | A larger dataset needs to be established to reduce errors and improve the accuracy of the model. |
| [49] | KNN/SVM/LDA | 97.58 ± 3% (SVM) 95.68% (KNN) 92.38% (LDA) | The nine extracted features were supplied to the LDA, KNN, and SVM. A five-fold cross-validation method divided the feature data and action labels into five equal groups. Four groups were used to train the classifiers and the other group was used to verify the accuracy of the classifiers. | It Is necessary to conduct actual clinical trials on patients to further verify the universality of detection equipment and prediction methods in identifying the abnormal movement patterns of patients. |
| [50] | ELM | - | Five characteristics were extracted for each exercise. Each exercise had 240 data samples, of which 200 samples served as the training set and the remaining 40 samples served as the test set. | The ceiling effect makes it difficult for doctors to accurately assess the patient’s motor functions. |
| [51] | SVM/RF/GBC/NN | 97.9% (SVM) 97.9% (RF) 97.2% (GBC) 98.6% (NN) | The input features were the means and standard deviations of the pressure data of the sensor, and then the features were transferred to the multi-class support vector machine (SVM) with the radial basis function (RBF) core as the classifier. | Only simple-activity testing was conducted, lacking complex-activity detection. |
| [42] | KNN/SVM/DT | 99.65% (SVM) 96.27% (KNN) 81.73% (DT) | The optimal feature subset was selected from the original features and each feature was tested independently to evaluate the combination of different features using the 10 × 10 cross-validation. | Similar actions are easily mistaken. |
| [52] | RFR | - | Each model trains 90% of the data and tests the remaining 10% of the data. The hyperparameters of the multivariate machine learning regression were optimized using grid search and multivariate Bayesian optimization methods. | It is not possible to fully capture the peaks and troughs of all knee joint flexions or the magnitude of internal/external rotational degrees of freedom. |
| [53] | DT/SVM/KNN/RF | 90.9% (DT) 95.7% (KNN) 97.2% (SVM) 96.4% (RF) | Training used a subset of high-level features; two different validation methods were used to evaluate the prediction performance. Ten-fold cross-validation distributed all labeled data segments randomly and evenly across ten sections. The data contained in the nine folds were trained and the remaining data were tested. | Some actions are misclassified as junk activities, and similar activities are easily confused. |
| [54] | DT/RF/SVM/LDA/MLP | 76.6% (DT) 91.8% (RF) 71.8% (SVM) 80.6% (LDA) 82.6% (MLP) | The performance of the classifier was tested by leave-one cross-validation. Each classifier was tested under four different conditions to determine an optimal classifier. | Due to the limited sample size, it is not guaranteed to perform well on larger datasets. |
| [55] | SVM | 95.0% | The training data set with the kernel function was used to train the SVM model, and the test data set was input into the model to check the accuracy. The model was optimized by punishing parameter C and gamma parameter g, which could test the probability of misclassifications. | When the cough is not strong enough, it is impossible to measure the amplitude in the signal, which can lead to an incorrect judgment. |
| [56] | SVM/ANN | 81.20% (SVM) 97.06% (ANN) | Purposeful events were randomly selected to evaluate the generalization ability of the machine learning model, and then the classifier was trained using all the parameters. The ten-fold cross-validation method was used to train and test the data. | There is a lack of data on other fingers and an age mismatch among participants in this study. |
| [57] | SVM/NBC/MLR | 73.6% (SVM) 73.6% (NBC) 66.0% (MLR) | Recursive feature elimination was performed on each model to study the relationship between the number of features and the accuracy, and to find the optimal feature selection. | The evaluation and data collection are not synchronized, which may lead to errors. The dataset is small and unevenly distributed, making it prone to overfitting and resulting in errors. The selection of gait features is not comprehensive. |
| [58] | LR/SVM/AB/RF/DT | SKE:86.05% (LR) 96.70% (SVM) 94.13% (AB) 93.11% (RF) 91.75% (DT) | Each model was evaluated through a five-step cross-validation process. To avoid overfitting, the folds were generated by dividing the date set by the patient. In the classification process, the data set contained only duplicates that were correctly segmented. | Partial actions obtained the less satisfying results in the laboratory dataset. |
| [59] | SVM | - | The set of training vectors was created based on EMG signal data collected from two different patients, and then an SVM was trained, and the resulting structure could be stored by significant settings. | The results cannot represent all individual diseases. |
| [60] | SVM/DT/RF/KNN/MLP/GP | 85.8% (SVM) 74.4% (DT) 82.8% (RF) 92.9% (KNN) 85.8% (MLP) 86.8% (GP) | The data were randomly divided into two parts, training and testing, with a ratio of four to one. The data were normalized to between 0 and 1 using min–max scaling. Five cross-validations were used for each classifier’s training dataset. | More datasets are needed to achieve a better classification performance. |
| [61] | SVM/KNN/NB/ECOC/DA/DT/ensemble | 88.42% (SVM) 80.09% (KNN) 73.04% (NB) 84.34% (ECOC) 81.73% (DA) 82.60% (DT) 85.65% (Ensemble) | The ratio of training to testing was 4:1, and the test set accuracy was displayed as the average accuracy of 10 trials. In order to achieve the best result, the linear kernel function was used. | The placement position of the armband has a significant impact on signal recognition. |
| [62] | SVM/RF/XGBoost/CNN/GRU | 65.4% (SVM) 57% (RF) 47.7% (XGBoost) 83.6% (CNN) 79% (GRU) | The classifier was trained and tested using TD and FD features. The integrated approach was built with the four models with the best training performance to evaluate methods that could improve the performance of individual models. | Similar movements with both hands can easily lead to confusion. |
| [63] | CNN | 92.63% | The leave-one session-out (LOSO) policy was adopted. The data obtained from one session were used as the test dataset, and the data collected from the remaining three sessions were used as the training dataset. This procedure was repeated four times until the data for each session were considered as one test dataset. | - |
| [64] | MP-CNN | 90.63% | Depending on the number of layers in the middle path, the correlation of the output of the last pooling layer was captured, and the accuracy was highest when D-CNN and S-CNN were combined. | More action data needs to be collected. |
| [65] | CNN | 85.6~100% | Cross-validation was performed on different input and sensor data, and the model with the most accurate data was determined. | There is some degree of data loss. |
| [66] | ANN | 94% | Flatten layer was used to convert the image of the plantar region into a one-dimensional value sequence. The sequence was then used as input data for the ANN model. Hidden layers to propagate training mechanisms. | The data set used is not comprehensive and the plantar features are not detailed. |
| [67] | CNN | 97.49% | A feature fusion model containing nuclei of different sizes was used. After signal normalization and conversion into a fixed format, the inertial data were divided into three partitions, which were composed of three convolution layers and one flattened layer, respectively. | When the data characteristics of two actions are similar, classification errors are prone to occur. |
| [68] | ANN | 91% | For ANN training and testing, a 3:1 ratio was used. The training and verification errors were reduced in a certain number of iterations. | No wrist motion and no force control. |
| [69] | RF/KNN/SVM/NB | 80.07% (RF) 94.20% (KNN) 75.35% (SVM) 82.43% (NB) | A cross-validation approach was used to evaluate the predictive performance of the classification model. The leave-one-subject-out strategy was used to divide the data into training and test sets. | - |
| [70] | RF/CNN | Home-Home: 77.1% (RF) 76.6% (CNN) | A validation dataset was generated by separating 20% of the continuous portion of the training dataset obtained from each participant in a random location. The results of the validation dataset were used to tune the classifier hyperparameters. | Datasets are small and unrepresentative. |
| [71] | DCNN | 98% | Training, validation, and test data were randomly divided into 70%, 15%, and 15%, respectively. The adaptive moment estimation method was used for optimization. The stop-loss criterion was applied to the training progress by evaluating the validation loss. | Lack of more detailed analysis of DCNN input data and gait kinematics data during rehabilitation process. |
| [72] | NN | - | The Bayesian regularization algorithm was used to train the neural network to minimize the internal parameters and model errors and avoid overfitting. | The data set is small and the system is not a fully closed loop. |
| [73] | SVM | 90.6% (layer 1) 92.7% (layer 2) | 10x cross-validation was used to validate the data. A total of 10 rounds were performed, with 1 subset of the 10 subjects selected for each round as the training data and the other 9 subsets as the test data. | Patient movements cannot be fully simulated, and the data are not accurate enough. |
| [74] | XGBoost | 86.4% | The feature data consisted of vectors representing all zeros in the elements that characterized the target surface EMG signal. | Need to improve silent speech recognition algorithm to realize the translation of silent speech into personalized synthetic speech. |
| [75] | K-means | HS: 88% (DOA) 83% (DOG) SP: 70% (DOA) 66% (DOG) | The clustering was formed using a sorted list of features; therefore, a combination of 2–30 features was selected in turn in each iteration, and 10-fold cross-validations were performed on the selected feature vector ten times. | The effects of sensor fusion and other attachment positions need to be observed in larger sample populations. |
| [76] | RF | 93% | The random forest algorithm was a collection of 10 classification decision trees, with 90% of the data randomly selected for building the tree and 10% for testing the algorithm. | Test data should include other activities of daily living to allow for a more comprehensive classification of activities of daily living. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Wu, Z. The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review. Sensors 2023, 23, 7667. https://doi.org/10.3390/s23187667
Wei S, Wu Z. The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review. Sensors. 2023; 23(18):7667. https://doi.org/10.3390/s23187667
Chicago/Turabian StyleWei, Suyao, and Zhihui Wu. 2023. "The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review" Sensors 23, no. 18: 7667. https://doi.org/10.3390/s23187667
APA StyleWei, S., & Wu, Z. (2023). The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review. Sensors, 23(18), 7667. https://doi.org/10.3390/s23187667






