Predictability of Fall Risk Assessments in Community-Dwelling Older Adults: A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Analysis
3. Results
3.1. Study Selection
3.2. Clinical Assessments without Sensors
3.3. Questionnaires
3.4. Physical Performance
3.5. Sensor-Based Clinical Assessments
3.6. Sensor-Based ADL Assessments
4. Discussion
5. Limitations and Future Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Falls. April 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 13 June 2023).
- VeiligheidNL. Feiten Cijfers—Valpreventie—VeiligheidNL. September 2020. Available online: https://www.veiligheid.nl/valpreventie/feiten-cijfers (accessed on 13 June 2023).
- Stam, C.; Blatter, B. Letsels 2020: Kerncijfers LIS. Tech. Rep. 2021, 2, 11. [Google Scholar]
- Vellas, B.J.; Wayne, S.J.; Romero, L.J.; Baumgartner, R.N.; Garry, P.J. Fear of falling and restriction of mobility in elderly fallers. Age Ageing 1997, 26, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.; Peterson, E.W.; Levin, W.C.; Fried, L.; Pordon, D.; Bak, S. Fear of falling among the community-dwelling elderly. J. Aging Health 1993, 5, 229–243. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Williams, C.S. The effect of falls and fall injuries on functioning in community-dwelling older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M112–M119. [Google Scholar] [CrossRef]
- Faulkner, K.A.; Cauley, J.A.; Studenski, S.A.; Landsittel, D.P.; Cummings, S.R.; Ensrud, K.E.; Donaldson, M.; Nevitt, M. Lifestyle predicts falls independent of physical risk factors. Osteoporos. Int. 2009, 20, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.F. Falls in the elderly. Am. Fam. Physician 2000, 61, 2159. [Google Scholar] [PubMed]
- Pasquetti, P.; Apicella, L.; Mangone, G. Pathogenesis and treatment of falls in elderly. Clin. Cases Miner. Bone Metab. 2014, 11, 222. [Google Scholar] [CrossRef]
- Boelens, C.; Hekman, E.E.; Verkerke, G.J. Risk factors for falls of older citizens. Technol. Health Care 2013, 21, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Cavanillas, A.; Padilla-Ruiz, F.; Jime’nez-Mole, J.J.; Peinado-Alonso, C.; Ga, R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur. J. Epidemiol. 2000, 16, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Van Diee, J.H.; Pijnappels, M. Balance control in older adults. In Locomotion and Posture in Older Adults; Springer: Berlin/Heidelberg, Germany, 2017; pp. 237–262. [Google Scholar]
- van der Kruk, E.; Silverman, A.K.; Koizia, L.; Reilly, P.; Fertleman, M.; Bull, A.M.J. Agerelated compensation: Neuromusculoskeletal capacity, reserve movement objectives. J. Biomech. 2021, 122, 110385. [Google Scholar] [CrossRef]
- Clemson, L.; Cumming, R.G.; Kendig, H.; Swann, M.; Heard, R.; Taylor, K. The effectiveness of a community-based program for reducing the incidence of falls in the elderly: A randomized trial. J. Am. Geriatr. Soc. 2004, 52, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the falls efficacy scale-international (fes-i). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (abc) scale. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Williams, T.F.; Mayewski, R. Fall risk index for elderly patients based on number of chronic disabilities. Am. J. Med. 1986, 80, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Marschollek, M.; Rehwald, A.; Wolf, K.; Gietzelt, M.; Nemitz, G.; Schwabedissen, H.M.Z.; Haux, R. Sensor-based fall risk assessment–an expert ‘to go’. Methods Inf. Med. 2011, 50, 420–426. [Google Scholar]
- Zhao, G.; Chen, L.; Ning, H. Sensor-based fall risk assess-ment: A survey. Healthcare 2021, 9, 1448. [Google Scholar] [CrossRef]
- Bizovska, L.; Svoboda, Z.; Janura, M.; Bisi, M.C.; Vuillerme, N. Local dynamic stability during gait for predicting falls in elderly people: A one-year prospective study. PLoS ONE 2018, 13, e0197091. [Google Scholar] [CrossRef]
- Greene, B.R.; McGrath, D.; Caulfield, B. A comparison of cross-sectional and prospective algorithms for falls risk assessment. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4527–4530. [Google Scholar]
- Ihlen, E.A.; Van Schooten, K.S.; Bruijn, S.M.; Van Dieen, J.H.; Vereijken, B.; Helbostad, J.L.; Pijnappels, M. Improved prediction of falls in community-dwelling older adults through phase-dependent entropy of daily-life walking. Front. Aging Neurosci. 2018, 10, 44. [Google Scholar] [CrossRef]
- Weiss, A.; Brozgol, M.; Dorfman, M.; Herman, T.; Shema, S.; Giladi, N.; Hausdorff, J.M. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabilit. Neural Repair 2013, 27, 742–752. [Google Scholar] [CrossRef]
- Kristoffersson, A.; Du, J.; Ehn, M. Performance and characteristics of wearable sensor systems discriminating and classifying older adults according to fall risk: A systematic review. Sensors 2021, 21, 5863. [Google Scholar] [CrossRef] [PubMed]
- Bezold, J.; Krell-Roesch, J.; Eckert, T.; Jekauc, D.; Woll, A. Sensor-based fall risk assessment in older adults with or without cognitive impairment: A systematic review. Eur. Rev. Aging Phys. Act. 2021, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Strini, V.; Schiavolin, R.; Prendin, A. Fall risk assessment scales: A systematic literature review. Nurs. Rep. 2021, 11, 430–443. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Yu, L.; Yeung EH, K.; Luo, J.; Tsui, K.L.; Zhao, Y. A systematic review of wearable sensor-based technologies for fall risk assessment in older adults. Sensors 2022, 22, 6752. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.N.; Ribeiro, N.F.; Santos, C.P. Fall risk assessment using wearable sensors: A narrative review. Sensors 2022, 22, 984. [Google Scholar] [CrossRef]
- Beauchet, O.; Fantino, B.; Allali, G.; Muir, S.; Odasso, M.M.; Annweiler, C. Timed up and go test and risk of falls in older adults: A systematic review. J. Nutr. Health Aging 2011, 15, 933–938. [Google Scholar] [CrossRef]
- Cummings, S.R.; Nevitt, M.C.; Kidd, S. Forgetting falls: The limited accuracy of recall of falls in the elderly. J. Am. Geriatr. Soc. 1988, 36, 613–616. [Google Scholar] [CrossRef]
- Ganz, D.A.; Higashi, T.; Rubenstein, L.Z. Monitoring falls in cohort studies of community-dwelling older people: Effect of the recall interval. J. Am. Geriatr. Soc. 2005, 53, 2190–2194. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.M.; Fritz, S.; Middleton, A.; Allison, L.; Wingood, M.; Phillips, E.; Criss, M.; Verma, S.; Osborne, J.; Chui, K.K. Determining risk of falls in community dwelling older adults: A systematic review and meta-analysis using posttest probability. J. Geriatr. Phys. Ther. 2017, 40, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, T.S.; Meira, D.M.; Rico, N.C.; Mizuta, S.K. Accuracy of timed up and go test for screening risk of falls among community-dwelling elderly. Braz. J. Phys. Ther. 2012, 16, 381–388. [Google Scholar] [CrossRef]
- Hnizdo, S.; Archuleta, R.A.; Taylor, B.; Kim, S.C. Validity and reliability of the modified john hopkins fall risk assessment tool for elderly patients in home health care. Geriatr. Nurs. 2013, 34, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Masud, T.; Kendrick, D.; Morris, R.; Gawler, S.; Treml, J.; Iliffe, S. Does the timed up and go test predict future falls among british community-dwelling older people? prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015, 15, 38. [Google Scholar] [CrossRef]
- Leclerc, S.; Be, C.; Cadieux, É.; Goulet, L.; Allaire, J.-F.; Meloche, J.; Leduc, N.; Kergoat, M.-J. A classification and regression tree for predicting recurrent falling among community-dwelling seniors using home-care services. Can. J. Public Health 2009, 100, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Moller, U.O.; Kristensson, J.; Midlo, P.; Ekdahl, C.; Jakobsson, U. Predictive validity and cut-off scores in four diagnostic tests for falls–a study in frail older people at home. Phys. Occup. Ther. Geriatr. 2012, 30, 189–201. [Google Scholar] [CrossRef]
- Muhaidat, J.; Kerr, A.; Evans, J.J.; Pilling, M.; Skelton, D.A. Validity of simple gait-related dual-task tests in predicting falls in community-dwelling older adults. Arch. Phys. Med. Rehabil. 2014, 95, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Samah, Z.A.; Singh, D.K.A.; Murukesu, R.R.; Shahar, S.; Nordin, N.; Omar, M.A.; Chin, A. Discriminative and predictive ability of physical performance measures in identifying fall risk among older adults. Sains Malays. 2018, 47, 2769–2776. [Google Scholar] [CrossRef]
- Trueblood, P.R.; Hodson-Chennault, N.; McCubbin, A.; Youngclarke, D. Performance and impairment-based assessments among community dwelling elderly: Sensitivity and specificity. J. Geriatr. Phys. Ther. 2001, 24, 2–6. [Google Scholar] [CrossRef]
- Wrisley, D.M.; Kumar, N.A. Functional gait assessment: Concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys. Ther. 2010, 90, 761–773. [Google Scholar] [CrossRef]
- Tromp, A.; Pluijm, S.; Smit, J.; Deeg, D.; Bouter, L.; Lips, P. Fall-risk screening test: A prospective study on predictors for falls in community-dwelling elderly. J. Clin. Epidemiol. 2001, 54, 837–844. [Google Scholar] [CrossRef]
- Bongue, B.; Dupre, C.; Beauchet, O.; Rossat, A.; Fantino, B.; Colvez, A. A screening tool with five risk factors was developed for fall-risk prediction in community-dwelling elderly. J. Clin. Epidemiol. 2011, 64, 1152–1160. [Google Scholar] [CrossRef]
- Delbaere, K.; Close, J.C.; Brodaty, H.; Sachdev, P.; Lord, S.R. Determinants of disparities between perceived and physiological risk of falling among elderly people: Cohort study. BMJ 2010, 341, c4165. [Google Scholar] [CrossRef]
- Gerdhem, P.; Ringsberg, K.A.; Åkesson, K.; Obrant, K.J. Clinical history and biologic age predicted falls better than objective functional tests. J. Clin. Epidemiol. 2005, 58, 226–232. [Google Scholar] [CrossRef]
- Kwan, M.M.-S.; Lin, S.-I.; Close, J.C.; Lord, S.R. Depressive symptoms in addition to visual impairment, reduced strength and poor balance predict falls in older taiwanese people. Age Ageing 2012, 41, 606–612. [Google Scholar] [CrossRef]
- Laessoe, U.; Hoeck, H.C.; Simonsen, O.; Sinkjaer, T.; Voigt, M. Fall risk in an active elderly population–can it be assessed? J. Negat. Results Biomed. 2007, 6, 2. [Google Scholar] [CrossRef]
- Lindemann, U.; Lundin-Olsson, L.; Hauer, K.; Wengert, M.; Becker, C.; Pfeiffer, K. Maximum step length as a potential screening tool for falls in non-disabled older adults living in the community. Aging Clin. Exp. Res. 2008, 20, 394–399. [Google Scholar] [CrossRef]
- Muir, S.W.; Berg, K.; Chesworth, B.; Speechley, M. Use of the berg balance scale for predicting multiple falls in community-dwelling elderly people: A prospective study. Phys. Ther. 2008, 88, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Berg, K.; Chesworth, B.; Klar, N.; Speechley, M. Application of a fall screening algorithm stratified fall risk but missed preventive opportunities in community-dwelling older adults: A prospective study. J. Geriatr. Phys. Ther. 2010, 33, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Râıche, M.; He, R.; Prince, F.; Corriveau, H. Screening older adults at risk of falling with the tinetti balance scale. Lancet 2000, 356, 1001–1002. [Google Scholar] [CrossRef]
- Russell, M.A.; Hill, K.D.; Blackberry, I.; Day, L.M.; Dharmage, S.C. The reliability and predictive accuracy of the falls risk for older people in the community assessment (frop-com) tool. Age Ageing 2008, 37, 634–639. [Google Scholar] [CrossRef]
- Russell, M.A.; Hill, K.D.; Day, L.M.; Blackberry, I.; Gurrin, L.C.; Dharmage, S.C. Development of the falls risk for older people in the community (frop-com) screening tool. Age Ageing 2009, 38, 40–46. [Google Scholar] [CrossRef]
- Verghese, J.; Buschke, H.; Viola, L.; Katz, M.; Hall, C.; Kuslansky, G.; Lipton, R. Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. J. Am. Geriatr. Soc. 2002, 50, 1572–1576. [Google Scholar] [CrossRef]
- Zur, O.; Shaki, T.; Carmeli, E. Concurrent validity and reliability of a new balance scale used in older adults. In Respiratory Medicine and Science; Springer: Berlin/Heidelberg, Germany, 2015; pp. 63–70. [Google Scholar]
- Coll-Planas, L.; Kron, M.; Sander, S.; Rißmann, U.; Becker, C.; Nikolaus, T. Accidental falls among community-dwelling older adults. Z. Gerontol. Und Geriatr. 2006, 39, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, A.; Lord, S.R.; Sherrington, C. The development and validation of a brief performance-based fall risk assessment tool for use in primary care. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 896–903. [Google Scholar] [CrossRef]
- Buatois, S.; Miljkovic, D.; Manckoundia, P.; Gueguen, R.; Miget, P.; Vanc, G.; Perrin, P.; Benetos, A. Five times sit to stand test is a predictor of recurrent falls in healthy communityliving subjects aged 65 and older. J. Am. Geriatr. Soc. 2008, 56, 1575–1577. [Google Scholar] [CrossRef]
- Buatois, S.; Perret-Guillaume, C.; Gueguen, R.; Miget, P.; Vanc, G.; Perrin, P.; Benetos, A. A simple clinical scale to stratify risk of recurrent falls in community-dwelling adults aged 65 years and older. Phys. Ther. 2010, 90, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Stalenhoef, P.; Diederiks, J.; Knottnerus, J.; Kester, A.; Crebolder, H. A risk model for the prediction of recurrent falls in community-dwelling elderly: A prospective cohort study. J. Clin. Epidemiol. 2002, 55, 1088–1094. [Google Scholar] [CrossRef]
- Bergland, A.; Laake, K. Concurrent and predictive validity of “getting up from lying on the floor”. Aging Clin. Exp. Res. 2005, 17, 181–185. [Google Scholar] [CrossRef]
- Tiedemann, A.; Shimada, H.; Sherrington, C.; Murray, S.; Lord, S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing 2008, 37, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Hirata, S.; Ono, R.; Tsutsumimoto, K.; Misu, S.; Ando, H. The harmonic ratio of trunk acceleration predicts falling among older people: Results of a 1-year prospective study. J. Neuroeng. Rehabil. 2013, 10, 7. [Google Scholar] [CrossRef]
- Drover, D.; Howcroft, J.; Kofman, J.; Lemaire, E.D. Faller classification in older adults using wearable sensors based on turn and straight-walking accelerometer-based features. Sensors 2017, 17, 1321. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Prospective fallrisk prediction models for older adults based on wearable sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Lemaire, E.D.; Kofman, J. Prospective elderly fall prediction by older-adult fall-risk modeling with feature selection. Biomed. Signal Process. Control. 2018, 43, 320–328. [Google Scholar] [CrossRef]
- Lockhart, T.E.; Soangra, R.; Yoon, H.; Wu, T.; Frames, C.W.; Weaver, R.; Roberto, K. Prediction of fall risk among community-dwelling older adults using a wearable system. Sci. Rep. 2021, 11, 20976. [Google Scholar] [CrossRef]
- Atrsaei, A.; Paraschiv-Ionescu, A.; Krief, H.; Henchoz, Y.; Santos-Eggimann, B.; Büla, C.; Aminian, K. Instrumented 5-Time Sit-To-Stand Test: Parameters Predicting Serious Falls beyond the Duration of the Test. Gerontology 2022, 68, 587–600. [Google Scholar] [CrossRef]
- Bet, P.; Castro, P.C.; Ponti, M.A. Foreseeing future falls with accelerometer features in active community-dwelling older persons with no recent history of falls. Exp. Gerontol. 2021, 143, 111139. [Google Scholar] [CrossRef]
- Bayot, M.; Dujardin, K.; Dissaux, L.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Allali, G.; Delval, A. Can dual-task paradigms predict falls better than single task?–a systematic literature review. Neurophysiol. Clin. 2020, 50, 401–440. [Google Scholar] [CrossRef]

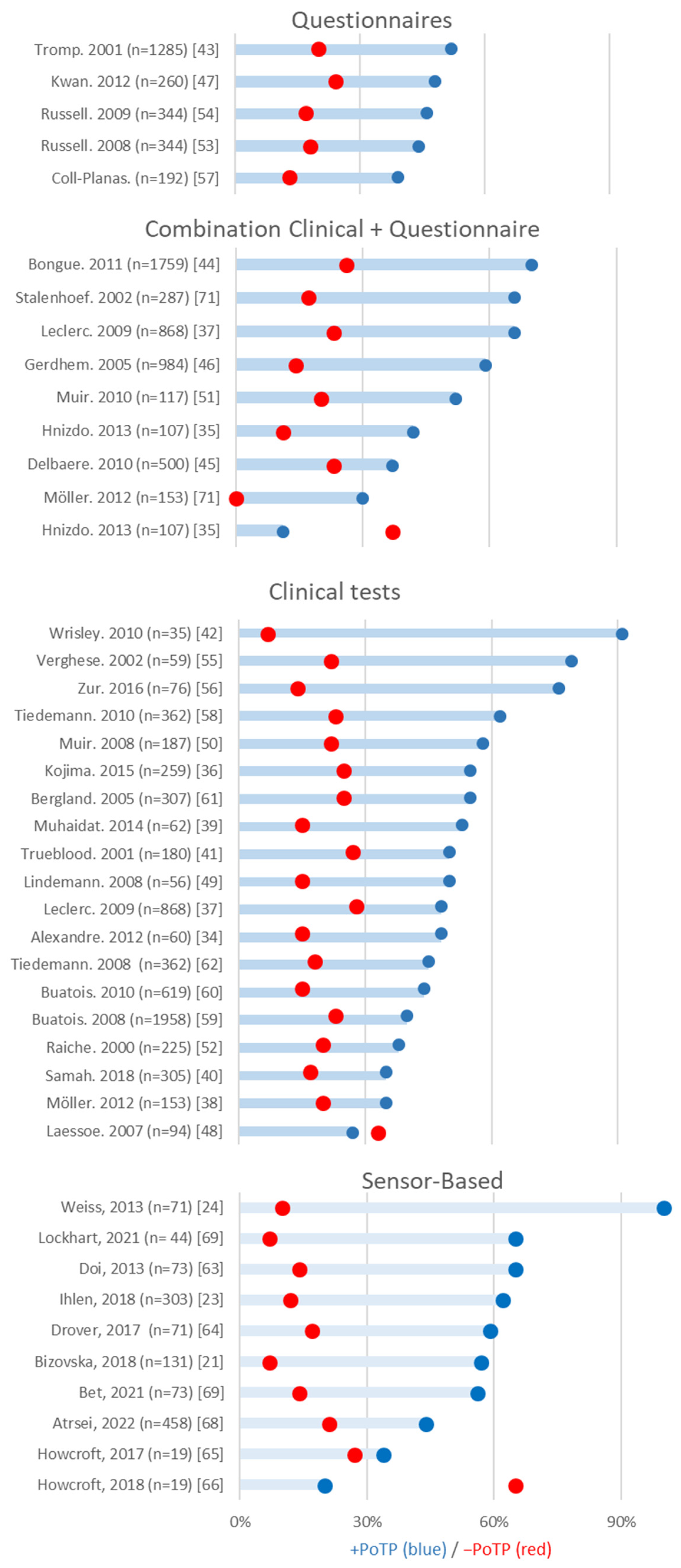
| Author | Total (n) | Female (%) | Mean Age (SD) | Fallers | Fall Criteria | Follow-Up Time (Months) | Type of Sensor | Sensor Position | Assessment | Analyzed | +PoTP | −PoTP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrsaei, 2021 [69] | 458 | 57 | 74.9 (1.4) | 108 | >=2 or >=1 injury due to fall | 12, fall calendar report monthly | 1 3D accelerometer 1 3D gyroscope | Sternum | 5xSTS | 44% | 21% | ||
| Bet, 2022 [70] | 73 | 56 | 70.2 (6.7) | 15 | >=1 | 12, fall journal contacted every 3 months | 1 3D accelerometer | Waist, L3 | TUG, TUG-DT | 56% | 14% | ||
| Bizovska, 2018 [21] | 131 | NR | NF: 70.5 (6.4) MF: 71.2 (5.3) | SF: 35 MF: 15 | >=2 | 12, every 14 days called to report | 3 3D accelerometers | Trunk (near L5) Left and right shank (15 cm above malleolus) | 25 m walking | Trunk stLE ML | 60% | 19% | |
| Tinetti balance score, trunk stLE ML | 47% | 20% | |||||||||||
| Tinetti total score, trunk stLE ML | 57% | 7% | |||||||||||
| Tinetti balance score, Tinetti total score, trunk stLE ML | 55% | 11% | |||||||||||
| Doi, 2013 [64] | 73 | 78.1 | 80.3 | SF: 16 | >=1 | 12, self-reporting weekly collection | 2 3D accelerometers | Upper trunk (C7) Lower trunk (L3) | 15 m walking | 65% | 14% | ||
| Drover, 2017 [65] | 71 | NR | 74.15 (7.0) | SF: 28 | >=1 | 6, fall occurrence survey after 6 months | 3 accelerometers | Lower back Left and right lateral shank Acc: posterior head | 6MWT | 57% | 18% | ||
| Howcroft, 2017 [66] | 19 | 58.7 | 75.2 (6.6) | SF: 7 | >=1 | 6, fall calendar report monthly | 1 accelerometer, 1 pressure sensor | Lower back Lateral shank just above the ankle Pressure insole: plantar H-RS H-P-LS Acc: posterior head | 7.62 m walking (ST, DT and 6MWT (ST) | single-task walking dual-task walking | 34% | 27% | |
| single-task walking | 33% | 28% | |||||||||||
| dual-task walking | 36% | 27% | |||||||||||
| Howcroft, 2018 [67] | 19 | 58.7 | 75.2 (6.6) | SF: 7 | >=1 | 6, fall calendar report monthly | 1 accelerometer, 1 pressure sensor | Lower back Lateral shank just above the ankle Pressure insole: plantar | ST Walking | 20% | 65% | ||
| Ihlen, 2018 [23] | 303 | SF: 51 ME: 48.8 | SF:76 (6.8) MF: 75.9 (6.7) | SF: 58 MF: 46 | >=1 >=2 | 12, monthly phone calls | 1 3D accelerometer | Lower back | 1-week ADL | PGME | 60% | 13% | |
| Conventional gait and demographic variables | 52% | 18% | |||||||||||
| Fall history | 38% | 25% | |||||||||||
| All combined | 62% | 12% | |||||||||||
| PGME | 74% | 14% | |||||||||||
| Conventional gait and demographic variables | 59% | 11% | |||||||||||
| Fall history | 40% | 23% | |||||||||||
| Lockhart 2021 [68] | 44 | NR | 73.0 (8.0) | SF: 9 | >=1 | 6, self-report | 1 3D accelerometer | Sternum | 10 m walk | 65% | 7% | ||
| Weiss, 2013 [24] | 71 | 65 | 78.36 (4.71) | MF: 12 | >=2 | 6 | 1 3D accelerometer 1 3D gyroscope | Lower back | 3-day ADL | All combined | 64% | 8% | |
| Dynamic gait index (without sensors) | 87% | 23% | |||||||||||
| DGI (without sensors) + 3-day acceleration-derived | 100% | 10% | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waterval, N.F.J.; Claassen, C.M.; van der Helm, F.C.T.; van der Kruk, E. Predictability of Fall Risk Assessments in Community-Dwelling Older Adults: A Scoping Review. Sensors 2023, 23, 7686. https://doi.org/10.3390/s23187686
Waterval NFJ, Claassen CM, van der Helm FCT, van der Kruk E. Predictability of Fall Risk Assessments in Community-Dwelling Older Adults: A Scoping Review. Sensors. 2023; 23(18):7686. https://doi.org/10.3390/s23187686
Chicago/Turabian StyleWaterval, N. F. J., C. M. Claassen, F. C. T. van der Helm, and E. van der Kruk. 2023. "Predictability of Fall Risk Assessments in Community-Dwelling Older Adults: A Scoping Review" Sensors 23, no. 18: 7686. https://doi.org/10.3390/s23187686
APA StyleWaterval, N. F. J., Claassen, C. M., van der Helm, F. C. T., & van der Kruk, E. (2023). Predictability of Fall Risk Assessments in Community-Dwelling Older Adults: A Scoping Review. Sensors, 23(18), 7686. https://doi.org/10.3390/s23187686








