The Use of Sensors to Prevent, Predict Transition to Chronic and Personalize Treatment of Low Back Pain: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Data Extraction Process
2.5. Data Items
2.6. Assessment of Methodological Quality and Risk of Bias
3. Results
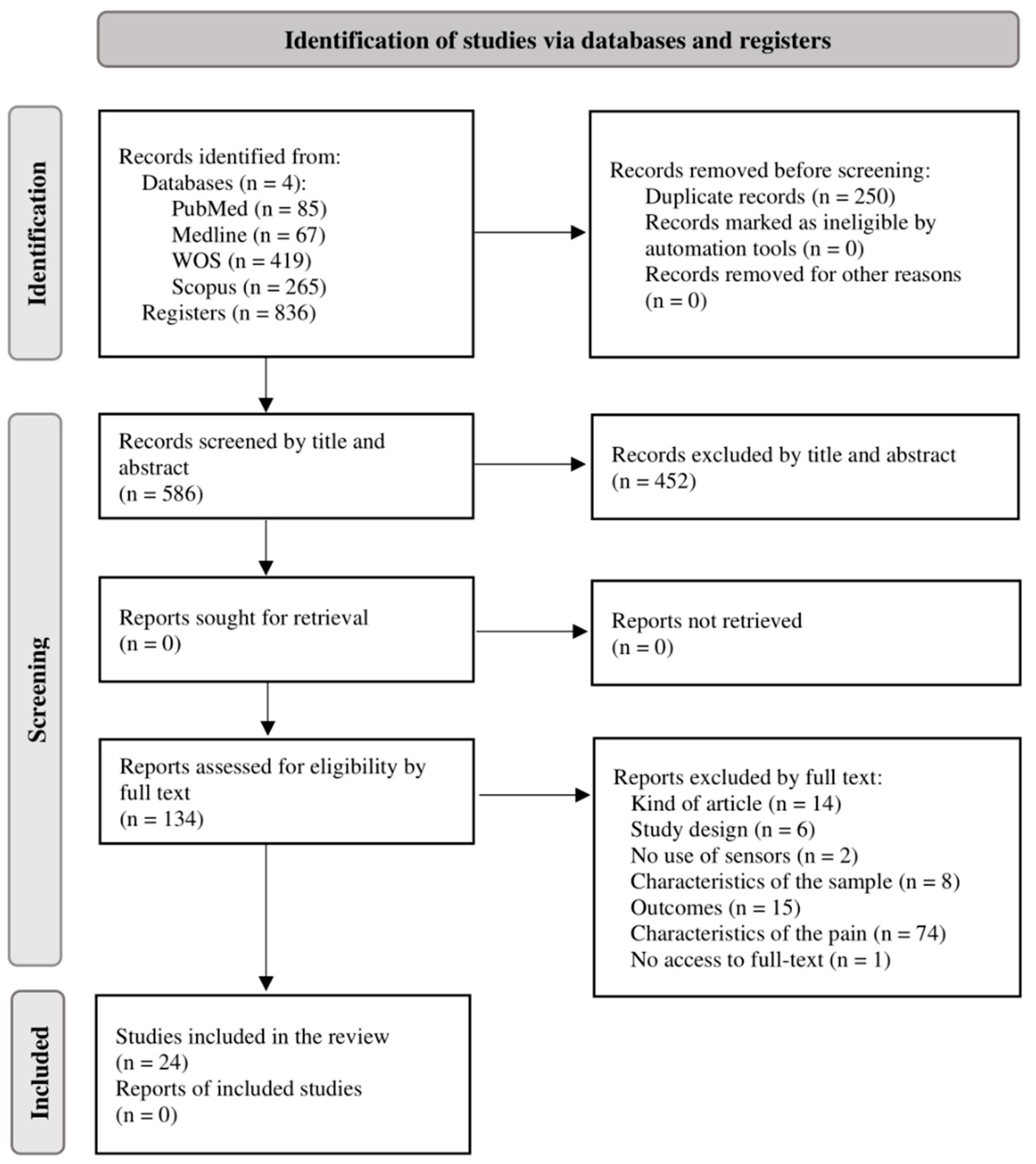
3.1. Study Selection
3.2. Study Characteristics
3.3. Synthesis of Results
3.3.1. Studies Comparing Population with Chronic NSLBP and Healthy Population
3.3.2. Studies Comparing Outcomes between Groups of NSLBP
3.3.3. Studies Comparing Outcomes before and after Treatment
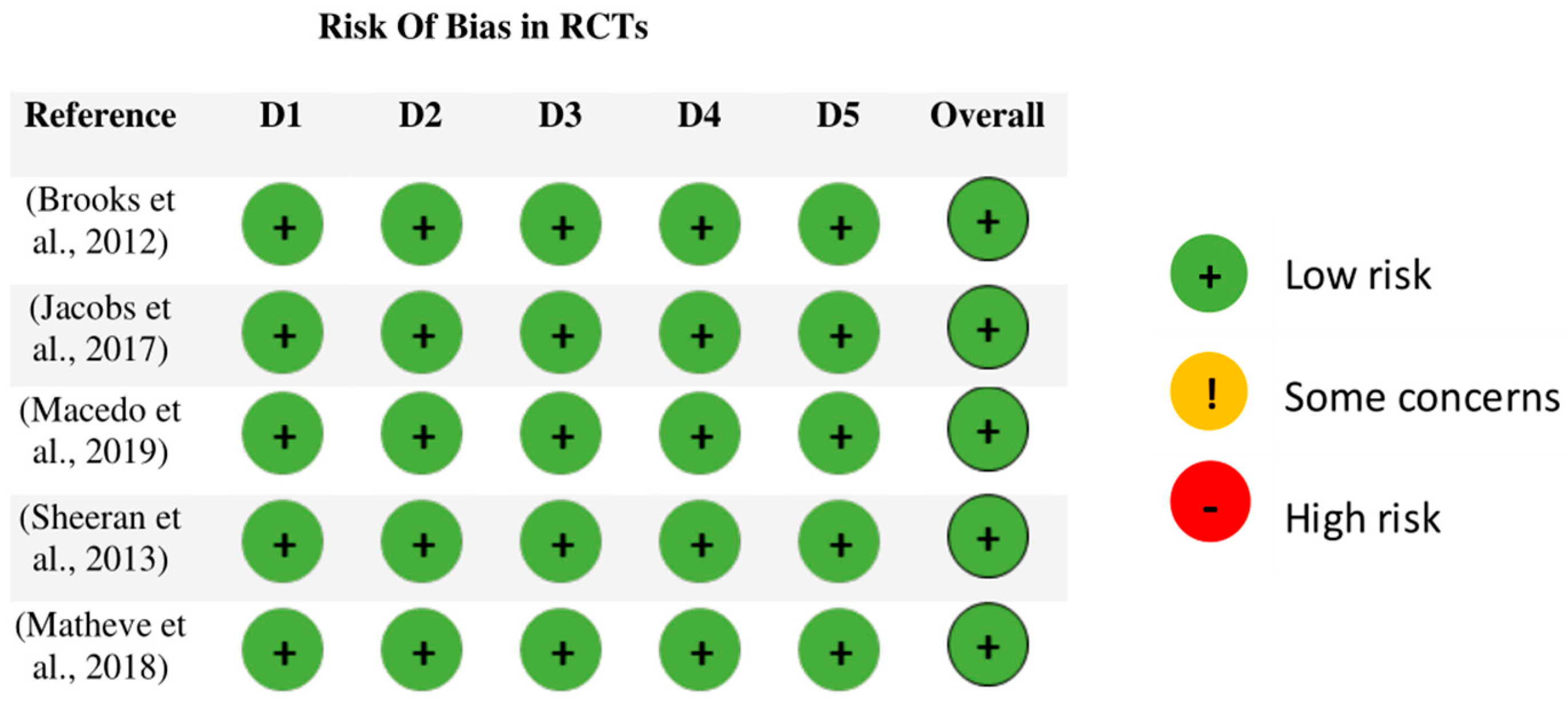
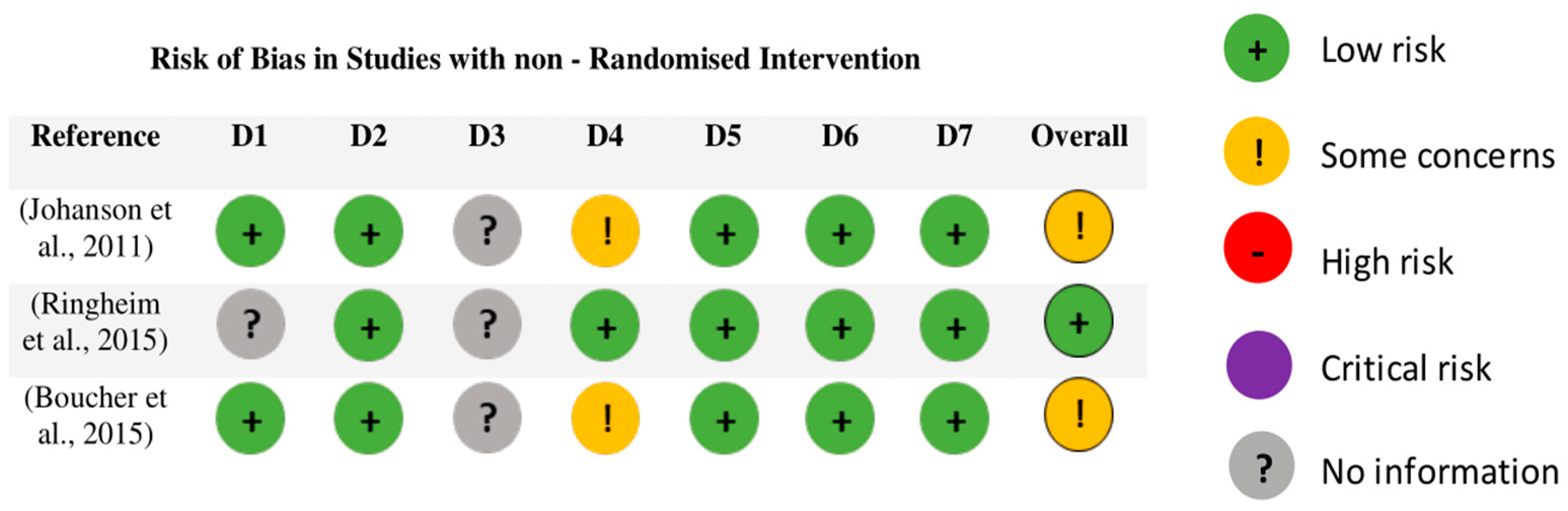
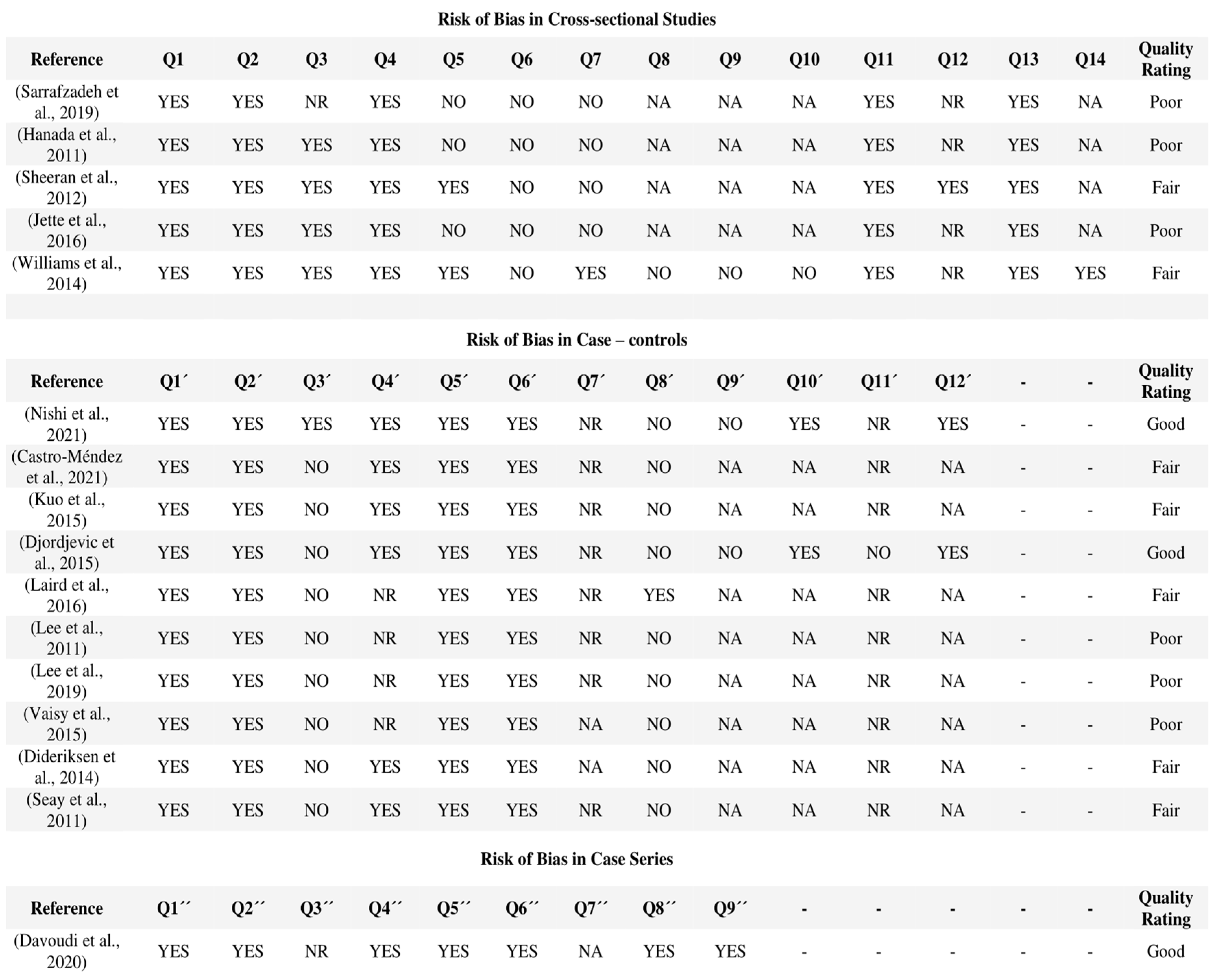
3.4. Assessment of Methodological Quality and Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leach, M.J.; Climstein, M.; Fryer, G.; Ziaian, T.; Lauche, R.; Kumar, S.; Agnew, T. Developing a needs-based integrative service delivery model to deliver best practice care for chronic nonspecific low back pain. Eur. J. Integr. Med. 2022, 53, 102153. [Google Scholar] [CrossRef]
- Meucci, R.D.; Fassa, A.G.; Xavier Faria, N.M. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 1. [Google Scholar] [CrossRef]
- AlMazrou, S.H.; Elliott, R.A.; Knaggs, R.D.; AlAujan, S.S. Cost-effectiveness of pain management services for chronic low back pain: A systematic review of published studies. BMC Health Serv. Res. 2020, 20, 194. [Google Scholar] [CrossRef]
- Bardin, L.D.; King, P.; Maher, C.G. Diagnostic triage for low back pain: A practical approach for primary care. Med. J. Aust. 2017, 206, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Goldstein, P.; Ashar, Y.; Tesarz, J.; Kazgan, M.; Cetin, B.; Wager, T.D. Emerging Clinical Technology: Application of Machine Learning to Chronic Pain Assessments Based on Emotional Body Maps. Neurotherapeutics 2020, 17, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.D.; Angelova, M.; Zhao, X.; Owen, P.J.; Miller, C.T.; Wilkin, T.; Belavy, D.L. Artificial intelligence to improve back pain outcomes and lessons learnt from clinical classification approaches: Three systematic reviews. NPJ Digit. Med. 2020, 3, 93. [Google Scholar] [CrossRef]
- Caviedes, J.E.; Li, B.; Jammula, V.C. Wearable Sensor Array Design for Spine Posture Monitoring during Exercise Incorporating Biofeedback. IEEE Trans. Biomed. Eng. 2020, 67, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Papi, E.; Koh, W.S.; McGregor, A.H. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef]
- De Carvalho, D.E.; de Luca, K.; Funabashi, M.; Breen, A.; Wong, A.Y.; Johansson, M.S.; Ferreira, M.L.; Swab, M.; Kawchuk, G.N.; Adams, J.; et al. Association of Exposures to Seated Postures with Immediate Increases in Back Pain: A Systematic Review of Studies with Objectively Measured Sitting Time. J. Manip. Physiol. Ther. 2020, 43, 1–12. [Google Scholar] [CrossRef]
- Laird, R.A.; Gilbert, J.; Kent, P.; Keating, J.L. Comparing lumbo-pelvic movement in people with and without back pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2014, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Ranavolo, A.; Ajoudani, A.; Cherubini, A.; Bianchi, M.; Fritzsche, L.; Iavicoli, S.; Sartori, M.; Silvetti, A.; Vanderborght, B.; Varrecchia, T.; et al. The sensor-based biomechanical risk assessment at the base of the need for revising of standards for human ergonomics. Sensors 2020, 20, 5750. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH [Internet]. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 April 2022).
- Nishi, Y.; Shigetoh, H.; Fujii, R.; Osumi, M.; Morioka, S. Changes in trunk variability and stability of gait in patients with chronic low back pain: Impact of laboratory versus daily-living environments. J. Pain Res. 2021, 14, 1675–1686. [Google Scholar] [CrossRef]
- Castro-Méndez, A.; Requelo-Rodríguez, I.; Pabón-Carrasco, M.; González-Elena, M.L.; Ponce-Blandón, J.A.; Palomo-Toucedo, I.C. A case–control study of the effects of chronic low back pain in spatiotemporal gait parameters. Sensors 2021, 21, 5247. [Google Scholar] [CrossRef]
- Sheeran, L.; Sparkes, V.; Caterson, B.; Busse-Morris, M.; Van Deursen, R. Spinal position sense and trunk muscle activity during sitting and standing in nonspecific chronic low back pain: Classification analysis. Spine 2012, 37, E486–E495. [Google Scholar] [CrossRef]
- Laird, R.A.; Kent, P.; Keating, J.L. How consistent are lordosis, range of movement and lumbo-pelvic rhythm in people with and without back pain? BMC Musculoskelet. Disord. 2016, 17, 403. [Google Scholar] [CrossRef]
- Lee, J.K.; Desmoulin, G.T.; Khan, A.H.; Park, E.J. Comparison of 3D spinal motions during stair-climbing between individuals with and without low back pain. Gait Posture 2011, 34, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, C.; Kim, K.; Cho, M.; Kim, H.C.; Chung, S.G. Lumbar Stability in Healthy Individuals and Low Back Pain Patients Quantified by Wall Plank-and-Roll Test. PM R 2019, 11, 483–494. [Google Scholar] [CrossRef]
- Matheve, T.; Brumagne, S.; Demoulin, C.; Timmermans, A. Sensor-based postural feedback is more effective than conventional feedback to improve lumbopelvic movement control in patients with chronic low back pain: A randomised controlled trial. J. Neuroeng. Rehabil. 2018, 15, 85. [Google Scholar] [CrossRef]
- Vaisy, M.; Gizzi, L.; Petzke, F.; Consmüller, T.; Pfingsten, M.; Falla, D. Measurement of Lumbar Spine Functional Movement in Low Back Pain. Clin. J. Pain 2015, 31, 876–885. [Google Scholar] [CrossRef]
- Dideriksen, J.L.; Gizzi, L.; Petzke, F.; Falla, D. Deterministic accessory spinal movement in functional tasks characterizes individuals with low back pain. Clin. Neurophysiol. 2014, 125, 1663–1668. [Google Scholar] [CrossRef]
- Seay, J.F.; Van Emmerik, R.E.A.; Hamill, J. Influence of low back pain status on pelvis-trunk coordination during walking and running. Spine 2011, 36, E1070–E1079. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.L.; Huang, K.Y.; Chiang, P.T.; Lee, P.Y.; Tsai, Y.J. Steadiness of spinal regions during single-leg standing in older adults with and without chronic low back pain. PLoS ONE 2015, 10, e0128318. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadeh, J.; Ebrahimi, I.; Ahmadi, A.; Negahban, H. Comparison of Postural Balance between Subgroups of Nonspecific Low-back Pain Patients Based on O’Sullivan Classification System and Normal Subjects during Lifting. Arch. Bone Jt. Surg. 2019, 7, 52–60. [Google Scholar]
- Hanada, E.Y.; Johnson, M.; Hubley-Kozey, C. A comparison of trunk muscle activation amplitudes during gait in older adults with and without chronic low back pain. PM R 2011, 3, 920–928. [Google Scholar] [CrossRef]
- Johanson, E.; Brumagne, S.; Janssens, L.; Pijnenburg, M.; Claeys, K.; Pääsuke, M. The effect of acute back muscle fatigue on postural control strategy in people with and without recurrent low back pain. Eur. Spine J. 2011, 20, 2152–2159. [Google Scholar] [CrossRef]
- Ringheim, I.; Austein, H.; Indahl, A.; Roeleveld, K. Postural strategy and trunk muscle activation during prolonged standing in chronic low back pain patients. Gait Posture 2015, 42, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.A.; Abboud, J.; Nougarou, F.; Normand, M.C.; Descarreaux, M. The effects of vibration and muscle fatigue on trunk sensorimotor control in low back pain patients. PLoS ONE 2015, 10, e0135838. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Lomond, K.V.; Hitt, J.R.; DeSarno, M.J.; Bunn, J.Y.; Henry, S.M. Effects of low back pain and of stabilization or movement- system-impairment treatments on induced postural responses: A planned secondary analysis of a randomized controlled trial. Man. Ther. 2016, 21, 210–219. [Google Scholar] [CrossRef]
- Djordjevic, O.; Konstantinovic, L.; Miljkovic, N.; Bijelic, G. Relationship between Electromyographic Signal Amplitude and Thickness Change of the Trunk Muscles in Patients with and Without Low Back Pain. Clin. J. Pain 2015, 31, 893–902. [Google Scholar] [CrossRef]
- Brooks, C.; Kennedy, S.; Marshall, P.W.M. Specific trunk and general exercise elicit similar changes in anticipatory postural adjustments in patients with chronic low back pain: A randomized controlled trial. Spine 2012, 37, 1543–1550. [Google Scholar] [CrossRef]
- Macedo, L.; Richards, J.; Borges, D.T.; Melo, S.A.; Brasileiro, J.S. Kinesio Taping reduces pain and improves disability in low back pain patients: A randomised controlled trial. Physiotherapy 2019, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, L.; Van Deursen, R.; Caterson, B.; Sparkes, V. Classification-Guided Versus Generalized Postural Intervention in Subgroups of Nonspecific Chronic Low Back Pain: A pragmatic randomized controlled study. Spine 2013, 38, 1613–1625. [Google Scholar] [CrossRef]
- Davoudi, M.; Shokouhyan, S.M.; Abedi, M.; Meftahi, N.; Rahimi, A.; Rashedi, E.; Hoviattalab, M.; Narimani, R.; Parnianpour, M.; Khalaf, K. A practical sensor-based methodology for the quantitative assessment and classification of chronic non specific low back patients (NSLBP) in clinical settings. Sensors 2020, 20, 2902. [Google Scholar] [CrossRef]
- Williams, J.M.; Haq, I.; Lee, R.Y. An experimental study investigating the effect of pain relief from oral analgesia on lumbar range of motion, velocity, acceleration and movement irregularity. BMC Musculoskelet. Disord. 2014, 15, 304. [Google Scholar] [CrossRef] [PubMed]
- Jette, N.G.; Lim, Y.L.; Lim, H.L.; Mokhtar, S.A.; Gan, K.B.; Singh, D.K.A. Lumbar kinematics, functional disability and fear avoidance beliefs among adults with nonspecific chronic low back pain. Sultan Qaboos Univ. Med. J. 2016, 16, e430–e436. [Google Scholar] [CrossRef]
- Papi, E.; Bull, A.M.J.; McGregor, A.H. Is there evidence to use kinematic/kinetic measures clinically in low back pain patients? A systematic review. Clin. Biomech. 2018, 55, 53–64. [Google Scholar] [CrossRef]
- Koch, C.; Hänsel, F. Non-specific low back pain and postural control during quiet standing—A systematic review. Front. Psychol. 2019, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Stabbert, H.; Bagwell, J.J.; Teng, H.-L.; Wade, V.; Lee, S.-P. Do people with low back pain walk differently? A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 450–465. [Google Scholar] [CrossRef]
- Claeys, K.; Brumagne, S.; Dankaerts, W.; Kiers, H.; Janssens, L. Decreased variability in postural control strategies in young people with non-specific low back pain is associated with altered proprioceptive reweighting. Eur. J. Appl. Physiol. 2011, 111, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ghamkhar, L.; Kahlaee, A.H. Trunk Muscles Activation Pattern during Walking in Subjects with and without Chronic Low Back Pain: A Systematic Review. PM R 2015, 7, 519–526. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4: European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. S2), 192–300. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Lind, J.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; et al. The Oxford 2011 Levels of Evidence. Oxford Cent. Evid.-Based Med. 2011, 1, 53. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 15 May 2022).
| Reference | Study Design | Participants Characteristics | Assessments | Outcome Parameter | Sensor | Scales | Results |
|---|---|---|---|---|---|---|---|
| [18] | Case-control | LBP group n = 20 Age 54.05 (10.76) Female 9/20 CG n = 20 Age 56.75 (9.43) Female 8/20 | Assessment of gait | Accelerations of trunk in A-P (anterior-posterior) and M-L (medium-lateral) MSE (multiscale sample entropy) Stability of gait: LyE (maximum Lyapunov exponent) | Wearable tri-axial accelerometer | NPRS TSK RMDQ |
|
| [19] | Case-control, cross-sectional | LBP group n = 75 Female 40.27% CG n = 72 Female 59.72% | Assessment of spatiotemporal gait parameters | Spatiotemporal gait parameters | OptoGait optical sensor system | VAS ODI |
|
| [20] | Cross-sectional | FP (flexor pattern) n = 51 Age 33.0 (10.3) Female 56.9% AEP (active extensor pattern) n = 39 Age 37.0 (11.4) Female 76.9% CG n = 38 Age 36.0 (10.3) Female 62.9% | Assessment sitting and standing | Spinal repositioning sense: thoracic and lumbar curvatures (AE (absolute error), VE (variable error), CE (constant error)) EMG in LM, ILPT, EO, TrIO, subMVC (Maximal Voluntary contraction) | EMG 3-D kinematic motion analysis system | VAS RMDQ |
|
| [21] | Case-control | LBP group n = 30 Age 45.8 (11.6) Female 50% CG n = 32 Age 35.5 (12.4) Female 42% | Assessment of lumbar kinematics | ROM, lumbopelvic angles, lumbo-pelvic rhythm (% contribution of the lumbar region to the ROM of the trunk) | ViMove system (Inertial Measurement System) | NPRS RMDQ |
|
| [22] | Case-control | LBP group n = 10 Age 43.2 (12.5) Female 40% CG n = 10 Age 35.9 (16.6) Female 30% | Assessment stair-climbing test, single and double steps | Spatiotemporal stride parameters, ROM in relation to the stride cycle, movement patterns | Spinal motion measurement (3 inertial/magnetic MTx sensors) Stride cycle detection | ODI |
|
| [23] | Case-control | LBP group n = 17 Age 38.0 (12.0) Female 12/17 CG n = 57 Age 40.1 (16.9) Female 34/57 | Assessment during the WPR (Wall Plank-and-Roll) test | General motor patterns (relative movement of thorax and pelvis) Lumbar posture: 3 relative angles (axial twist, kyphosis-lordosis, and lateral bending) | 2 inertial sensors | None |
|
| [24] | RCT (Randomized Controlled Trial) | LBP group n = 44 (Control n = 15; Mirror n = 15; Sensor n = 14) Age 39.7 Female 18/44 CG n = 47 (Control n = 17; Mirror n = 15; Sensor n = 15) Age 36.7 Female 24/47 | Assessment at baseline, during and after intervention (lumbopelvic control task with feedback from sensors, mirror or no feedback) | Lumbopelvic kinematics measurements in the sagittal plane | Laptop 3 wireless inertial measurement sensors (Valedo motion research tool) | NPRS RMDQ TSK |
|
| [25] | Case-control | LBP group n = 20 Age 32.9 (9.6) Female 55% CG n = 19 Age 29.1 (7.1) Female 48% | Assessment of analytic movements in two phases: ascendant and descendent | Maximal ROM, time to maximal ROM, maximal and average angular velocity of spinal movement | Epionics SPINE (two strips with 12 angle sensors per strip) | SF-STAI ODI TSK PCS NPRS SF-36 |
|
| [26] | Case-control | LBP group n = 17 Age 32.5 (9.6) Female 59% CG n = 17 Female 53% | Assessment of spine kinematics during a repetitive lifting task | Angles in sagittal plane, variance and offset (average) of spinal angles % of determination of accessory movement | Epionics SPINE | TSK PCS ODI SF-STAI SF-36 NPRS |
|
| [27] | Case-control | LBP group n = 14 Age 35.71 (10.90) Female 6/14 Recovered from LBP n = 14 Age 32.56 (9.42) Female 5/14 CG n = 14 Age 29.90 (8.45) Female 8/14 | Assessment while walking at systematically increased speed | 3-D pelvis and trunk segment angles and coordination between pelvis and trunk (changes in angle-angle diagrams) | 8 high-speed cameras | ODI VAS |
|
| [28] | Case-control | LBP group n = 13 Age 60.5 (4.1) Female 9/13 CG n = 13 Age 59.7 (3.0) | Assessment during single leg-standing, TUG (Timed Up and Go) and 5TSTS (Five Times to Sit To Stand test) | Steadiness index of spinal regions RHT (relative holding time) and RST (relative standstill time) Time TUG and 5TSTS | 6-camera motion analysis system (Vicon) AMTI Force platform | ODI |
|
| [29] | Cross-sectional | FP group n = 20 Age 32.42 (8.36) AEP group n = 20 Age 33.05 (9.01) CG n = 20 Age 31.06 (8) | Assessment of lifting test (static and dynamic phase) | Postural balance: SD.apx (SD (standard deviation) of COP amplitude in the frontal plane); SD.Apy (SD of COP amplitude in the sagittal plane); SD.APvx (SD of COP velocity in frontal plane); SD.SPvy (SD of COP velocity in sagittal plane), MTV (mean total velocity) | 6-camera motion analysis system Force plate system | VAS |
|
| [30] | Cross-sectional | LBP group n = 9 Age 61.4 (9.8) Female 5/9 CG n = 9 Age 64.9 (8.8) Female 5/9 | Assessment during 4 walking trials, at rest and at the MVIC (Maximal Voluntary Isometric Contraction) | EMG in low RA, LES, IO, and LM MVIC Spatiotemporal gait parameters | GAITRite mat with pressure sensors EMG (surface electrodes) | ABCSQ RMDQ |
|
| [31] | Intervention no randomized | LBP n = 16 Age 22.0 (1.1) Female 11/16 CG n = 16 Age 22.7 (1.7) Female 11/16 | Assessment of an endurance test, with and without BMF (back muscle fatigue) | Displacement of COP, positions of COP in A-P, RMS (root mean square)-COP, ratio of COP displacement | Force plate EMG mean power frequency | ODI NPRS Borg Scale |
|
| [32] | Intervention no randomized | LBP group n = 17 Age 39.0 (5.4) Female 10/17 CG n = 20 Age 40.2 (5.4) Female 13/20 | Assessment of three standing tests: quiet standing, prolonged standing and quiet standing | EMG in ES, GM, RA, EO MVC in flexo-extension Force reaction in quiet standing (global COP, COP RMS, COP speed A-P and M-L, COP area) Number of shifts in body weight | Force sensor Force plates EMG (surface electrodes) | VAS ODI TSK Borg Scale NPRS |
|
| [33] | Intervention no randomized | LBP group n = 20 Age 33.7 (14.4) Female 7/20 CG n = 20 Age 29.1 (7.8) Female 7/20 | Assessment during isometric contractions and during the fatigue protocol | EMG in LES Force data: TPT (time to peak torque), CE, VE, AE | EMG (surface electrodes) | ODI TSK VAS |
|
| [34] | Planned secondary analysis of a prospectively registered RCT | LBP group n = 68 Age 41.1 (38.8–44.0) Female 44% CG n = 27 Age 32.5 (28.8–36.2) Female 67% | Assessment 1 week before/after treatment (stabilisation protocol and MSI-directed protocol) | EMG in EO, IO, RA Responses to lateral, forward, and backward perturbations | EMG (surface electrodes) Force platform (disturbances) | ODI NPRS |
|
| [35] | Case-control | LBP group n = 36 Age 53.22 (8.12) Female 18/36 CG n = 37 Age 52.55 (9.45) Female 22/37 | Assessment at rest and contraction | EMG in TrA, LM Muscle thickness | Wireless LUMBIA System Ultrasound | VAS ODI |
|
| [36] | RCT | SEG group n = 32 Age 36.2 (8.2) Female 20/32 GEG group n = 32 Female 20/32 | Assessment of APAs (Anticipatory postural Adjustments) in rapid shoulder flexion, before and after intervention | APAs EMG in RA, LES, TrA, IO | EMG (surface electrodes) | VAS ODI |
|
| [37] | Assessor blinded prospective RCT | Kinesiotaping + tension (KTT) n = 27 Age 25 (6) Kinesiotaping no tension (KTNT) n = 27 Age 24(5) Micropore (MP) n = 27 Age 25 (5) CG n = 27 Age 24 (4) | Assessment pre, 3 and 10 days after intervention (Kinesiotaping or Micropore tape) | Trunk ROM (range of movement) EMG in longissimus muscles MVIC | EMG (surface electrodes) iHandy level (iPhone app, ROM) | NPRS RMDQ |
|
| [38] | Pragmatic RCT single-blinded | CSPI group n = 25 Age 35.9 (10.13) Female 64% GPI group n = 24 Age 37.1 (11.1) Female 54.2% | Assessment sitting and standing Treatment: CSPI/GPI | EMG in LM, ILPT, EO, TrIO Spinal repositioning sense: thoracic and lumbar curvatures (AE, VE, CE) | 8-channel EMG (surface electrodes) 3-D kinematic Vicon motion analysis system | VAS RMDQ |
|
| [39] | Case series | LR (Low risk) n = 33 Age 46.1 (3.2) MR (Medium risk) n = 35 Age 44.8 (4.4) HR (High risk) n = 32 Age 44.3 (3.4) | Assessment of trunk flexo-extension in 5 planes of movement at maximum speed without pain | Maximal and average angular velocity, linear acceleration and maximal jerk (2nd derivate of the angular velocity) | Inertial sensor | VAS |
|
| [40] | Prospective, cross-sectional, experimental repeated-measures design | Acute LBP n = 20 Age 42.7 (6.8) Female 9/20 Chronic LBP n = 20 Age 36.6 (10.8) Female 9/20 | Assessment before and after intervention (oral analgesia) | Lumbar ROM, angular velocity, angular acceleration | Two wired 3DM-GX3-25 inertial sensors | VAS TSK |
|
| [41] | Cross-sectional | n = 32 Age 32.94 (7.83) Female 59.4% | Assessment of lumbar kinematics | Maximal ROM, maximal minimum angular velocity, acceleration | Inertial Measurement System | VAS ODI FABQ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, P.; Ríos-Asín, I.; Lapuente-Hernández, D.; Pérez, L.; Calvo, S.; Gil-Calvo, M. The Use of Sensors to Prevent, Predict Transition to Chronic and Personalize Treatment of Low Back Pain: A Systematic Review. Sensors 2023, 23, 7695. https://doi.org/10.3390/s23187695
Herrero P, Ríos-Asín I, Lapuente-Hernández D, Pérez L, Calvo S, Gil-Calvo M. The Use of Sensors to Prevent, Predict Transition to Chronic and Personalize Treatment of Low Back Pain: A Systematic Review. Sensors. 2023; 23(18):7695. https://doi.org/10.3390/s23187695
Chicago/Turabian StyleHerrero, Pablo, Izarbe Ríos-Asín, Diego Lapuente-Hernández, Luis Pérez, Sandra Calvo, and Marina Gil-Calvo. 2023. "The Use of Sensors to Prevent, Predict Transition to Chronic and Personalize Treatment of Low Back Pain: A Systematic Review" Sensors 23, no. 18: 7695. https://doi.org/10.3390/s23187695
APA StyleHerrero, P., Ríos-Asín, I., Lapuente-Hernández, D., Pérez, L., Calvo, S., & Gil-Calvo, M. (2023). The Use of Sensors to Prevent, Predict Transition to Chronic and Personalize Treatment of Low Back Pain: A Systematic Review. Sensors, 23(18), 7695. https://doi.org/10.3390/s23187695






