Temperature-Compensated Solution Concentration Measurements Using Photonic Crystal Fiber-Tip Sensors
Abstract
:1. Introduction
2. Materials and Methods
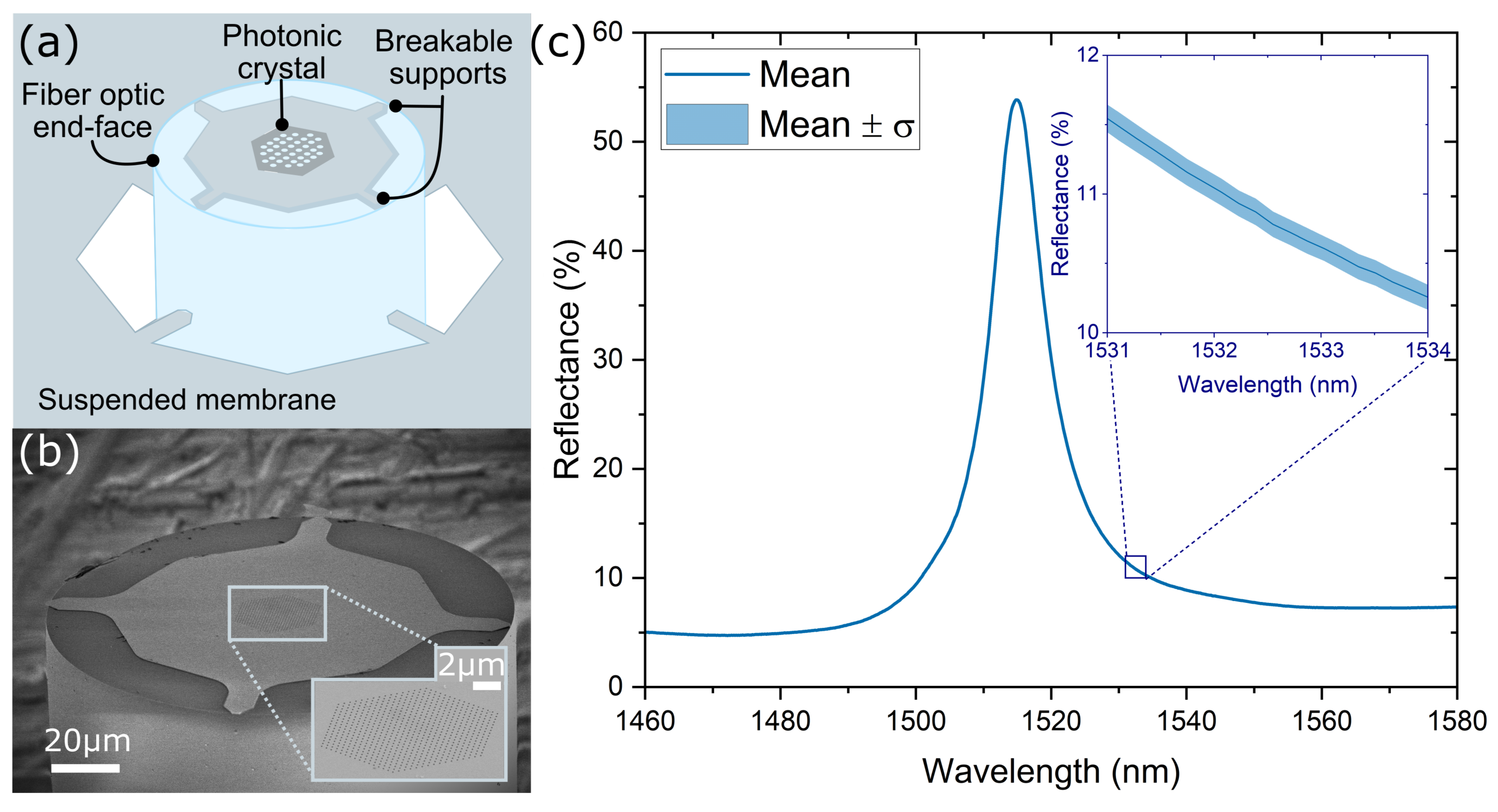
2.1. PhC Fiber-Tip Sensor Fabrication
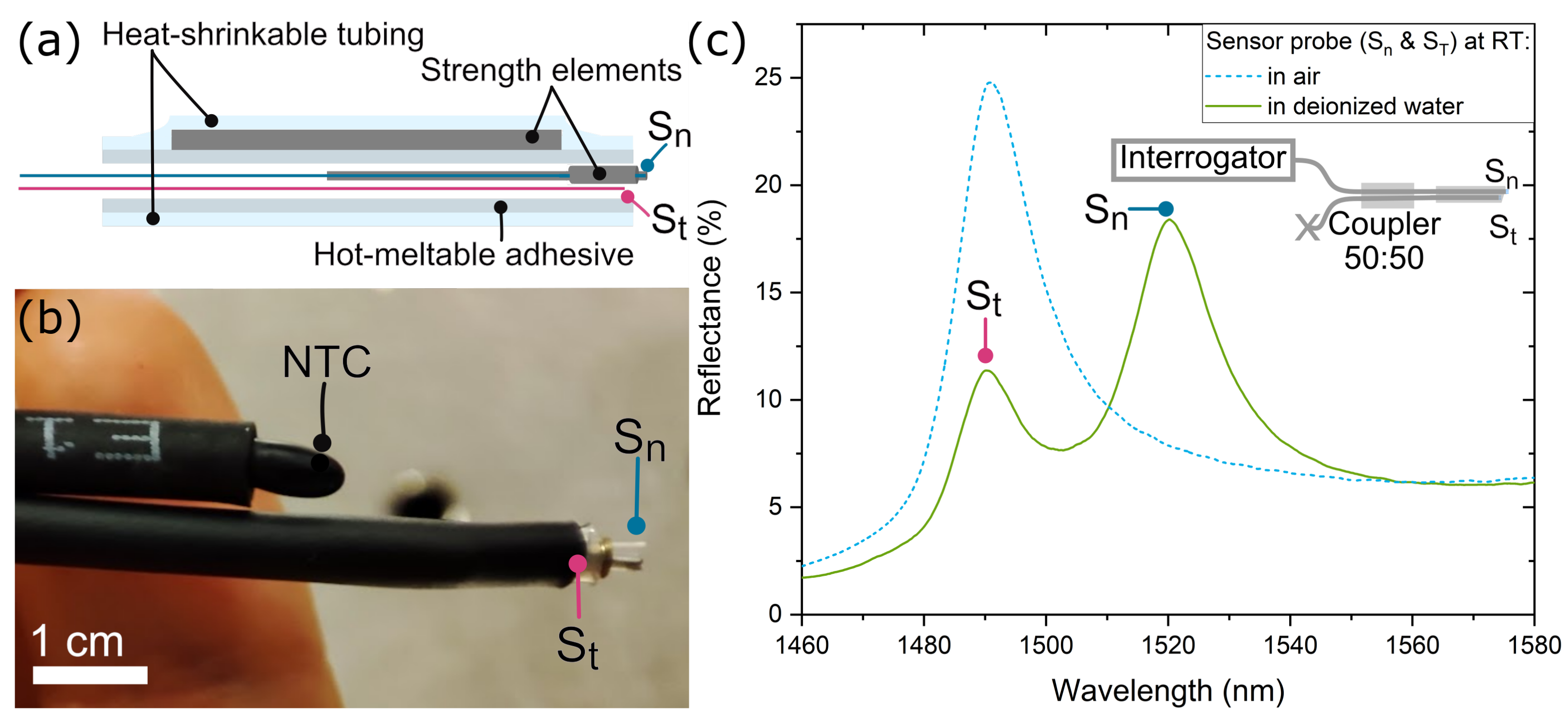
2.2. Sensor Probe Design and Spectral Response
3. Results and Discussion
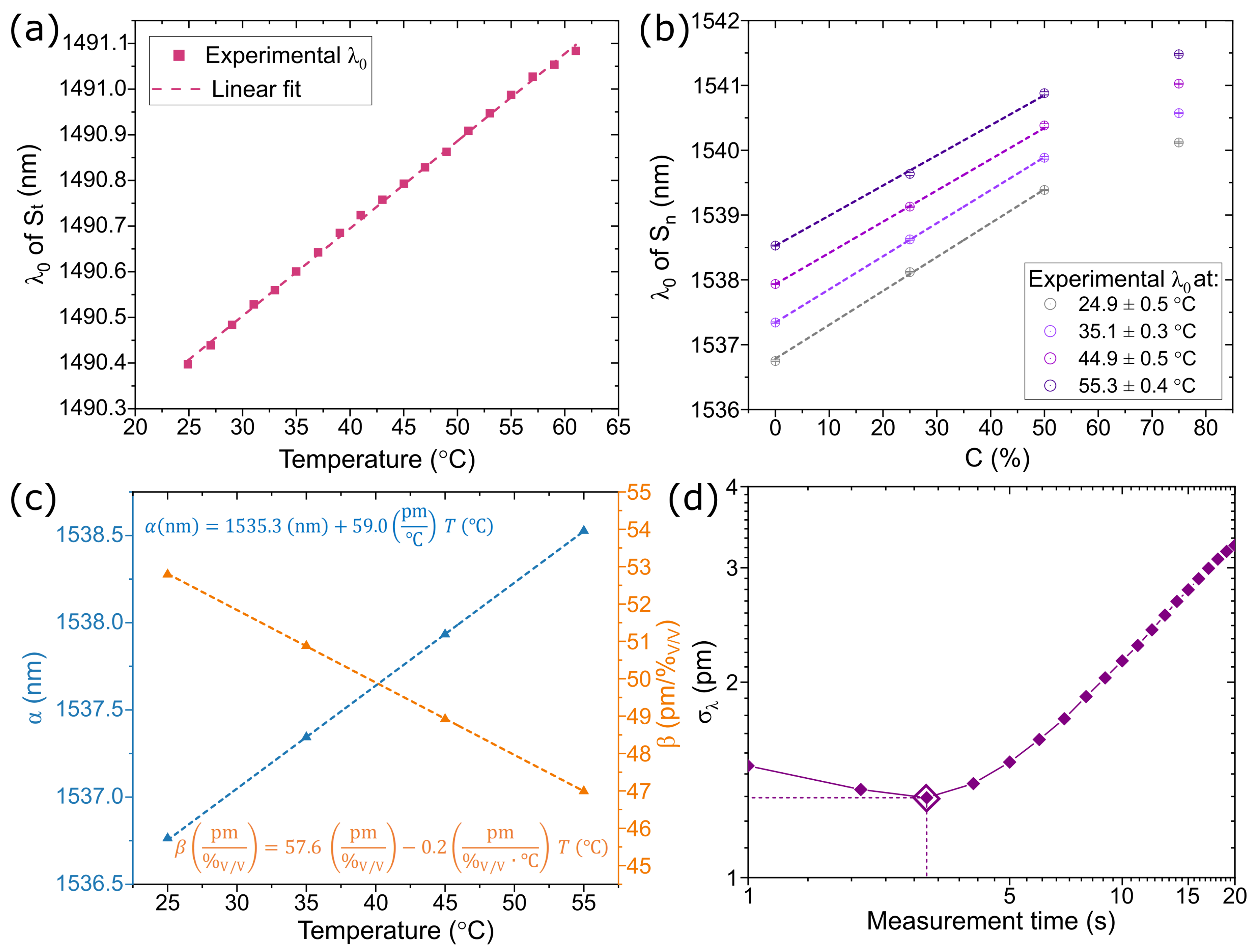
3.1. Sensor Probe Characterization
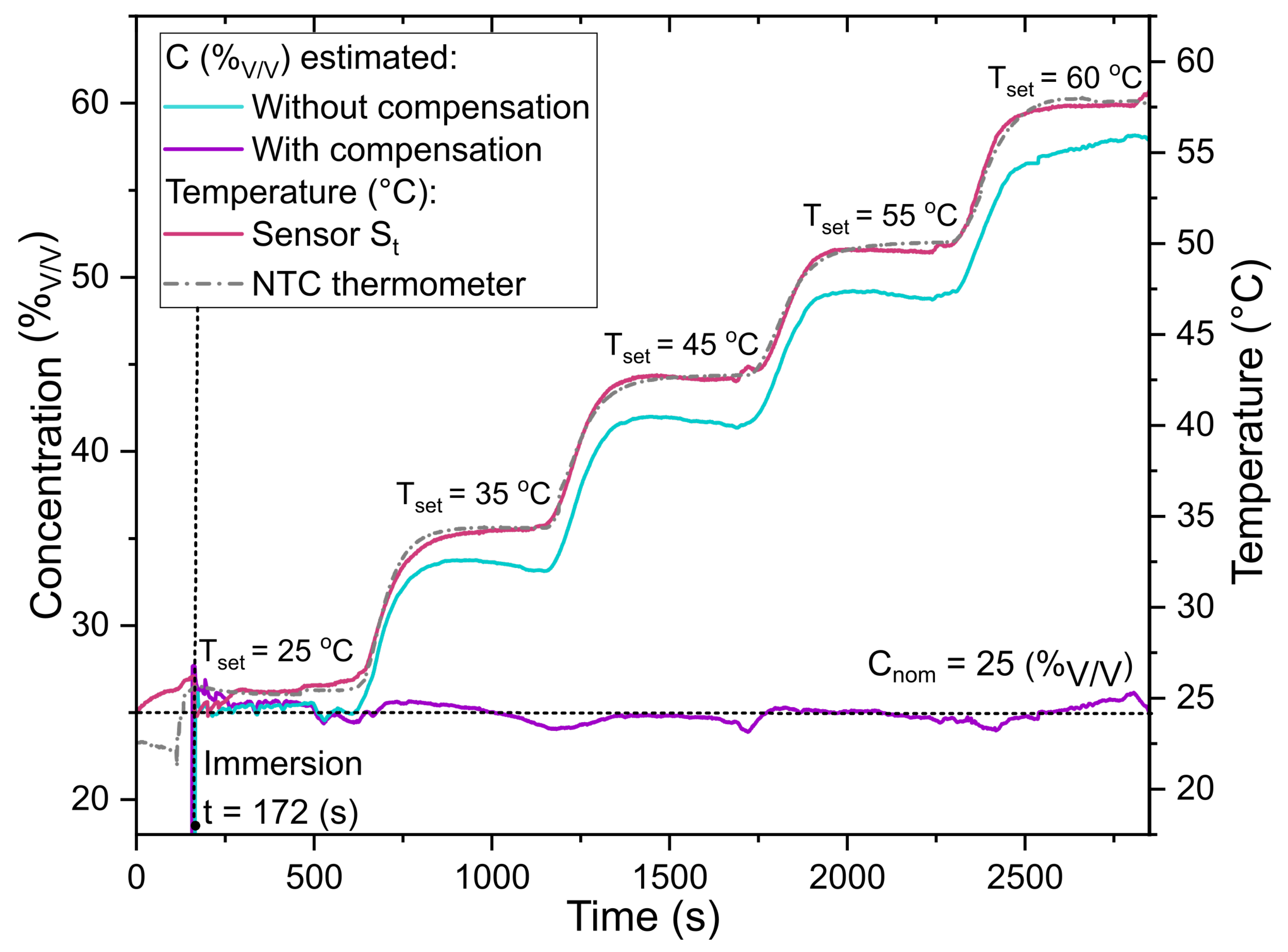
3.2. Ethanol Concentration Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, M.J.; Gu, B.; An, Q.F.; Yang, C.; Guan, Y.L.; Yong, K.T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Urrutia, A.; Del Villar, I.; Zubiate, P.; Zamarreño, C.R. A comprehensive review of optical fiber refractometers: Toward a standard comparative criterion. Laser Photon. Rev. 2019, 13, 1900094. [Google Scholar] [CrossRef]
- Li, J. A review: Development of novel fiber-optic platforms for bulk and surface refractive index sensing applications. Sensors Actuators Rep. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Memon, S.F.; Wang, R.; Strunz, B.; Chowdhry, B.S.; Pembroke, J.T.; Lewis, E. A Review of Optical Fibre Ethanol Sensors: Current State and Future Prospects. Sensors 2022, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Novais, S.; Ferreira, M.S.; Pinto, J.L. Determination of thermo-optic coefficient of ethanol-water mixtures with optical fiber tip sensor. Opt. Fiber Technol. 2018, 45, 276–279. [Google Scholar] [CrossRef]
- Coradin, F.K.; Possetti, G.R.C.; Kamikawachi, R.C.; Muller, M.; Fabris, J.L. Etched fiber bragg gratings sensors for water-ethanol mixtures: A comparative study. J. Microw. Optoelectron. Electromagn. Appl. 2010, 9, 131–143. [Google Scholar] [CrossRef]
- Liao, C.; Zhu, F.; Zhou, P.; Wang, Y. Fiber Taper-Based Mach-Zehnder Interferometer for Ethanol Concentration Measurement. Micromachines 2019, 10, 741. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Han, Y.; Gao, S.; Sheng, Y.; Zhang, S.; Lu, Z.; Man, B.; Jiao, Y.; Jiang, S. Evanescent wave absorption sensor with direct-growth MoS2 film based on U-bent tapered multimode fiber. J. Phys. D Appl. Phys. 2017, 50, 315302. [Google Scholar] [CrossRef]
- Fujiwara, E.; Takeishi, R.T.; Hase, A.; Ono, E.; Santos, J.S.; Suzuki, C.K. Real-time optical fibre sensor for hydro-alcoholic solutions. Meas. Sci. Technol. 2010, 21, 094035. [Google Scholar] [CrossRef]
- Fujiwara, E.; Ono, E.; Suzuki, C.K. Application of an Optical Fiber Sensor on the Determination of Sucrose and Ethanol Concentrations in Process Streams and Effluents of Sugarcane Bioethanol Industry. IEEE Sens. J. 2012, 12, 2839–2843. [Google Scholar] [CrossRef]
- Alemohammad, H.; Toyserkani, E.; Pinkerton, A.J. Femtosecond laser micromachining of fibre Bragg gratings for simultaneous measurement of temperature and concentration of liquids. J. Phys. D Appl. Phys. 2008, 41, 185101. [Google Scholar] [CrossRef]
- Aristilde, S.; Cordeiro, C.M.B.; Osório, J.H. Gasoline Quality Sensor Based on Tilted Fiber Bragg Gratings. Photonics 2019, 6, 51. [Google Scholar] [CrossRef]
- Monteiro-Silva, F.; Santos, J.L.; de Almeida, J.M.M.M.; Coelho, L. Quantification of Ethanol Concentration in Gasoline Using Cuprous Oxide Coated Long Period Fiber Gratings. IEEE Sens. J. 2018, 18, 1493–1500. [Google Scholar] [CrossRef]
- Mitsushio, M.; Higo, M. A gold-deposited surface plasmon resonance-based optical fiber sensor system using various light-emitting diodes. Anal. Sci. 2011, 27, 247–252. [Google Scholar] [CrossRef]
- Sharif, V.; Pakarzadeh, H. High-performance surface plasmon resonance fiber sensor based on cylindrical vector modes. Sci. Rep. 2023, 13, 4563. [Google Scholar] [CrossRef]
- Tian, J.; Lu, Z.; Quan, M.; Jiao, Y.; Yao, Y. Fast response Fabry-Perot interferometer microfluidic refractive index fiber sensor based on concave-core photonic crystal fiber. Opt. Express 2016, 24, 20132–20142. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Z.; Zhang, A.P.; Guan, B.O.; Tam, H.Y. In-line open-cavity Fabry-Pérot interferometer formed by C-shaped fiber fortemperature-insensitive refractive index sensing. Opt. Express 2014, 22, 21757–21766. [Google Scholar] [CrossRef]
- Zhou, F.; Su, H.; Joe, H.E.; Jun, M.B.G. Temperature insensitive fiber optical refractive index probe with large dynamic range at 1550 nm. Sens. Actuators A Phys. 2020, 312, 112102. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, Q.; Wang, Y.; Lewis, E.; Yang, M. Enhanced sensitivity of heterocore structure surface plasmon resonance sensors based on local microstructures. Organ. Ethic. 2018, 57, 076105. [Google Scholar] [CrossRef]
- Picelli, L.; van Klinken, A.; Lindgren, G.; Hakkel, K.D.; Pagliano, F.; Fiaschi, N.; Sersic-Vollenbroek, I.; van Veldhoven, P.J.; van der Heijden, R.W.; Fiore, A. Scalable wafer-to-fiber transfer method for lab-on-fiber sensing. Appl. Phys. Lett. 2020, 117, 151101. [Google Scholar] [CrossRef]
- Micron Optics Inc. User Guide. Optical Sensing Instrumentation and Software. Available online: https://lunainc.com/sites/default/files/assets/files/resource-library/Optical%20Sensing%20Instrumentation%20and%20Software%20-%20rev20170912.pdf (accessed on 29 August 2023).
- Conteduca, D.; Barth, I.; Pitruzzello, G.; Reardon, C.P.; Martins, E.R.; Krauss, T.F. Dielectric nanohole array metasurface for high-resolution near-field sensing and imaging. Nat. Commun. 2021, 12, 3293. [Google Scholar] [CrossRef] [PubMed]
- Eppendorf ThermoMixer® C. Available online: https://www.eppendorf.com/nl-en/eShop-Products/Temperature-Control-and-Mixing/Instruments/Eppendorf-ThermoMixerC-p-PF-19703 (accessed on 29 August 2023).
- Jerath, K.; Brennan, S.; Lagoa, C. Bridging the gap between sensor noise modeling and sensor characterization. Measurement 2018, 116, 350–366. [Google Scholar] [CrossRef]

| Sensing Principle | T Compensation | Sensitivities | Ranges |
|---|---|---|---|
| Etched FBG [6] | No | 3.6 pm/%V/V 7.2 pm/°C | 0–80%V/V 3 & 20 °C |
| Oxide coated LPG [13] | No | 760 pm/%V/V 110 pm/°C | 0–30%V/V - |
| FBG (dual) [11] | No | 3.6 pm/%V/V 10 pm/°C | 95–100%V/V 35–60 °C |
| Tilted FBG [12] | No | 3.2 pm/%V/V 10 pm/°C | 0–60%V/V 25–50 °C |
| PhC sensor probe (this work) | Yes | 53 pm/%V/V 19.1 pm/°C | 0–60%V/V 25–60 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Velázquez, M.S.; Hendriks, A.L.; Picelli, L.; van Veldhoven, R.P.J.; Fiore, A. Temperature-Compensated Solution Concentration Measurements Using Photonic Crystal Fiber-Tip Sensors. Sensors 2023, 23, 7703. https://doi.org/10.3390/s23187703
Cano-Velázquez MS, Hendriks AL, Picelli L, van Veldhoven RPJ, Fiore A. Temperature-Compensated Solution Concentration Measurements Using Photonic Crystal Fiber-Tip Sensors. Sensors. 2023; 23(18):7703. https://doi.org/10.3390/s23187703
Chicago/Turabian StyleCano-Velázquez, Mildred S., Arthur L. Hendriks, Luca Picelli, Rene P. J. van Veldhoven, and Andrea Fiore. 2023. "Temperature-Compensated Solution Concentration Measurements Using Photonic Crystal Fiber-Tip Sensors" Sensors 23, no. 18: 7703. https://doi.org/10.3390/s23187703






