Effect of Forearm Postures and Elbow Joint Angles on Elbow Flexion Torque and Mechanomyography in Neuromuscular Electrical Stimulation of the Biceps Brachii
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
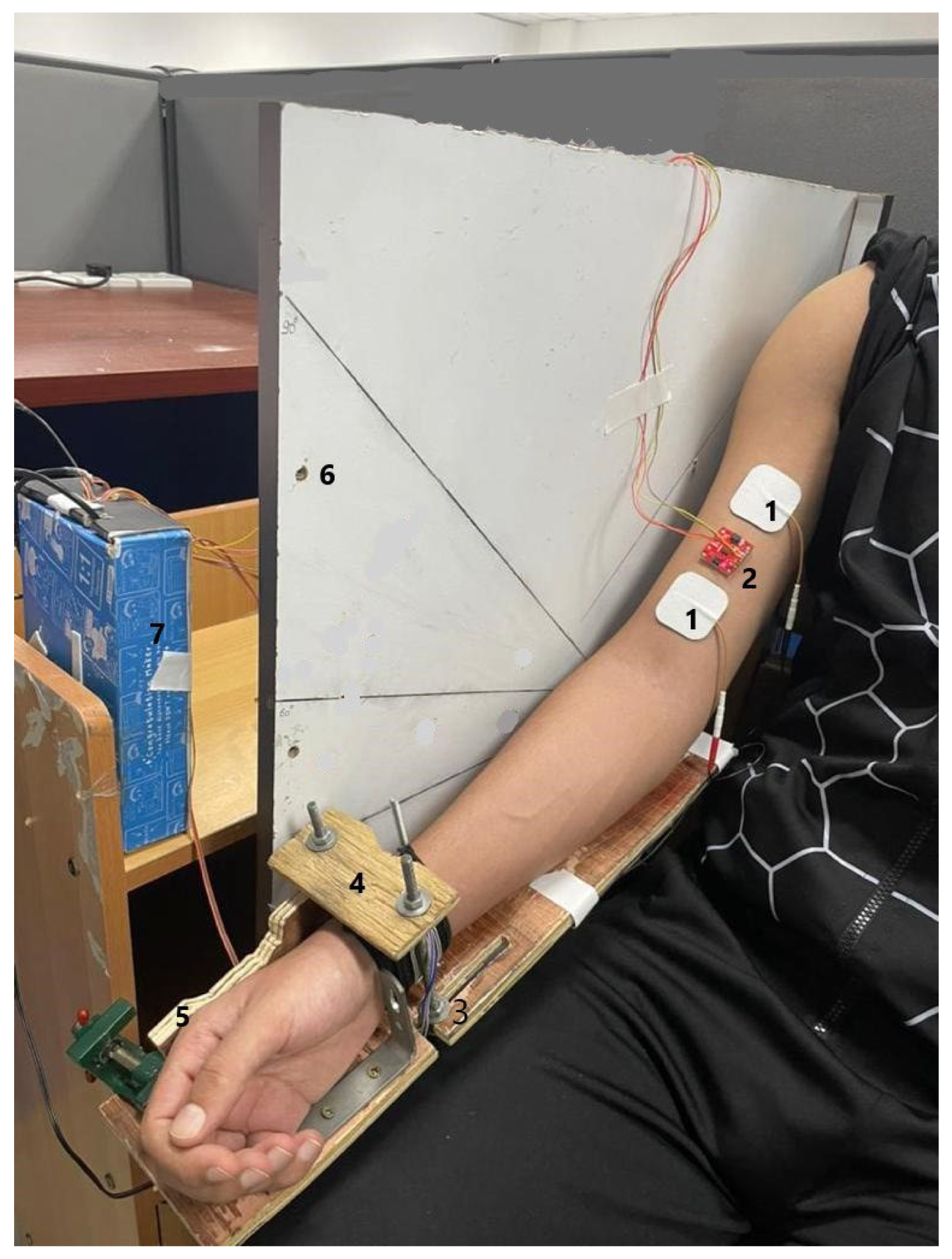
2.2. Experimental Protocol
2.3. Data Acquisition and Signal Processing
2.4. Statistical Analysis
3. Results
3.1. Effect of the Forearm Posture and Joint Angle on Torque
3.2. Effect of the Elbow Joint Angle and Forearm Posture on the MMG Responses to NMES
3.2.1. MMG Amplitude
3.2.2. MMG Frequency
4. Discussion
4.1. Effect of the Forearm Posture and Elbow Joint Angle on Torque
4.2. Effect of the Forearm Posture and Elbow Joint Angle on the MMG Amplitude
4.3. Effect of the Forearm Posture and Elbow Joint Angle on the MMG Frequency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akima, H.; Maeda, H.; Koike, T.; Ishida, K. Effect of elbow joint angles on electromyographic activity versus force relationships of synergistic muscles of the triceps brachii. PLoS ONE 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.J.; Downey, R.J.; Rouse, C.A.; Dixon, W.E. Influence of Elbow Flexion and Stimulation Site on Neuromuscular Electrical Stimulation of the Biceps Brachii. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.F.; Ikoma, K.; Chuma, T.; Mano, Y. Mechanomyographic response to transcranial magnetic stimulation from biceps brachii and during transcutaneous electrical nerve stimulation on extensor carpi radialis. J. Neurosci. Methods 2005, 149, 164–171. [Google Scholar] [CrossRef]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U.; Ali, M.A. Mechanomyography sensor development, related signal processing, and applications: A systematic review. IEEE Sens. J. 2013, 13, 2499–2516. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Zuniga, J.M.; Abdul Wahab, A.K. Mechanomyography and muscle function assessment: A review of current state and prospects. Clin. Biomech. 2014, 29, 691–704. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Hasnan, N.; Khairi, A.; Wahab, A. Torque and mechanomyogram relationships during electrically evoked isometric quadriceps contractions in persons with spinal cord injury. Med. Eng. Phys. 2016, 38, 767–775. [Google Scholar] [CrossRef]
- Kleiber, T.; Kunz, L.; Disselhorst-Klug, C. Muscular coordination of biceps brachii and brachioradialis in elbow flexion with respect to hand position. Front. Physiol. 2015, 6, 215. [Google Scholar] [CrossRef]
- Forman, D.A.; Abdel-Malek, D.; Bunce, C.M.; Holmes, M.W. Muscle length and joint angle influence spinal but not corticospinal excitability to the biceps brachii across forearm postures. J. Neurophysiol. 2019, 122, 413–423. [Google Scholar] [CrossRef]
- Akataki, K.; Mita, K.; Watakabe, M.; Itoh, K. Mechanomyogram and force relationship during voluntary isometric ramp contractions of the biceps brachii muscle. Eur. J. Appl. Physiol. 2001, 84, 19–25. [Google Scholar] [CrossRef]
- Uwamahoro, R.; Sundaraj, K.; Subramaniam, I.D. Assessment of muscle activity using electrical stimulation and mechanomyography: A systematic review. Biomed. Eng. Online 2021, 20, 1. [Google Scholar] [CrossRef]
- Flodin, J.; Mikkelsen, C.; Ackermann, P.W. Knee extensor force production and discomfort during neuromuscular electrical stimulation of quadriceps with and without gluteal muscle co-stimulation. Eur. J. Appl. Physiol. 2022, 122, 1521–1530. [Google Scholar] [CrossRef]
- Uwamahoro, R.; Sundaraj, K.; Subramaniam, I.D. Analysis of upper limb rehabilitation using muscle mechanics: Current and future perspectives using Mechanomyography signals. In Proceedings of the 12th Biomedical Engineering International Conference, Ubon Ratchathani, Thailand, 19–22 November 2019; pp. 1–5. [Google Scholar]
- Esposito, F.; Cè, E.; Rampichini, S.; Veicsteinas, A. Acute passive stretching in a previously fatigued muscle: Electrical and mechanical response during tetanic stimulation. J. Sports Sci. 2009, 27, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Ferrarin, M.; Pedotti, A. The relationship between electrical stimulus and joint torque: A dynamic model. IEEE Trans. Rehabil. Eng. 2000, 8, 342–352. [Google Scholar] [CrossRef]
- Gerrits, K.H.; Maganaris, C.N.; Reeves, N.D.; Sargeant, A.J.; Jones, D.A.; De Haan, A. Influence of knee joint angle on muscle properties of paralyzed and nonparalyzed human knee extensors. Muscle Nerve 2005, 32, 73–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamoto, N.; Oda, S. Mechanomyographic and electromyographic responses of the triceps surae during maximal voluntary contractions. J. Electromyogr. Kinesiol. 2003, 13, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U. Mechanomyogram for Muscle Function Assessment: A Review. PLoS ONE 2013, 8, e58902. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, Y.P.; Huang, Q.H.; Chen, X. Continuous monitoring of sonomyography, electromyography and torque generated by normal upper arm muscles during isometric contraction: Sonomyography assessment for arm muscles. IEEE Trans. Biomed. Eng. 2008, 55, 1191–1198. [Google Scholar]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Cramer, J.T.; Weir, J.P.; Coburn, J.W.; Malek, M.H. Does the frequency content of the surface mechanomyographic signal reflect motor unit firing rates? A brief review. J. Electromyogr. Kinesiol. 2007, 17, 1–13. [Google Scholar] [CrossRef]
- Gobbo, M.; Maffiuletti, N.A.; Orizio, C.; Minetto, M.A. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J. Neuroeng. Rehabil. 2014, 11, 17. [Google Scholar] [CrossRef]
- Madeleine, P.; Cescon, C.; Farina, D. Spatial and force dependency of mechanomyographic signal features. J. Neurosci. Methods 2006, 158, 89–99. [Google Scholar] [CrossRef]
- Lei, K.F.; Cheng, S.C.; Lee, M.Y.; Lin, W.Y. Measurement and estimation of muscle contraction strength using mechanomyography based on artificial neural network algorithm. Biomed. Eng. Singap. 2013, 25, 1350020. [Google Scholar] [CrossRef]
- Miyamoto, N.; Oda, S. Effect of joint angle on mechanomyographic amplitude during unfused and fused tetani in the human biceps brachii muscle. Eur. J. Appl. Physiol. 2005, 95, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Retortillo, S.; Rizzo, R.; Wang, J.W.; Sitges, C.; Ivanov, P.C. Universal spectral profile, and dynamic evolution of muscle activation. J. Appl. Physiol. 2020, 129, 419–441. [Google Scholar] [CrossRef]
- Talib, I.; Sundaraj, K.; Lam, C.K. Association of anthropometric parameters with amplitude and crosstalk of mechanomyographic signals during forearm flexion, pronation, and supination torque tasks. Sci. Rep. 2019, 9, 16166. [Google Scholar] [CrossRef]
- Szumilas, M.; Lewenstein, K.; Ślubowska, E. Verification of the functionality of device for monitoring human tremor. Biocybern. Biomed. Eng. 2015, 35, 240–246. [Google Scholar] [CrossRef]
- Shin, I.; Ahn, S.; Choi, E.; Ryu, J.; Park, S.; Son, J.; Kim, Y. Fatigue analysis of the quadriceps femoris muscle based on mechanomyography. Int. J. Precis. Eng. Manuf. 2016, 17, 473–478. [Google Scholar] [CrossRef]
- Latella, C.; Ruas, C.V.; Mesquita, R.N.; Nosaka, K.; Taylor, J.L. Test-retest reliability of elbow flexor contraction characteristics with tensiomyography for different elbow joint angles. J. Electromyogr. Kinesiol. 2019, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.G.; Housh, T.J.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Anders, J.P.V.; Schmidt, R.J.; Johnson, G.O. Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men. J. Funct. Morphol. Kinesiol. 2023, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T. Training based on electrical stimulation superimposed onto voluntary contraction would be relevant only as part of submaximal contractions in healthy subjects. Front. Physiol. 2018, 9, 1428. [Google Scholar] [CrossRef]
- Doheny, E.P.; Lowery, M.M.; FitzPatrick, D.P.; O’Malley, M.J. Effect of elbow joint angle on force–EMG relationships in human elbow flexor and extensor muscles. J. Electromyogr. Kinesiol. 2008, 18, 760–770. [Google Scholar] [CrossRef]
- Nunes, J.P.; Jacinto, J.L.; Ribeiro, A.S.; Mayhew, J.L.; Nakamura, M.; Capel, D.M.; Santos, L.R.; Santos, L.; Cyrino, E.S.; Aguiar, A.F. Placing greater torque at shorter or longer muscle lengths? Effects of cable vs. barbell preacher curl training on muscular strength and hypertrophy in young adults. Int. J. Environ. Res. Public Health 2020, 17, 5859. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Phillips, C.A. The effect of elbow angle on the isometric strength and endurance of the elbow flexors in men and women. J. Hum. Ergol. 1980, 9, 125–131. [Google Scholar]
- Hou, J.; Sun, Y.; Sun, L.; Pan, B.; Huang, Z.; Wu, J.; Zhang, Z. A pilot study of individual muscle force prediction during elbow flexion and extension in the neurorehabilitation field. Sensors 2016, 16, 2018. [Google Scholar] [CrossRef] [PubMed]
- Thepaut-Mathieu, C.; Van Hoecke, J.; Maton, B. Myoelectrical and mechanical changes linked to length specificity during isometric training. J. Appl. Physiol. 1988, 64, 1500–1505. [Google Scholar] [CrossRef]
- Krueger, E.; Scheeren, E.M.; Nogueira-Neto, G.N.; Button, V.L.D.S.N.; Nohama, P. Advances and perspectives of mechanomyography. Rev. Bras. Eng. Biomed. 2014, 30, 384–401. [Google Scholar] [CrossRef][Green Version]
- Kubo, K.; Ohgo, K.; Takeishi, R.; Yoshinaga, K.; Tsunoda, N.; Kanehisa, H.; Fukunaga, T. Effects of isometric training at different knee angles on the muscle–tendon complex in vivo. Scand. J. Med. Sci. Sports 2006, 16, 159–167. [Google Scholar] [CrossRef]
- Martin, S.; MacIsaac, D. Innervation zone shift with changes in joint angle in the brachial biceps. J. Electromyogr. Kinesiol. 2006, 16, 144–148. [Google Scholar] [CrossRef]
- Son, J.; Lee, D.; Kim, Y. Effects of involuntary eccentric contraction training by neuromuscular electrical stimulation on the enhancement of muscle strength. Clin. Biomech. 2014, 29, 767–772. [Google Scholar] [CrossRef]
- He, L.; Mathieu, P.A. Static hand posture classification based on the biceps brachii muscle synergy features. J. Electromyogr. Kinesiol. 2018, 43, 201–208. [Google Scholar] [CrossRef]
- Talib, I.; Sundaraj, K.; Lam, C.K.; Sundaraj, K. A systematic review of muscle activity assessment of the biceps brachii muscle using mechanomyography. J. Musculoskelet. Neuronal Interact. 2018, 18, 446. [Google Scholar]
- Mogk, J.P.; Rogers, L.M.; Murray, W.M.; Perreault, E.J.; Stinear, J.W. Corticomotor excitability of arm muscles modulates according to static position and orientation of the upper limb. Clin. Neurophysiol. 2014, 125, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.T. Acoustic signals from frog skeletal muscle. Biophys. J. 1987, 51, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P.; Johnson, G.O.; Coburn, J.W.; Malek, M.H.; Mielke, M. Mechanomyographic amplitude and frequency responses during dynamic muscle actions: A comprehensive review. Biomed. Eng. Online 2005, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, B.; Carpentier, A.; Duchateau, J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J. Neurophysiol. 2005, 94, 3126–3133. [Google Scholar] [CrossRef]
- Frangioni, J.V.; Kwan-Gett, T.S.; Dobrunz, L.E.; McMahon, T.A. The Mechanism of Low-Frequency Sound Production in Muscle. Biophys. J. 1987, 51, 775–783. [Google Scholar] [CrossRef]
- Qi, L.; Wakeling, J.M.; Ferguson-Pell, M. Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J. Electromyogr. Kinesiol. 2011, 21, 1056–1063. [Google Scholar] [CrossRef]
- Vaz, M.A.; Herzog, W.; Zhang, Y.T.; Leonard, T.R.; Hlhmnn, H.N. The Effect of Muscle Length on Electrically Elicited Muscle Vibrations in the In-Situ Cat Soleus Muscle. J. Electromyogr. Kinesiol. 1997, 7, 113–121. [Google Scholar] [CrossRef]
- Krishnan, C.; Allen, E.J.; Williams, G.N. Effect of knee position on quadriceps muscle force steadiness and activation strategies. Muscle Nerve 2011, 43, 563–573. [Google Scholar] [CrossRef]
- Kong, P.W.; Pan, J.; Chu, D.P.; Cheung, P.M.; Lau, P.W.C. Acquiring Expertise in Precision Sport—What Can We Learn from an Elite Snooker Player? Muscle Nerve 2021, 5, 98–106. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.; Koo, B.; Lee, S.; Park, S.; Kim, S.; Cho, K.H.; Kim, Y. sEMG-Based Hand Posture Recognition and Visual Feedback Training for the Forearm Amputee. Sensors 2022, 22, 7984. [Google Scholar] [CrossRef]








| Elbow Joint Angle | 10° | 30° | 60° | 90° | |||||
|---|---|---|---|---|---|---|---|---|---|
| Forearm Posture | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| N | 0.4516 | 0.1775 | 0.5707 | 0.1990 | 0.7416 | 0.1839 | 0.6029 | 0.2051 | |
| P | 0.4325 | 0.1724 | 0.5108 | 0.1700 | 0.6500 | 0.1704 | 0.5957 | 0.1797 | |
| S | 0.4002 | 0.1678 | 0.4524 | 0.1970 | 0.5041 | 0.2033 | 0.4639 | 0.1939 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwamahoro, R.; Sundaraj, K.; Feroz, F.S. Effect of Forearm Postures and Elbow Joint Angles on Elbow Flexion Torque and Mechanomyography in Neuromuscular Electrical Stimulation of the Biceps Brachii. Sensors 2023, 23, 8165. https://doi.org/10.3390/s23198165
Uwamahoro R, Sundaraj K, Feroz FS. Effect of Forearm Postures and Elbow Joint Angles on Elbow Flexion Torque and Mechanomyography in Neuromuscular Electrical Stimulation of the Biceps Brachii. Sensors. 2023; 23(19):8165. https://doi.org/10.3390/s23198165
Chicago/Turabian StyleUwamahoro, Raphael, Kenneth Sundaraj, and Farah Shahnaz Feroz. 2023. "Effect of Forearm Postures and Elbow Joint Angles on Elbow Flexion Torque and Mechanomyography in Neuromuscular Electrical Stimulation of the Biceps Brachii" Sensors 23, no. 19: 8165. https://doi.org/10.3390/s23198165
APA StyleUwamahoro, R., Sundaraj, K., & Feroz, F. S. (2023). Effect of Forearm Postures and Elbow Joint Angles on Elbow Flexion Torque and Mechanomyography in Neuromuscular Electrical Stimulation of the Biceps Brachii. Sensors, 23(19), 8165. https://doi.org/10.3390/s23198165




