Characterization of an Impedance-Type Humidity Sensor Based on Porous SnO2/TiO2 Composite Ceramics Modified with Molybdenum and Zinc
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure and Morphology
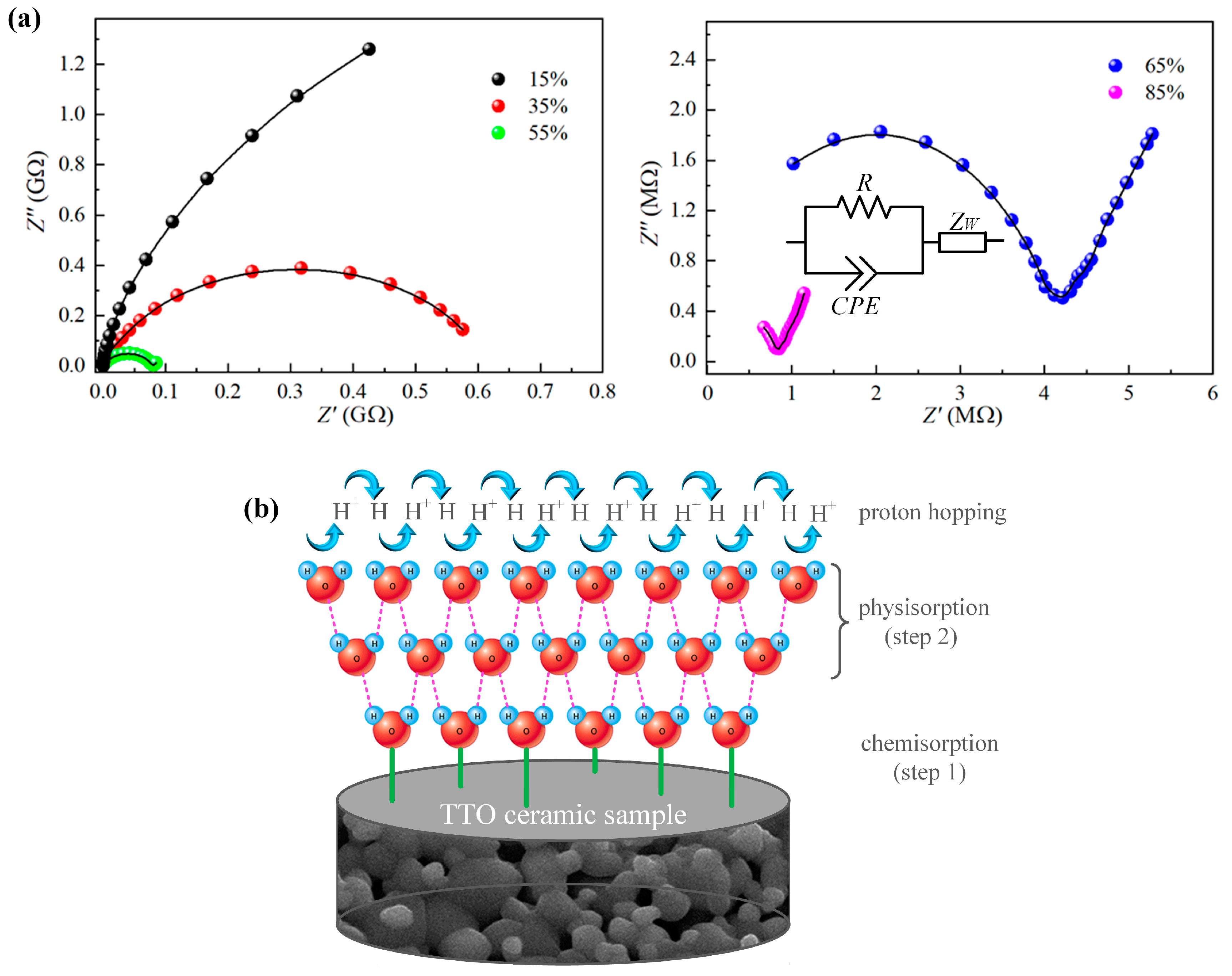
3.2. Impedance Spectroscopy
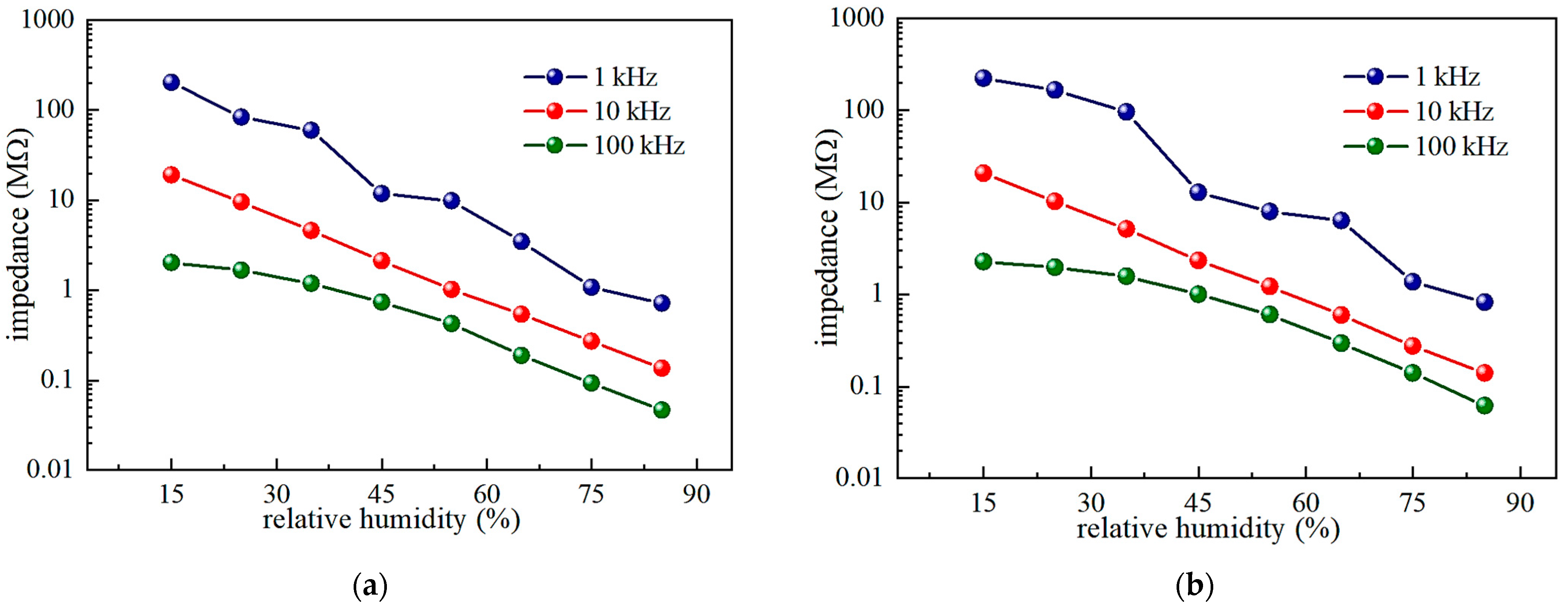
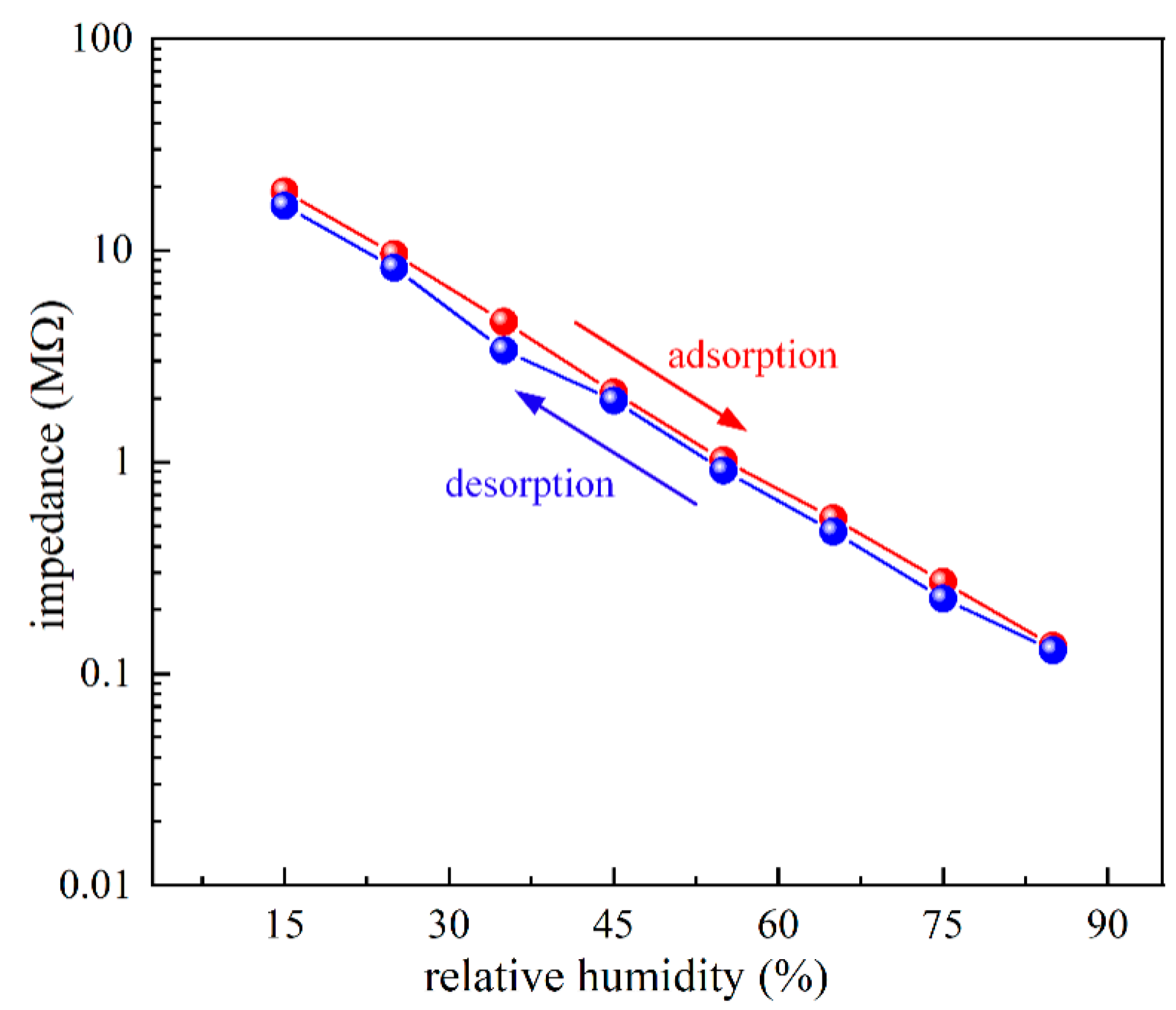
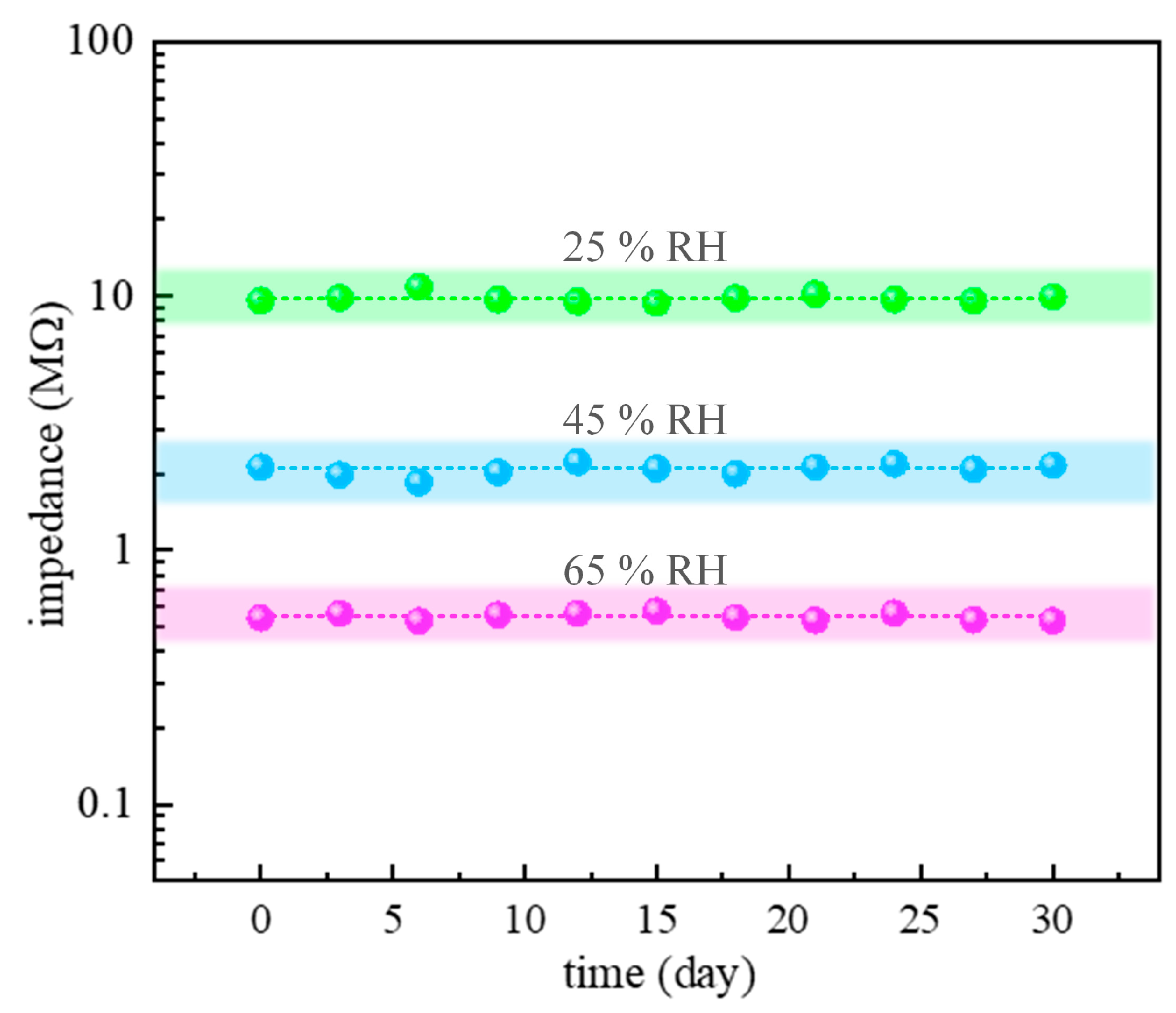
3.3. Humidity-Sensing Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blansk, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B-Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M.V. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensor. Sens. Actuators B-Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Zhao, B.; Sivasankar, V.S.; Subudhi, S.K.; Dasgupta, A.; Das, S. Printed carbon nanotube-based humidity sensors deployable on surfaces of widely varying curvatures. ACS Appl. Nano Mater. 2023, 6, 1459–1474. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D.D. Semiconductor-metal-oxide-based nano-composites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112053. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B-Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Pan, S.; Gayathri, G.; Reshma, T.S.; Mangamma, G.; Prasad, A.K.; Das, A. A sensitive humidity sensor at low pressure with SnO2 QDs. Sens. Actuators A-Phys. 2022, 346, 113835. [Google Scholar] [CrossRef]
- Li, H.; Meng, B.; Jia, H.; Wang, D.; Wei, Z.; Li, R.; Chen, R. Optical humidity sensor based on ZnO nanomaterials. In Proceedings of the 2020 IEEE 5th Optoelectronics Global Conference (OGC), Shenzhen, China, 7–11 September 2020; pp. 169–172. [Google Scholar] [CrossRef]
- Li, F.; Li, P.; Zhang, H. Preparation and research of a high-performance ZnO/SnO2 humidity sensor. Sensors 2022, 22, 293. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Fatima, Q.; Li, Z.; Yao, Z. Improving the humidity sensing below 30% RH of TiO2 with GO modification. Mater. Res. Bull. 2018, 99, 124–131. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Li, C.; Men, G.; Han, D.; Gu, F. Humidity-sensing performance of 3DOM WO3 with controllable structural modification. ACS Appl. Mater. Interfaces 2018, 10, 3776–3783. [Google Scholar] [CrossRef]
- Khan, M.U.; Hassan, G.; Awais, M.; Bae, J. All printed full range humidity sensor based on Fe2O3. Sens. Actuators A 2022, 311, 112072. [Google Scholar] [CrossRef]
- Ateia, M.A.; Ateia, E.E.; Mosry, M.; Arman, M.M. Synthesis and characterization of non-stoichiometric Li1.1Co0.3Fe2.1O4 ferrite nanoparticles for humidity sensors. Appl. Phys. A 2022, 128, 884. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, Y.; Shirage, P.M. Synthesis of humidity sensitive zinc stannate nanomaterials and modelling of Freundlich adsorption isotherm model. AIP Conf. Proc. 2018, 1942, 050095. [Google Scholar] [CrossRef]
- Prasad, N.V.K.; Venkata Prasad, K.; Ramesh, S.; Phanidhar, S.V.; Venkata Ratnam, K.; Janardhan, S.; Manjunatha, H.; Sarma, M.S.S.R.K.N.; Srinivaset, K. Ceramic Sensors: A mini-review of their applications. Front. Mater. 2020, 7, 593342. [Google Scholar] [CrossRef]
- Laobuthee, A.; Wongkasemjit, S.; Traversa, E.; Laine, R.M. MgAl2O4 spinel powders from oxide one pot synthesis (OOPS) process for ceramic humidity sensors. J. Eur. Ceram. Soc. 2000, 20, 91–97. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Cosentino, I.C.; Muccillo, E.N.S.; Muccillo, R. Development of zirconia-titania porous ceramics for humidity sensors. Sens. Actuators B-Chem. 2003, 96, 677–683. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.Z.; Qu, W.; Du, B.; et al. An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B-Chem. 2021, 337, 129772. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Aryaet, S. Review-Recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Bueno, P.B.; Varela, J.A. Electronic ceramics based on poly crystalline SnO2, TiO2 and (SnxTi1-x)O2 solid solution. Mater. Res. 2006, 9, 293–300. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius’ J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2(n)-NiO(p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sensor. Actuators B-Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Araújo, E.S.; Leão, V.N.S. TiO2/WO3 heterogeneous structures prepared by electrospinning and sintering steps: Characterization and analysis of the impedance variation to humidity. J. Adv. Ceram. 2019, 8, 238–246. [Google Scholar] [CrossRef]
- Marzec, A.; Radecka, M.; Maziarzc, W.; Kusior, A.; Pedzich, Z. Structural, optical and electrical properties of nanocrystalline TiO2, SnO2 and their composites obtained by the sol-gel method. J. Eur. Ceram. Soc. 2016, 36, 2981–2989. [Google Scholar] [CrossRef]
- Kumar, V.; Chauhan, V.; Ram, J.; Gupta, R.; Kumar, S.; Chaudhary, P.; Yadav, B.C.; Ojha, S.; Sulania, I.; Kumar, R. Study of humidity sensing properties and ion beam induced modifications in SnO2-TiO2 nanocomposite thin films. Surf. Coat. Technol. 2020, 392, 125768. [Google Scholar] [CrossRef]
- Chaisan, W.; Yimnirun, R.; Ananta, S.; Cann, D.P. The effects of the spinodal microstructure on the electrical properties of TiO2-SnO2 ceramics. J. Solid State Chem. 2005, 178, 613–620. [Google Scholar] [CrossRef]
- Radecka, M.; Zakrzewska, K.; Rękas, M. SnO2-TiO2 solid solutions for gas sensors. Sens. Actuators B-Chem. 1998, 47, 194–204. [Google Scholar] [CrossRef]
- Lustosa, G.M.M.M.; da Costa, J.P.C.; Perazolli, L.A.; Stojanovic, B.D.; Zaghete, M.A. Electrophoretic deposition of (Zn,Nb)SnO2-films varistor superficially modified with Cr3+. J. Eur. Ceram. Soc. 2015, 35, 2083–2089. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Xia, Y.; Benzine, O.; Petrović, J.; Papan, J.; Lukić-Petrović, S.R.; Litvinchuk, A.P. Structure, electrochemical impedance and Raman spectroscopy of lithium-niobium-titanium-oxide ceramics for LTCC technology. Ceram. Int. 2021, 47, 4944–4953. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Sekulić, D.L.; Papan, J.; Gúth, I.O.; Petrović, D.M.; Lukić-Petrović, S.R. Niobium and zinc doped titanium-tin-oxide solid-solution ceramics: Synthesis, structure and electrical characterization. Ceram. Int. 2018, 44, 18987–18995. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. EIS Spectrum Analyser (A Freeware Program for Analysis and Simulation of Impedance Spectra). 2008. Available online: https://www.abc.chemistry.bsu.by/vi/analyser/ (accessed on 18 August 2023).
- Raonić, R.R.; Sekulić, D.L.; Lukić-Petrović, S.R.; Ivetić, T.B. Lithium-niobium-titanium-oxide ceramics with ZnO as a functional additive: Structural and impedance characterization with humidity properties. Acta Phys. Pol. A 2022, 142, 457–463. [Google Scholar] [CrossRef]
- Hirata, T. Oxygen position, octahedral distortion, and bond-valence parameter from bond lengthsi n Ti1-xSnxO2 (0 < x < 1). J. Am. Ceram. Soc. 2000, 83, 3205–3207. [Google Scholar] [CrossRef]
- Edelman, F.; Hahn, H.; Seifried, S.; Alof, C.; Hoche, H.; Balogh, A.; Werner, P.; Zakrzewska, K.; Radecka, M.; Pasierb, P.; et al. Structural evolution of SnO2-TiO2 nanocrystalline films for gas sensors. Mater. Sci. Eng. B-Adv. 2000, 69–70, 386–391. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Hu, C.-C.; Hua, C.-C. Preparation and characterization of nanocrystalline TixSn1-xO2 solid solutions via a microwave-assisted hydrothermal synthesis process. Cryst. Eng. Comm. 2011, 13, 5638–5641. [Google Scholar] [CrossRef]
- Harunsani, M.H.; Oropeza, F.E.; Palgrave, R.G.; Egdell, R.G. Electronic and Structural Properties of SnxTi1-xO2 (0.0≤x≤0.1) Solid Solutions. Chem. Mater. 2010, 22, 1551–1558. [Google Scholar] [CrossRef]
- Hwang, T.J.; Choi, G.M. Electrical characterization of porous BaTiO3 using impedance spectroscopy in humid condition. Sens. Actuators B-Chem. 1997, 40, 87–191. [Google Scholar] [CrossRef]
- Sekulić, D.L.; Lazarević, Z.Z.; Jovalekić, C.D.; Milutinović, A.N.; Romcević, N.Z. Impedance spectroscopy of nanocrystalline MgFe2O4 and MnFe2O4 ferrite ceramics: Effect of grain boundaries on the electrical properties. Sci. Sinter. 2016, 48, 17–28. [Google Scholar] [CrossRef]
- Cantalini, C.; Sun, H.T.; Faccio, M.; Ferri, G.; Pelino, M. Niobium-doped α-Fe2O3 semiconductor ceramic sensors for the measurement of nitric oxide gases. Sens. Actuators B-Chem. 1995, 25, 673–677. [Google Scholar] [CrossRef]
- Bagum, N.; Gafur, M.A.; Bhuiyan, A.H.; Saha, D.K. MgCl2 doped CuxZn1-xFe2O4 ferrite humidity sensors. Phys. Status Solidi A 2010, 207, 986–992. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Zheng, X.; Jiang, B.; Li, P.; Deng, S. Humidity sensing properties of K0.5Na0.5NbO3 powder synthesized bymetal organic decomposition. Sens. Actuators B-Chem. 2015, 209, 252–257. [Google Scholar] [CrossRef]
- Sekulić, D.L.; Lazarević, Z.; Romcević, N.Z. Nanocrystalline porous nickel ferrite ceramicsfor humidity sensing applications. In Proceedings of the 2019 IEEE 31st International Conferenceon Microelectronics (MIEL), Niš, Serbia, 16–18 September2019; pp. 95–98. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-fast response humidity sensor based on La0.7Sr0.3MnO3 nanocrystals prepared by PVP-assisted sol-gel method. Sens. Actuators B-Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Arunachalam, S.; Izquierdo, R.; Nabki, F. Low-hysteresis and fast response time humidity sensors using suspended functionalized carbon nanotubes. Sensors 2019, 19, 680. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, X.; Wang, X.; Baranyi, K.M.; Zhang, X. Hysteresis dynamic modeling and analysis of flexible nano silver–polyvinyl alcohol humidity sensor based on the microscopic process and Langmuir–Fick theory. ACS Omega 2022, 7, 14994–15004. [Google Scholar] [CrossRef]
- Su, P.-G.; Lu, P.-H. Electrical and humidity-hensing properties of impedance-type humidity sensors that were made of Agmicrowires/PPy/SnO2 ternary composites. Chemosensors 2020, 8, 92. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S.; Adhyapak, P.V.; Mulla, I.S. Mn-loaded mesoporous silica nanocomposite: A highly efficient humidity sensor. J. Am. Ceram. Soc. 2015, 98, 741–747. [Google Scholar] [CrossRef]
- Tai, W.P.; Oh, J.H. Fabrication and humidity sensing properties of nanostructured TiO2-SnO2 thin films. Sens. Actuators B 2002, 85, 154–157. [Google Scholar] [CrossRef]
- Mi, Y.; Li, P. Preparation and performance of NaTaO3/TiO2 humidity sensors with high responsivity. Results Phys 2023, 50, 106560. [Google Scholar] [CrossRef]
- Yu, W.; Chen, D.; Li, J.; Zhang, Z. TiO2-SnS2 nanoheterostructures for high-performance humidity sensor. Crystals 2023, 13, 482. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, H.; Peng, J.; Duan, Z.; Ma, M.; Xin, X.; Li, W.; Zheng, X. Enhanced humidity sensing properties of SmFeO3-modified MoS2 nanocomposites based on the synergistic effect. Sens. Actuators B-Chem. 2018, 272, 459–467. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.; Cao, W.; Li, L.; Luo, M.; Wang, C. Humidity sensing properties of (In + Nb) doped HfO2 ceramics. Nanomaterials 2023, 13, 951. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Evans, R.A.; Shafiei, M.; Langford, S. J.Enhanced capacitive humidity sensing performance at room temperature via hydrogen bonding of cyanopyridone-based oligothiophene donor. Chemosensors 2021, 9, 320. [Google Scholar] [CrossRef]
- Bauskara, D.; Kaleb, B.B.; Patil, P. Synthesis and humidity sensing properties of ZnSnO3 cubic crystallites. Sens. Actuators B-Chem. 2012, 161, 396–400. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Xiao, Y.; Meng, B.; Xing, C.; Zhang, H.; Peng, Z. A fully inkjet-printed transparent humidity sensor based on a Ti3C2/Ag hybrid for touchless sensing of finger motio. Nanoscale 2019, 11, 21522. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of metal oxide semiconductor gas sensors: A review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]

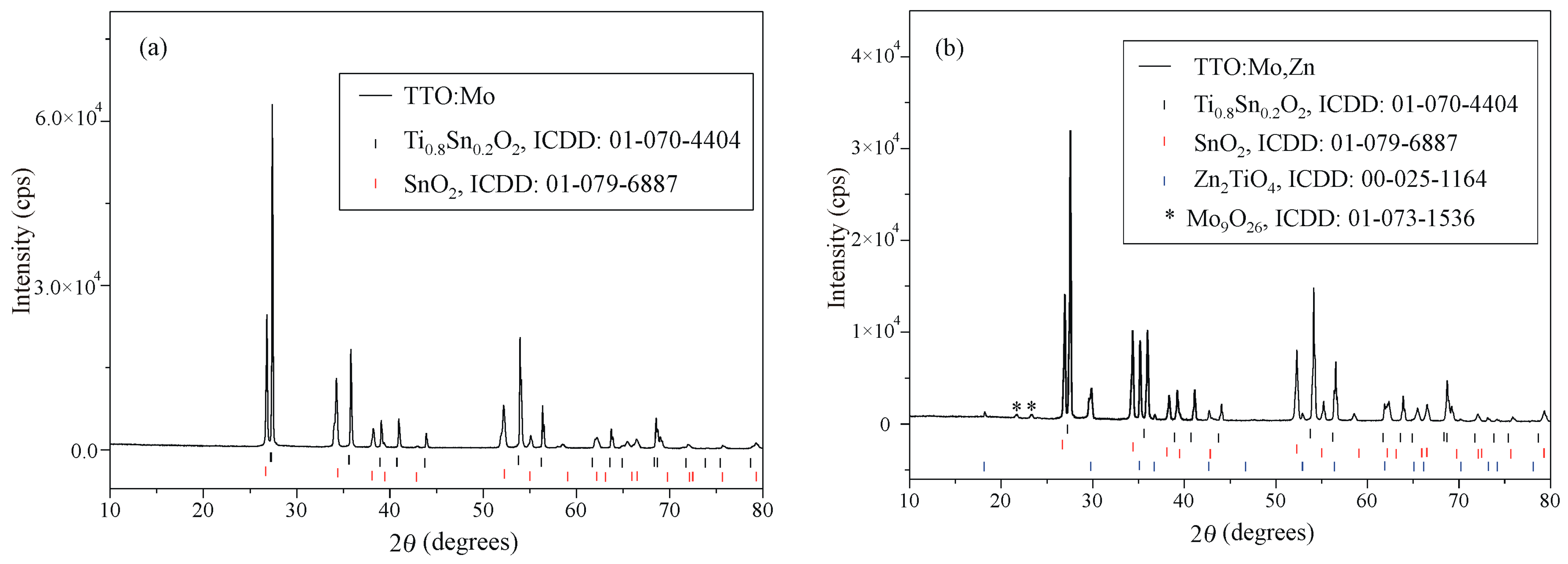
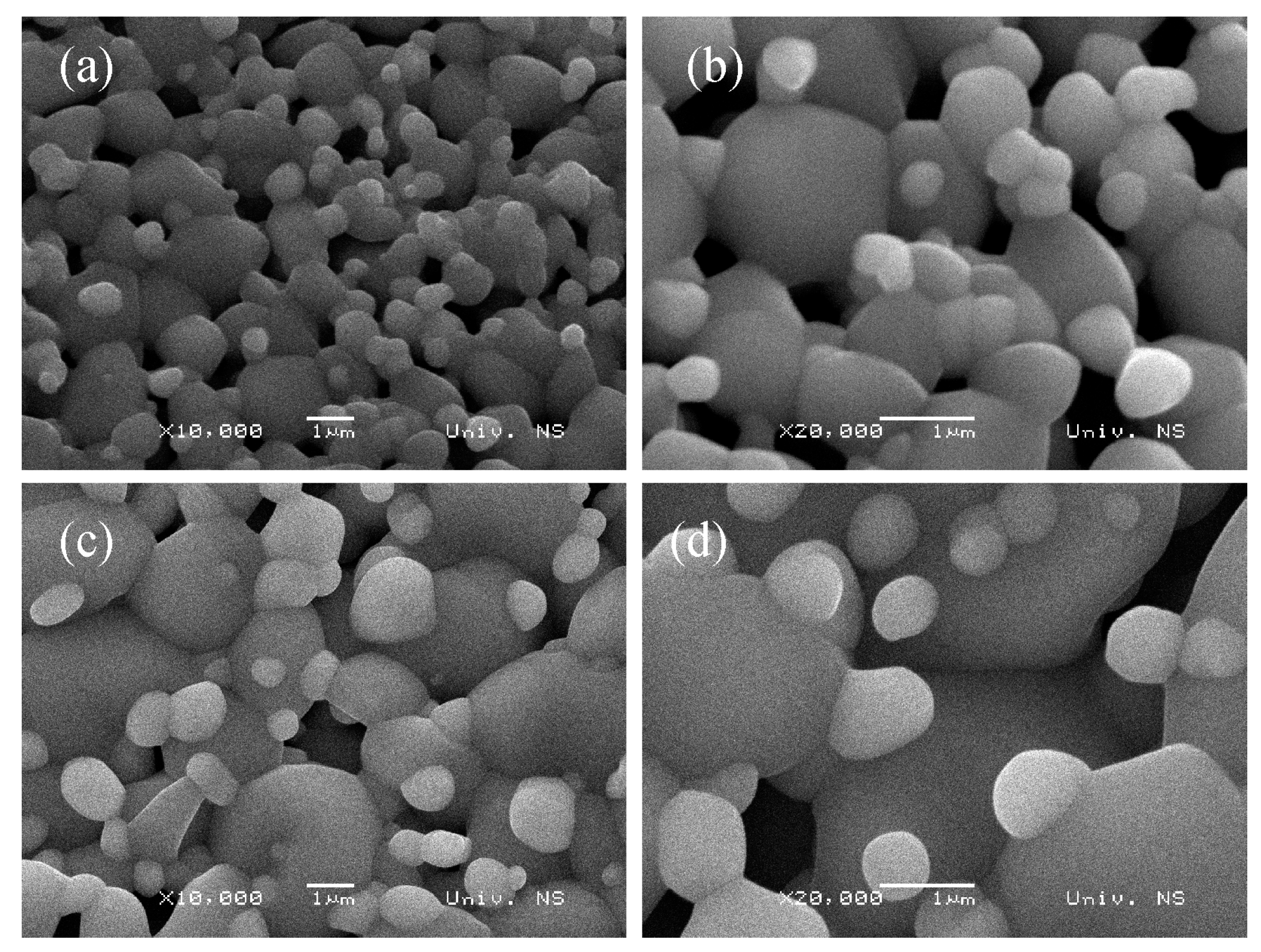
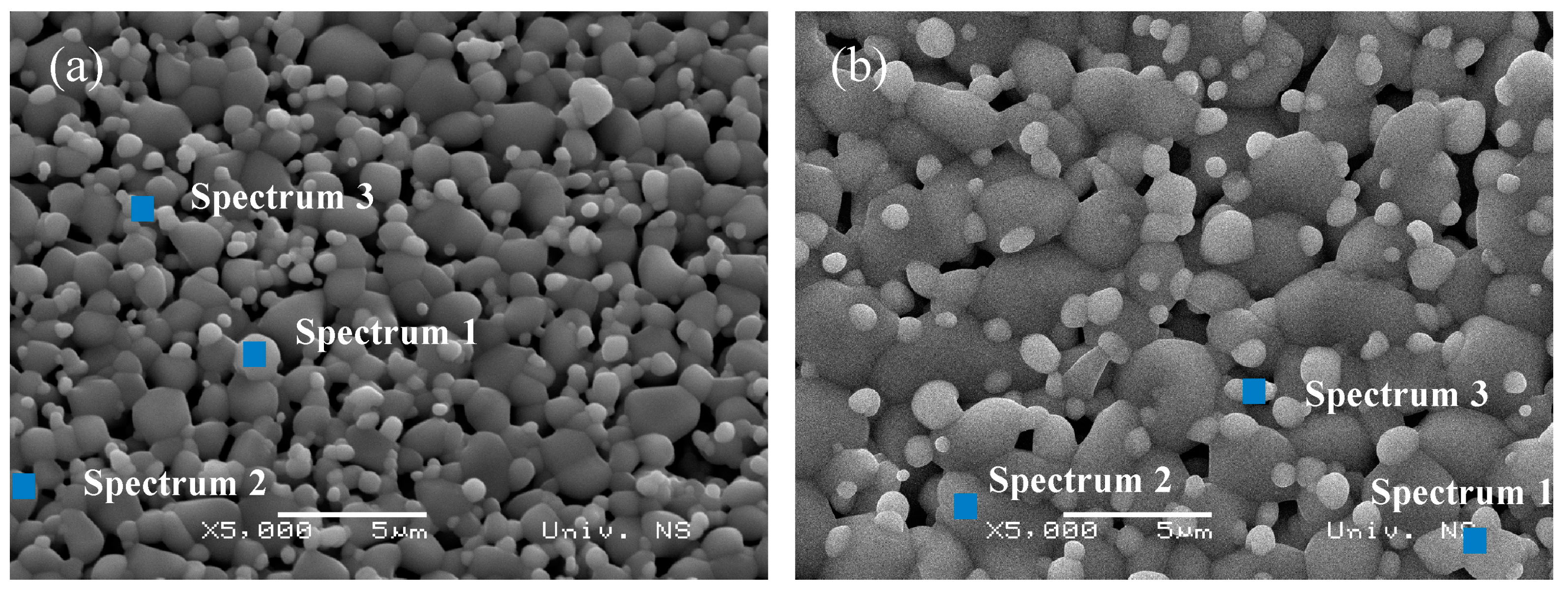
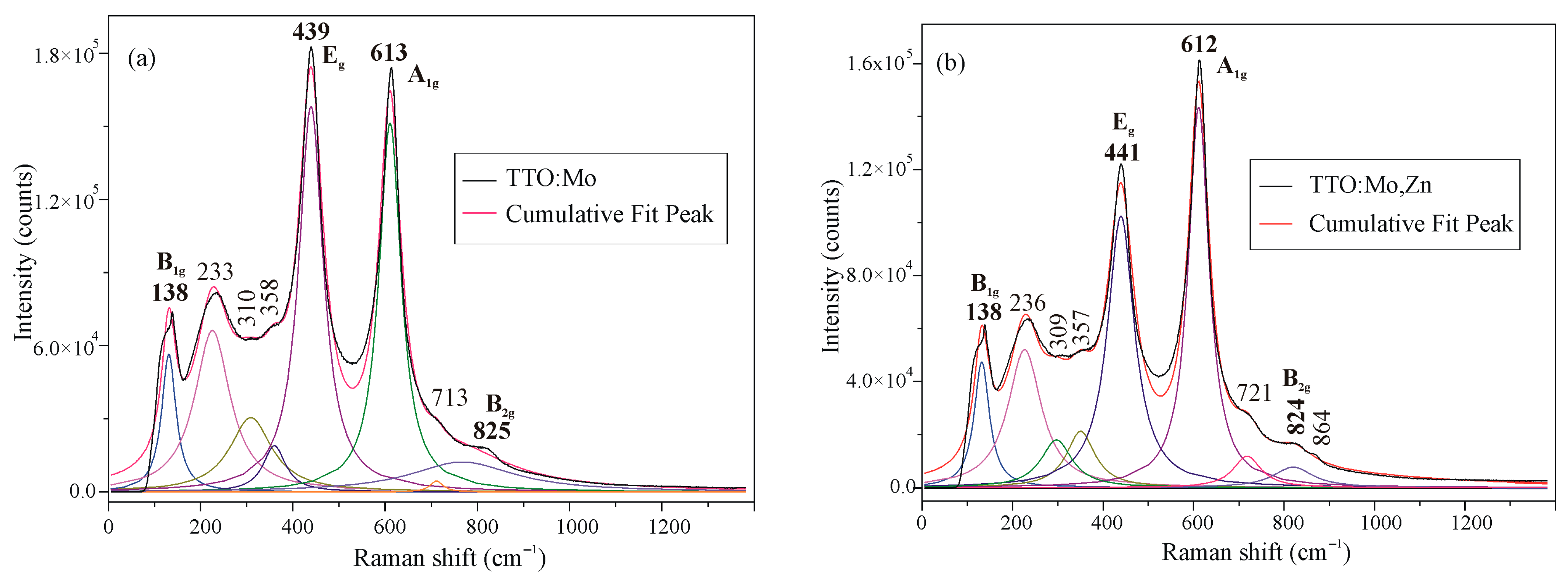


| Sample | TTO:Mo | TTO:Mo,Zn | ||||
|---|---|---|---|---|---|---|
| Spectrum | O | Ti | Sn | O | Ti | Sn |
| Spectrum 1 | 24.95 | 21.46 | 53.59 | 47.04 | 34.12 | 18.84 |
| Spectrum 2 | 35.66 | 40.28 | 24.06 | 41.50 | 41.72 | 16.79 |
| Spectrum 3 | 36.36 | 30.34 | 33.30 | 31.95 | 24.26 | 43.79 |
| Sensing Material | Detection Range | Sensitivity | Response Time | Recovery Time | Hysteresis Error | Reference |
|---|---|---|---|---|---|---|
| TiO2-SnO2 | 20–90% RH | ~103 | 20 s | - | - | [49] |
| SnO2-TiO2 | 10–95% RH | 12.374 | 19.1s | 181 s | - | [26] |
| ZnO/SnO2 | 11–95% RH | ~104 | 8 s | 35 s | 6.6% | [5] |
| NaTaO3/TiO2 | 11–95% RH | ~104 | 13 s | 9 s | 8.3% | [50] |
| TiO2-SnS2 | 11–93% RH | ~103 | 60 s | - | - | [51] |
| SmFeO3@MoS2 | 11–95% RH | ~105 | 1.5 s | 29.8 s | 2% | [52] |
| (In+Nb)-doped HfO2 | 11–94% RH | 3612 | 20 s | 50 s | 6.79% | [53] |
| CNT | 15–98% RH | 172 | 12 s | 47 s | 3.6% | [45] |
| TTO:Mo | 15–85% RH | ~102 | 18 s | 27 s | 2.8% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekulić, D.L.; Ivetić, T.B. Characterization of an Impedance-Type Humidity Sensor Based on Porous SnO2/TiO2 Composite Ceramics Modified with Molybdenum and Zinc. Sensors 2023, 23, 8261. https://doi.org/10.3390/s23198261
Sekulić DL, Ivetić TB. Characterization of an Impedance-Type Humidity Sensor Based on Porous SnO2/TiO2 Composite Ceramics Modified with Molybdenum and Zinc. Sensors. 2023; 23(19):8261. https://doi.org/10.3390/s23198261
Chicago/Turabian StyleSekulić, Dalibor L., and Tamara B. Ivetić. 2023. "Characterization of an Impedance-Type Humidity Sensor Based on Porous SnO2/TiO2 Composite Ceramics Modified with Molybdenum and Zinc" Sensors 23, no. 19: 8261. https://doi.org/10.3390/s23198261
APA StyleSekulić, D. L., & Ivetić, T. B. (2023). Characterization of an Impedance-Type Humidity Sensor Based on Porous SnO2/TiO2 Composite Ceramics Modified with Molybdenum and Zinc. Sensors, 23(19), 8261. https://doi.org/10.3390/s23198261







