Cardiac Electromechanical Activity in Healthy Cats and Cats with Cardiomyopathies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
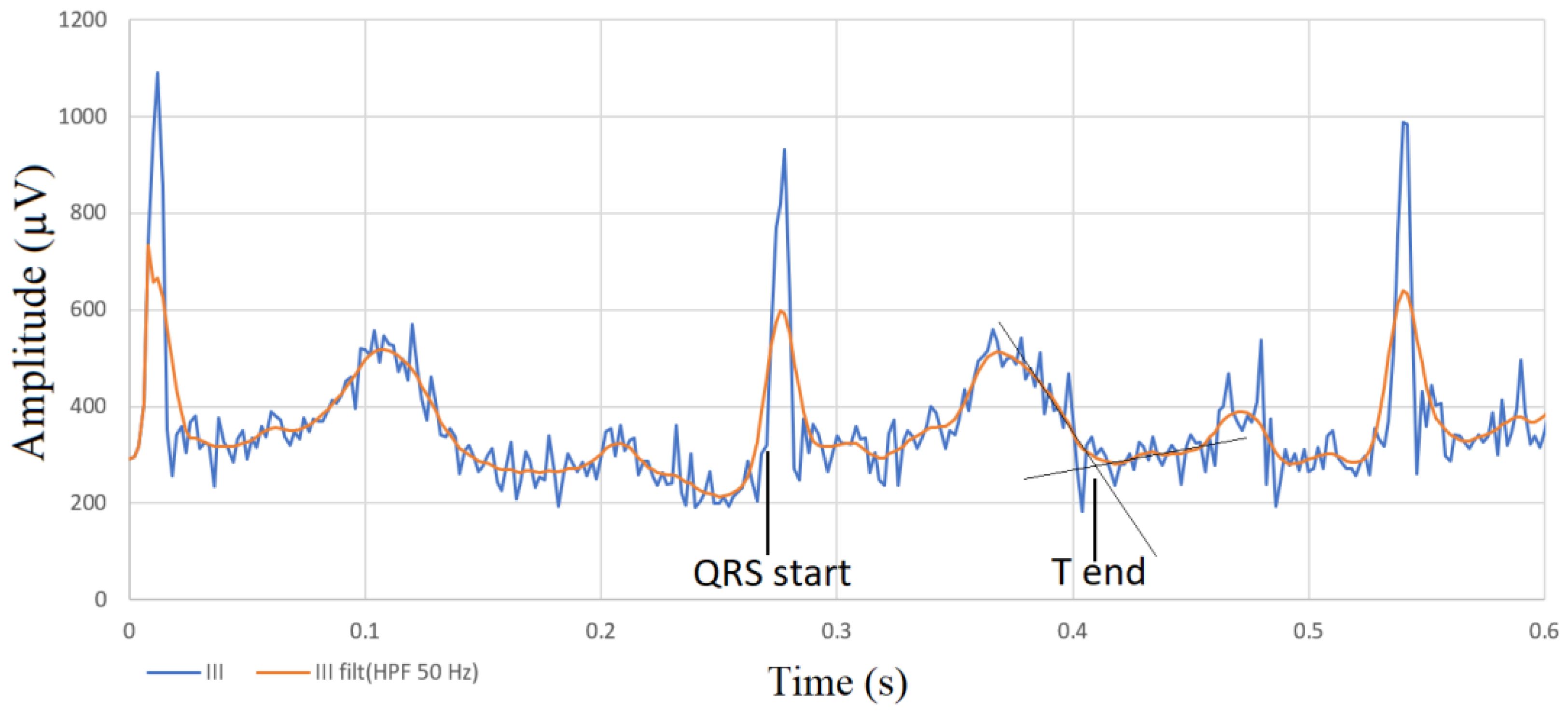
2.2. Electrocardiography
2.3. Phonocardiography
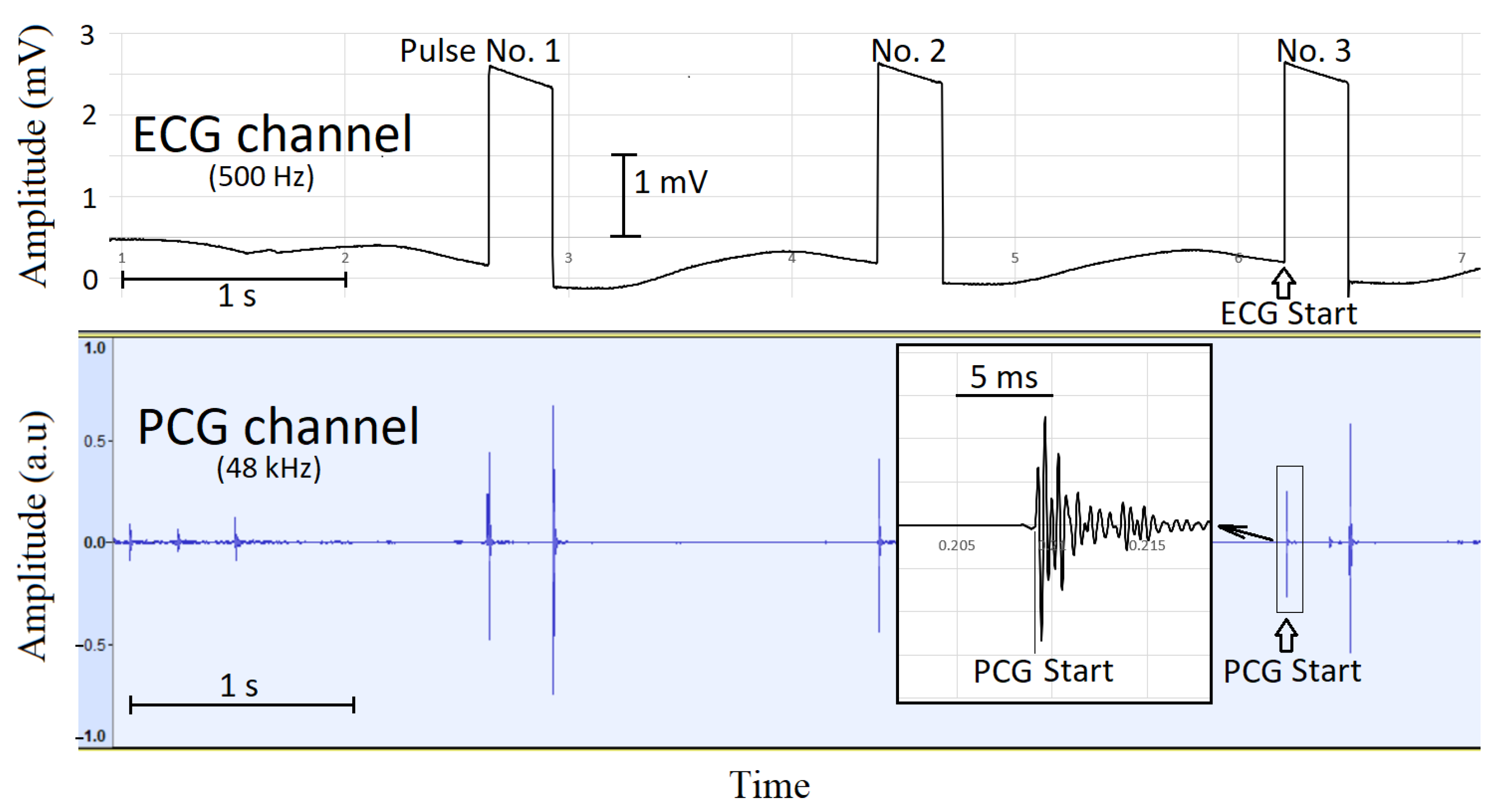
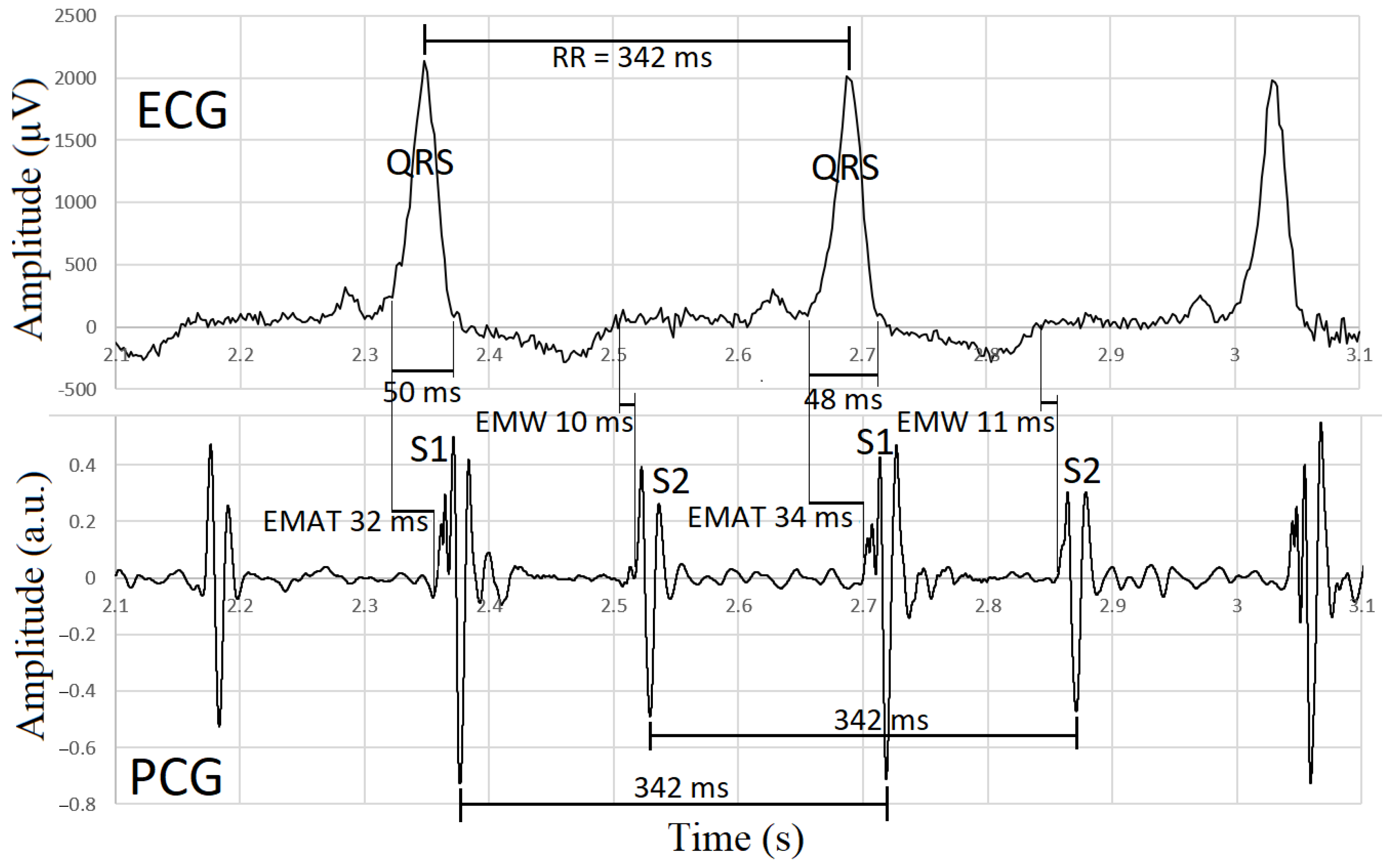
2.4. Synchronization of Electrocardiographic and Phonocardiographic Data
2.5. Echocardiography
2.6. Statistical Analysis
3. Results
- -
- In two cats with cardiomyopathy, synchronization of the ECG and PCG device failed; so, measurements of EMAT, EMW and QS2 were not possible.
- -
- In one cat with cardiomyopathy, CHF and atrial fibrillation, electromechanical activity could not be assessed because S1 and S2 heart sounds could not be determined.
- -
- In one healthy cat, the ECG could not be used for further analysis due to unclear borders of low-voltage complexes, and therefore QT, EMAT, QS2 and EMW could not be measured.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, R.A.; Arrand, P.; Myers, R. Electrocardiographic and acoustical data in the detection of left ventricular enlargement. Int. J. Bioelectromagn. 2003, 5, 193–196. Available online: http://www.ijbem.org/volume5/number1/193-196.htm (accessed on 11 August 2023).
- Zhang, J.; Liu, W.X.; Lyu, S.Z. Predictive value of Electromechanical Activation Time for in-hospital major cardiac adverse events in heart failure patients. Cardiovasc. Ther. 2020, 2020, 4532596. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Sung, S.H.; Cheng, H.M.; Yu, W.C.; Wang, K.L.; Huang, C.M.; Chen, C.H. Electromechanical activation time in the prediction of discharge outcomes in patients hospitalized with acute heart failure syndrome. Intern. Med. 2010, 49, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Huang, C.J.; Cheng, H.M.; Huang, W.M.; Yu, W.C.; Chen, C.H. Effect of Acoustic cardiography-guided management on 1-year outcomes in patients with acute heart failure. J. Card. Fail. 2020, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Efstratiadis, S.; Michaels, A.D. Computerized acoustic cardiographic electromechanical activation time correlates with invasive and echocardiographic parameters of left ventricular contractility. J. Card. Fail. 2008, 14, 577–582. [Google Scholar] [CrossRef]
- ter Bekke, R.M.; Haugaa, K.H.; van den Wijngaard, A.; Bos, J.M.; Ackerman, M.J.; Edvardsen, T.; Volders, P.G. Electromechanical window negativity in genotyped long-QT syndrome patients: Relation to arrhythmia risk. Eur. Heart J. 2015, 36, 179–186. [Google Scholar] [CrossRef]
- van der Linde, H.J.; Van Deuren, B.; Somers, Y.; Loenders, B.; Towart, R.; Gallacher, D.J. The Electro-Mechanical window: A risk marker for Torsade de Pointes in a canine model of drug induced arrhythmias. Br. J. Pharmacol. 2010, 161, 1444–1454. [Google Scholar] [CrossRef]
- Passini, E.; Trovato, C.; Morissette, P.; Sannajust, F.; Bueno-Orovio, A.; Rodriguez, B. Drug-induced shortening of the electromechanical window is an effective biomarker for in silico prediction of clinical risk of arrhythmias. Br. J. Pharmacol. 2019, 176, 3819–3833. [Google Scholar] [CrossRef]
- Zuber, N.; Zuber, M.; Schwarzwald, C.C. Assessment of systolic and diastolic function in clinically healthy horses using ambulatory acoustic cardiography. Equine Vet. J. 2019, 51, 391–400. [Google Scholar] [CrossRef]
- Gicana, K.R.B.; Lertwanakarn, T.; Tachampa, K. Novel approach to assess cardiac function using systolic performance and myocardial performance indices from simultaneous electrocardiography and phonocardiography recordings in dogs with various stages of myxomatous mitral valve disease. Front. Vet. Sci. 2021, 8, 741115. [Google Scholar] [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.D.; Côté, E. The feline cardiomyopathies: 1. General concepts. J. Feline Med. Surg. 2021, 23, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.D.; Côté, E. The feline cardiomyopathies: 2. Hypertrophic cardiomyopathy. J. Feline Med. Surg. 2021, 23, 1028–1051. [Google Scholar] [CrossRef] [PubMed]
- Romito, G.; Guglielmini, C.; Mazzarella, M.O.; Cipone, M.; Diana, A.; Contiero, B.; Toaldo, B.M. Diagnostic and prognostic utility of surface electrocardiography in cats with left ventricular hypertrophy. J. Vet. Cardiol. 2018, 20, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; Badilini, F.; Vaglio, M.; Lux, R.L.; Aysin, B.; Moon, T.E.; Heinz, B.; Strachan, I. A fundamental relationship between intraventricular conduction and heart rate. J. Electrocardiol. 2016, 49, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; Strauss, D.G.; Vaglio, M.; Badilini, F. Correction of the QRS duration for heart rate. J. Electrocardiol. 2019, 54, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.Q.; Silka, M.J.; Lan, Y.T.; Chang, R.K. Comparison of formulas for calculation of the corrected QT interval in infants and young children. J. Pediatr. 2015, 166, 960–964.e2. [Google Scholar] [CrossRef] [PubMed]
- Andršová, I.; Hnatkova, K.; Šišáková, M.; Toman, O.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Influence of heart rate correction formulas on QTc interval stability. Sci. Rep. 2021, 11, 14269. [Google Scholar] [CrossRef]
- Bazett, H.C. An analysis of the time-relations of electrocardiograms. Heart 1920, 7, 353–386. [Google Scholar] [CrossRef]
- Fridericia, L.S. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann. Noninvasive Electrocardiol. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Malik, M.; Garnett, C.; Hnatkova, K.; Johannesen, L.; Vicente, J.; Stockbridge, N. Importance of QT/RR hysteresis correction in studies of drug-induced QTc interval changes. J. Pharmacokinet. Pharmacodyn. 2018, 45, 491–503. [Google Scholar] [CrossRef]
- Matsunaga, T.; Mitsui, T.; Harada, T.; Inokuma, M.; Murano, H.; Shibutani, Y. QT corrected for heart rate and relation between QT and RR intervals in beagle dogs. J. Pharmacol. Toxicol. Methods 1997, 38, 201–209. [Google Scholar] [CrossRef]
- Ware, W.A.; Christensen, W.F. Duration of the QT interval in healthy cats. Am. J. Vet. Res. 1999, 60, 1426–1429. [Google Scholar] [PubMed]
- Kadunc Kos, V.; Brložnik, M.; Domanjko Petrič, A.; Avbelj, V. Simultaneous phonocardiography and electrocardiography using smartphone in dogs, cats and horses. In Proceedings of the 2020 43rd International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 28 September–2 October 2020; pp. 327–331. [Google Scholar] [CrossRef]
- Avbelj, V.; Brložnik, M. Phonocardiography and electrocardiography with a smartphone. In Proceedings of the 2020 43rd International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 28 September–2 October 2020; pp. 332–336. [Google Scholar] [CrossRef]
- Brložnik, M.; Kadunc Kos, V.; Kramarič, P.; Domanjko Petrič, A.; Avbelj, V. Electromechanical events in exercise induced remodeling of equine heart. In Proceedings of the 2021 44th International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 24–28 May 2021; pp. 352–356. [Google Scholar] [CrossRef]
- Ware, W.A.; Bonagura, J.D. Cardiovascular Disease in Companion Animals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022; p. 141. [Google Scholar]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J. Vet. Int. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Schober, K.; Savino, S.; Yildiz, V. Reference intervals and allometric scaling of two-dimensional echocardiographic measurements in 150 healthy cats. J. Vet. Med. Sci. 2017, 79, 1764–1771. [Google Scholar] [CrossRef]
- Domanjko Petrič, A.; Rishniw, M.; Thomas, W.P. Two-dimensionally-guided M-mode and pulsed wave Doppler echocardiographic evaluation of the ventricles of apparently healthy cats. J. Vet. Cardiol. 2012, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Drourr, L.; Lefbom, B.K.; Rosenthal, S.L.; Tyrrell, W.D., Jr. Measurement of M-mode echocardiographic parameters in healthy adult Maine Coon cats. J. Am. Vet. Med. Assoc. 2005, 226, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Rohrbaugh, M.N.; Schober, K.E.; Rhinehart, J.D.; Bonagura, J.D.; Habing, A.; Yildiz, V. Detection of congestive heart failure by Doppler echocardiography in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2020, 34, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Schober, K.E.; Chetboul, V. Echocardiographic evaluation of left ventricular diastolic function in cats: Hemodynamic determinants and pattern recognition. J. Vet. Cardiol. 2015, 17, S102–S133. [Google Scholar] [CrossRef]
- Peacock, W.F.; Harrison, A.; Moffa, D. Clinical and economic benefits of using AUDICOR S3 detection for diagnosis and treatment of acute decompensated heart failure. Congest. Heart Fail. 2006, 12, 32–36. [Google Scholar] [CrossRef]
- Trabelsi, I.; Msolli, M.A.; Sekma, A.; Fredj, N.; Dridi, Z.; Bzeouich, N.; Najjar, M.F.; Gannoun, I.; Mzali, M.; Laouiti, K.; et al. Value of systolic time intervals in the diagnosis of heart failure in emergency department patients with undifferentiated dyspnea. Int. J. Clin. Pract. 2020, 74, e13572. [Google Scholar] [CrossRef] [PubMed]
- Avbelj, V.; Trobec, R.; Gersak, B. Beat-to-beat repolarisation variability in body surface electrocardiograms. Med. Biol. Eng. Comput. 2003, 41, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Baumert, M.; Porta, A.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016, 18, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.M.; Armano, G.; Rahmani, A.M.; Mankodiya, K. PhonoSys: Mobile phonocardiography diagnostic system for newborns. In Proceedings of the 2015 5th EAI International Conference on Wireless Mobile Communication and Healthcare, London, UK, 14–16 October 2015. [Google Scholar] [CrossRef]
- Andrès, E.; Hajjam, A.; Brandt, C. Advances and innovations in the field of auscultation, with a special focus on the development of new intelligent communicating stethoscope systems. Health Technol. 2012, 2, 5–16. [Google Scholar] [CrossRef]
- Hoyte, H.; Jensen, T.; Gjesdal, K. Cardiac auscultation training of medical students: A comparison of electronic sensor-based and acoustic stethoscopes. BMC Med. Educ. 2005, 5, 14. [Google Scholar] [CrossRef]
- Thoms, L.J.; Colicchia, G.; Girwidz, R. Phonocardiography with a smartphone. Phys. Educ. 2017, 52, 023004. [Google Scholar] [CrossRef]
- Dahl, L.B.; Hasvold, P.; Arild, E.; Hasvold, T. Heart murmurs recorded by a sensor based electronic stethoscope and e-mailed for remote assessment. Arch. Dis. Child. 2002, 87, 297–301. [Google Scholar] [CrossRef]
- Abbasi Kesbi, R.; Valipour, A.; Imani, K. Cardiorespiratory system monitoring using a developed acoustic sensor. Health Technol. Lett. 2018, 5, 7–12. [Google Scholar] [CrossRef]
- Mondal, H.; Mondal, S.; Saha, K. Development of a low-cost wireless phonocardiograph with a bluetooth headset under resource-limited conditions. Med. Sci. 2018, 6, 117. [Google Scholar] [CrossRef]
- Sa-Ngasoongsong, A.; Kunthong, J.; Sarangan, V.; Cai, X.; Bukkapatnam, T.S. A low-cost, portable, high-throughput wireless sensor system for phonocardiography applications. Sensors 2012, 12, 10851–10870. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Pan, C.; Zhu, S.; Mead, R.H.; Li, R.; He, B. Mobile cardiac acoustic monitoring system to evaluate left ventricular systolic function in pacemaker patients. J. Clin. Med. 2022, 11, 3862. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.; Knaflitz, M. A novel method for measuring the timing of heart sound components through digital phonocardiography. Sensors 2019, 19, 1868. [Google Scholar] [CrossRef] [PubMed]

| Healthy Cats | Cats with Cardiomyopathy | |
|---|---|---|
| Number | 12 | 17 |
| Age (years; median, IQR) | 4.18 (2.55–5.78) | 7.80 (3.40–11.15) |
| Weight (kg; mean ± SD) | 4.74 ± 1.24 | 5.01 ± 1.84 |
| Female | 9 | 6 |
| Male | 3 | 11 |
| Breed | Domestic short-haired cat (n = 6), Siberian (n = 2), Maine Coon (n = 2), Bengal (n = 1), Sphynx (n = 1) | Domestic short-haired cat (n = 8), Sphynx (n = 6), Maine Coon (n = 1), British Shorthair (n = 2) |
| Variable | Healthy Cats (n = 12) | Cats with Cardiomyopathy (n = 17) | p |
|---|---|---|---|
| Heart rate (bpm) * | 192.9 ± 23.4 | 211.8 ± 25.4 | 0.052 |
| RR (ms) * | 315.5 ± 40.6 | 287.2 ± 35.1 | 0.055 |
| QRS (ms) ** | 24.0 (20.0–27.5) | 28.0 (23.0–30.0) | 0.070 |
| QT (ms) * | 161.6 ± 11.5 | 157.6 ± 13.6 | 0.462 |
| QTc1 (ms) * | 179.2 ± 10.6 | 178.2 ± 13.2 | 0.856 |
| QTc2 (ms) * | 159.7 ± 9.4 | 158.9 ± 11.8 | 0.856 |
| QTc3 (ms) * | 236.1 ± 12.6 | 239.1 ± 16.4 | 0.636 |
| QTc4 (ms) * | 285.6 ± 15.6 | 294.6 ± 20.3 | 0.250 |
| QTpredicted (ms) * | 187.8 ± 14.9 | 175.6 ± 13.4 | 0.048 |
| QTdiff (ms) * | −26.2 ± 1.3 | −18.0 ± 11.7 | 0.098 |
| EMAT (ms) * | 21.9 ± 9.3 | 28.4 ± 8.6 | 0.083 |
| EMAT/RR * | 0.069 ± 0.026 | 0.098 ± 0.028 | 0.017 |
| QS2 (ms) * | 171.1 ± 20.9 | 177.2 ± 19.2 | 0.467 |
| QS2/RR * | 0.542 ± 0.038 | 0.605 ± 0.048 | 0.002 |
| EMW (ms) * | 11.5 ± 17.6 | 17.6 ± 15.8 | 0.403 |
| EMW/RR * | 0.033 ± 0.057 | 0.058 ± 0.057 | 0.305 |
| S1S2 (ms) * | 149.2 ± 17.4 | 148.1 ± 15.7 | 0.873 |
| S1S2/RR * | 0.475 ± 0.034 | 0.514 ± 0.044 | 0.017 |
| QT/S1S2 * | 1.081 ± 0.130 | 1.082 ± 0.108 | 0.971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brložnik, M.; Lunka, E.; Avbelj, V.; Nemec Svete, A.; Domanjko Petrič, A. Cardiac Electromechanical Activity in Healthy Cats and Cats with Cardiomyopathies. Sensors 2023, 23, 8336. https://doi.org/10.3390/s23198336
Brložnik M, Lunka E, Avbelj V, Nemec Svete A, Domanjko Petrič A. Cardiac Electromechanical Activity in Healthy Cats and Cats with Cardiomyopathies. Sensors. 2023; 23(19):8336. https://doi.org/10.3390/s23198336
Chicago/Turabian StyleBrložnik, Maja, Ema Lunka, Viktor Avbelj, Alenka Nemec Svete, and Aleksandra Domanjko Petrič. 2023. "Cardiac Electromechanical Activity in Healthy Cats and Cats with Cardiomyopathies" Sensors 23, no. 19: 8336. https://doi.org/10.3390/s23198336
APA StyleBrložnik, M., Lunka, E., Avbelj, V., Nemec Svete, A., & Domanjko Petrič, A. (2023). Cardiac Electromechanical Activity in Healthy Cats and Cats with Cardiomyopathies. Sensors, 23(19), 8336. https://doi.org/10.3390/s23198336






