Impedimetric Bacterial Detection Using Random Antimicrobial Peptide Mixtures
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
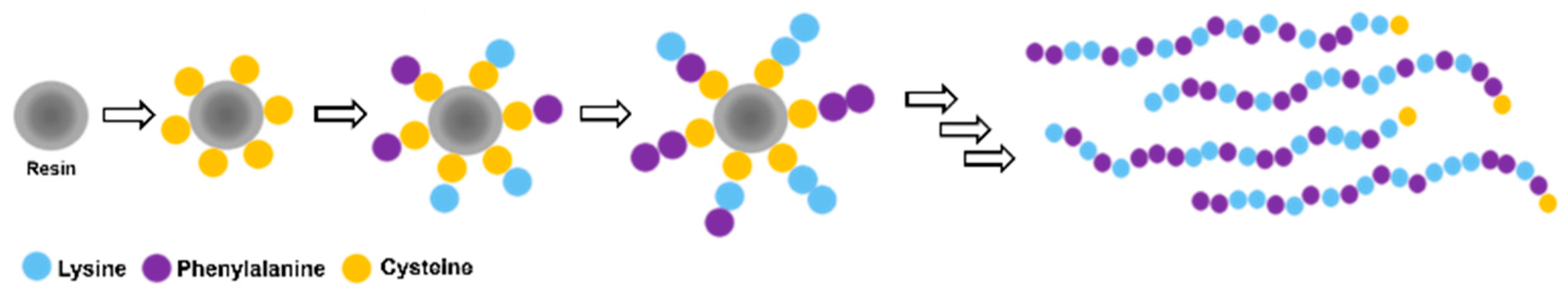
2.2.1. Synthesis of CFK Random Peptide Mixtures (RPMs)
2.2.2. CFK Assembly on a Gold Surface
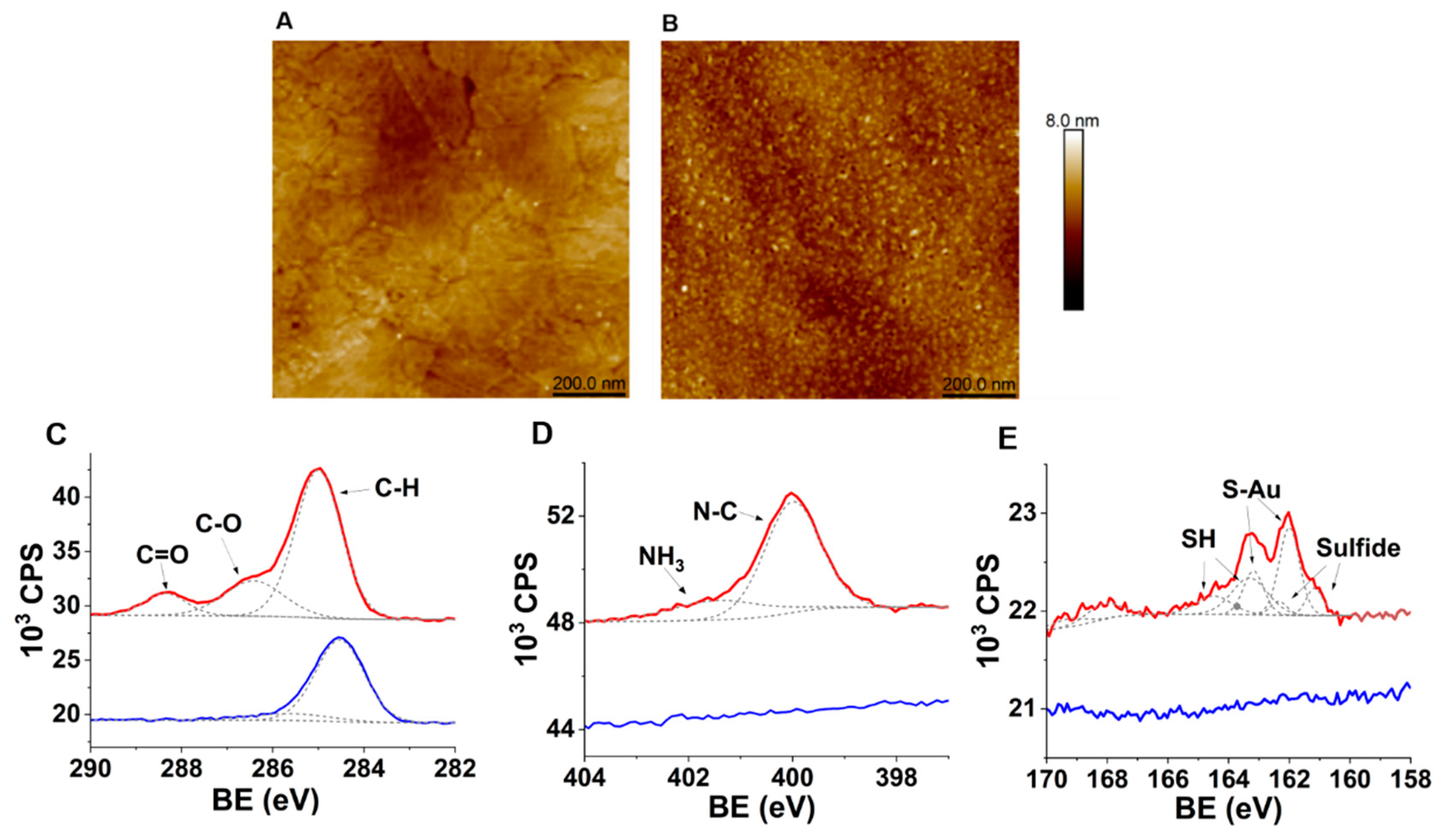
2.2.3. Surface Characterization
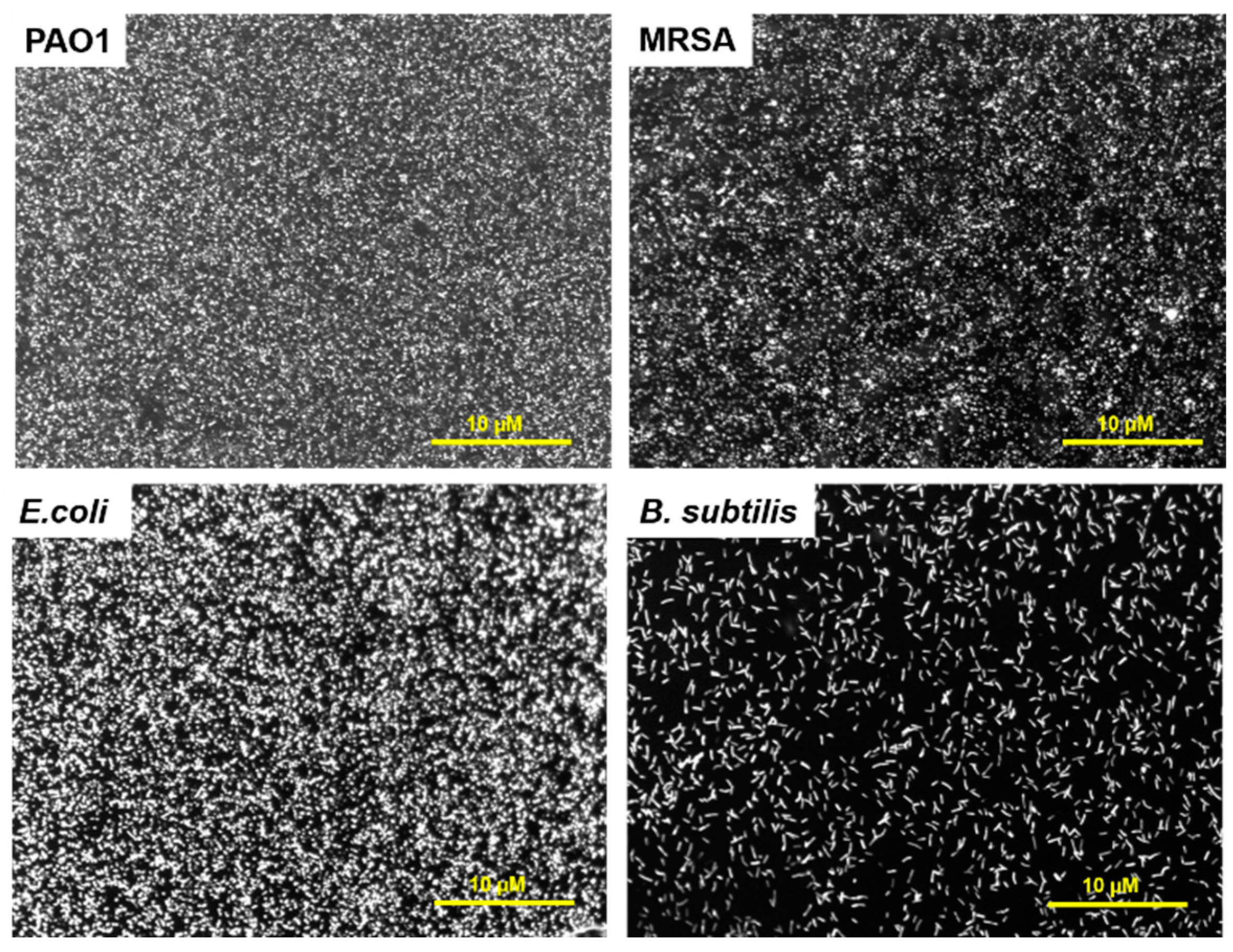
2.2.4. Florescence Microscopy
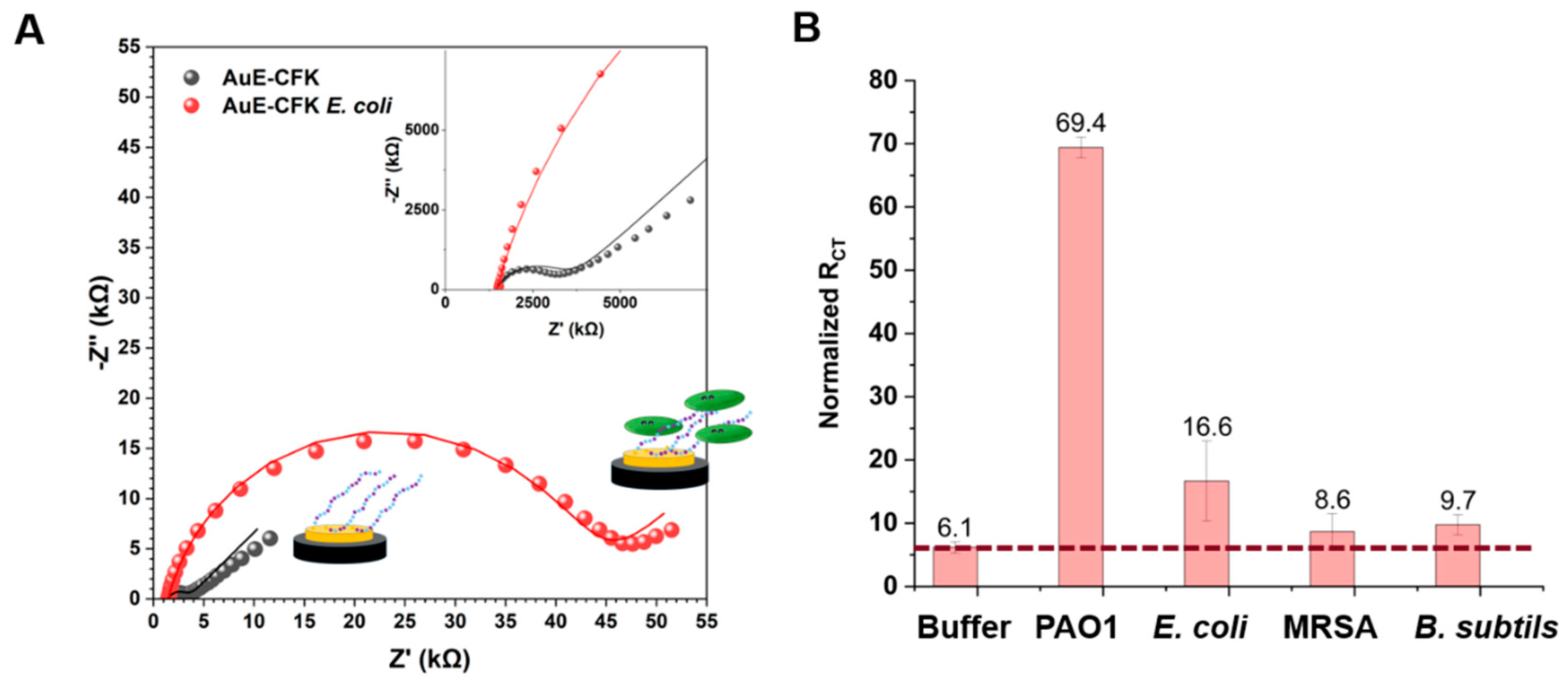
2.2.5. Gold Electrodes’ Surface Modifications and Impedimetric Sensing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of Multi-Drug Resistant Bacteria on Economic and Clinical Outcomes of Healthcare-Associated Infections in Adults: Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Rello, J.; Blasi, F.; Goossens, H.; Sotgiu, G.; Tavoschi, L.; Zasowski, E.J.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. Systematic Review of the Impact of Appropriate versus Inappropriate Initial Antibiotic Therapy on Outcomes of Patients with Severe Bacterial Infections. Int. J. Antimicrob. Agents 2020, 56, 106184. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A Review of Methods for the Detection of Pathogenic Microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Riu, J.; Giussani, B. Electrochemical Biosensors for the Detection of Pathogenic Bacteria in Food. TrAC-Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2018, 119, 700–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Z.; Ying, Y. New Trends in Impedimetric Biosensors for the Detection of Foodborne Pathogenic Bacteria. Sensors 2012, 12, 3449–3471. [Google Scholar] [CrossRef] [Green Version]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The Role of Peptides in the Design of Electrochemical Biosensors for Clinical Diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F. Self-Assembled Alkanethiol Monolayers on Gold Surfaces: Resolving the Complex Structure at the Interface. Phys. Chem. Chem. Phys. 2014, 16, 19074–19090. [Google Scholar] [CrossRef]
- Mandler, D.; Kraus-Ophir, S. Self-Assembled Monolayers (SAMs) for Electrochemical Sensing. J. Solid State Electrochem. 2011, 15, 1535–1558. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Venanzi, M. Self-Assembled Monolayers Formed by Helical Peptide Building Blocks: A New Tool for Bioinspired Nanotechnology. Polym. J. 2013, 45, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Hayouka, Z.; Chakraborty, S.; Liu, R.; Boersma, M.D.; Weisblum, B.; Gellman, S.H. Interplay among Subunit Identity, Subunit Proportion, Chain Length, and Stereochemistry in the Activity Profile of Sequence-Random Peptide Mixtures. J. Am. Chem. Soc. 2013, 135, 11748–11751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, T.; Zelinger, E.; Hayouka, Z.; Boersma, M.D.; Weisblum, B.; Gellman, S.H.; Welch, R.A.; Weisblum, B.; Masters, K.S.; Gellman, S.H.; et al. Random Peptide Mixtures Inhibit and Eradicate Methicillin-Resistant Staphylococcus Aureus Biofilms. Chem. Commun. 2016, 52, 7102–7105. [Google Scholar] [CrossRef] [PubMed]
- Hayouka, Z.; Bella, A.; Stern, T.; Ray, S.; Jiang, H.; Grovenor, C.R.M.M.; Ryadnov, M.G. Binary Encoding of Random Peptide Sequences for Selective and Differential Antimicrobial Mechanisms. Angew. Chem. 2017, 129, 8211–8215. [Google Scholar] [CrossRef]
- Topman, S.; Tamir-ariel, D.; Bochnic-, H.; Bauer, T.S.; Sha, S. Random Peptide Mixtures as New Crop Protection Agents. Microb. Biotechnol. 2018, 11, 1027–1036. [Google Scholar] [CrossRef]
- Topman-Rakover, S.; Malach, E.; Burdman, S.; Hayouka, Z. Antibacterial Lipo-Random Peptide Mixtures Exhibit High Selectivity and Synergistic Interactions. Chem. Commun. 2020, 56, 12053–12056. [Google Scholar] [CrossRef]
- Bauer Stern, T.; Hayouka, Z. Random Mixtures of Antimicrobial Peptides Inhibit Bacteria Associated with Pasteurized Bovine Milk. J. Pept. Sci. 2018, 24, e3088. [Google Scholar] [CrossRef]
- Cheriker, H.; Stern Bauer, T.; Oren, Y.; Nir, S.; Hayouka, Z. Immobilized Random Peptide Mixtures Exhibit Broad Antimicrobial Activity with High Selectivity. Chem. Commun. 2020, 56, 11022–11025. [Google Scholar] [CrossRef]
- Bennett, R.C.; Oh, M.W.; Kuo, S.H.; Belo, Y.; Maron, B.; Malach, E.; Lin, J.; Hayouka, Z.; Lau, G.W. Random Peptide Mixtures as Safe and Effective Antimicrobials against Pseudomonas Aeruginosa and MRSA in Mouse Models of Bacteremia and Pneumonia. ACS Infect. Dis. 2021, 7, 672–680. [Google Scholar] [CrossRef]
- Caraway, H.E.; Lau, J.Z.; Maron, B.; Oh, M.W.; Belo, Y.; Brill, A.; Malach, E.; Ismail, N.; Hayouka, Z.; Lau, G.W. Antimicrobial Random Peptide Mixtures Eradicate Acinetobacter Baumannii Biofilms and Inhibit Mouse Models of Infection. Antibiotics 2022, 11, 413. [Google Scholar] [CrossRef]
- Weiss, E.A.; Kaufman, G.K.; Kriebel, J.K.; Li, Z.; Schalek, R.; Whitesides, G.M. Si/SiO2-Templated Formation of Ultraflat Metal Surfaces on Glass, Polymer, and Solder Supports: Their Use as Substrates for Self-Assembled Monolayers. Langmuir 2007, 23, 9686–9694. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.M.; Popenoe, D.D.; Deinhammer, R.S.; Lamp, B.D.; Chung, C.; Porter, M.D. Reductive Desorption of Alkanethiolate Monolayers at Gold: A Measure of Surface Coverage. Langmuir 1991, 7, 2687–2693. [Google Scholar] [CrossRef]
- Widrig, C.A.; Chung, C.; Porter, M.D. The Electrochemical Desorption of N-Alkanethiol Monolayers from Polycrystalline Au and Ag Electrodes. J. Electroanal. Chem. 1991, 310, 335–359. [Google Scholar] [CrossRef]
- Imabayashi, S.I.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive Desorption of Carboxylic-Acid-Terminated Alkanethiol Monolayers from Au(111) Surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar] [CrossRef]
- Joshi, P.N.; Mervinetsky, E.; Solomon, O.; Chen, Y.J.; Yitzchaik, S.; Friedler, A. Electrochemical Biosensors Based on Peptide-Kinase Interactions at the Kinase Docking Site. Biosens. Bioelectron. 2022, 207, 114177. [Google Scholar] [CrossRef]
- Shamsi, F.; Coster, H.; Jolliffe, K.A. Characterization of Peptide Immobilization on an Acetylene Terminated Surface via Click Chemistry. Surf. Sci. 2011, 605, 1763–1770. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-Ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-Ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 7463, 5083–5086. [Google Scholar] [CrossRef]
- de la Llave, E.; Clarenc, R.; Schiffrin, D.J.; Williams, F.J. Organization of alkane amines on a gold surface: Structure, surface dipole, and electron transfer. J. Phys. Chem. C 2014, 118, 468–475. [Google Scholar] [CrossRef]
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization Reduces the Activity of Surface-Bound Cationic Antimicrobial Peptides with No Influence upon the Activity Spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Kanyong, P.; Davis, J.J. Homogeneous Functional Self-Assembled Monolayers: Faradaic Impedance Baseline Signal Drift Suppression for High-Sensitivity Immunosensing of C-Reactive Protein. J. Electroanal. Chem. 2020, 856, 113675. [Google Scholar] [CrossRef]
- Xu, X.; Makaraviciute, A.; Kumar, S.; Wen, C.; Sjödin, M.; Abdurakhmanov, E. Zhang, Z. Structural Changes of Mercaptohexanol Self-Assembled Monolayers on Gold and Their Influence on Impedimetric Aptamer Sensors. Anal. Chem. 2019, 91, 14697–14704. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia Coli, Pseudomonas Aeruginosa, and Staphylococcus Aureus Attachment Patterns on Glass Surfaces with Nanoscale Roughness. Curr. Microbiol. 2009, 58, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in Surface Properties between Escherichia Coli and Staphylococcus Aureus as Revealed by Electrophoretic Mobility Measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.G. Composition and Structure of Lipopolysaccharides from Pseudomonas Aeruginosa. Rev. Infect. Dis. 1983, 5, S941–S949. [Google Scholar] [CrossRef]
- Epand, R.F.; Savage, P.B.; Epand, R.M. Bacterial Lipid Composition and the Antimicrobial Efficacy of Cationic Steroid Compounds (Ceragenins). Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2500–2509. [Google Scholar] [CrossRef]
- McGroarty, E.J. Lipopolysaccharide and Capsular Polysaccharide. In Pseudomonas. Biotechnology Handbooks; Monties, T.C., Ed.; Springer: Boston, MA, USA, 1998; pp. 73–110. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern Bauer, T.; Yakobi, R.; Hurevich, M.; Yitzchaik, S.; Hayouka, Z. Impedimetric Bacterial Detection Using Random Antimicrobial Peptide Mixtures. Sensors 2023, 23, 561. https://doi.org/10.3390/s23020561
Stern Bauer T, Yakobi R, Hurevich M, Yitzchaik S, Hayouka Z. Impedimetric Bacterial Detection Using Random Antimicrobial Peptide Mixtures. Sensors. 2023; 23(2):561. https://doi.org/10.3390/s23020561
Chicago/Turabian StyleStern Bauer, Tal, Ravit Yakobi, Mattan Hurevich, Shlomo Yitzchaik, and Zvi Hayouka. 2023. "Impedimetric Bacterial Detection Using Random Antimicrobial Peptide Mixtures" Sensors 23, no. 2: 561. https://doi.org/10.3390/s23020561




