Ultrasensitive Detection of Interleukin 6 by Using Silicon Nanowire Field-Effect Transistors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Instrumentation
2.3. Chemical Modification of the SiNW-FET Decive
2.4. Immobilization of Anti-IL-6 Antibody or Aptamer
2.5. SiNW-FET Measurements
2.6. Preparation of AFM and XPS Samples
3. Results
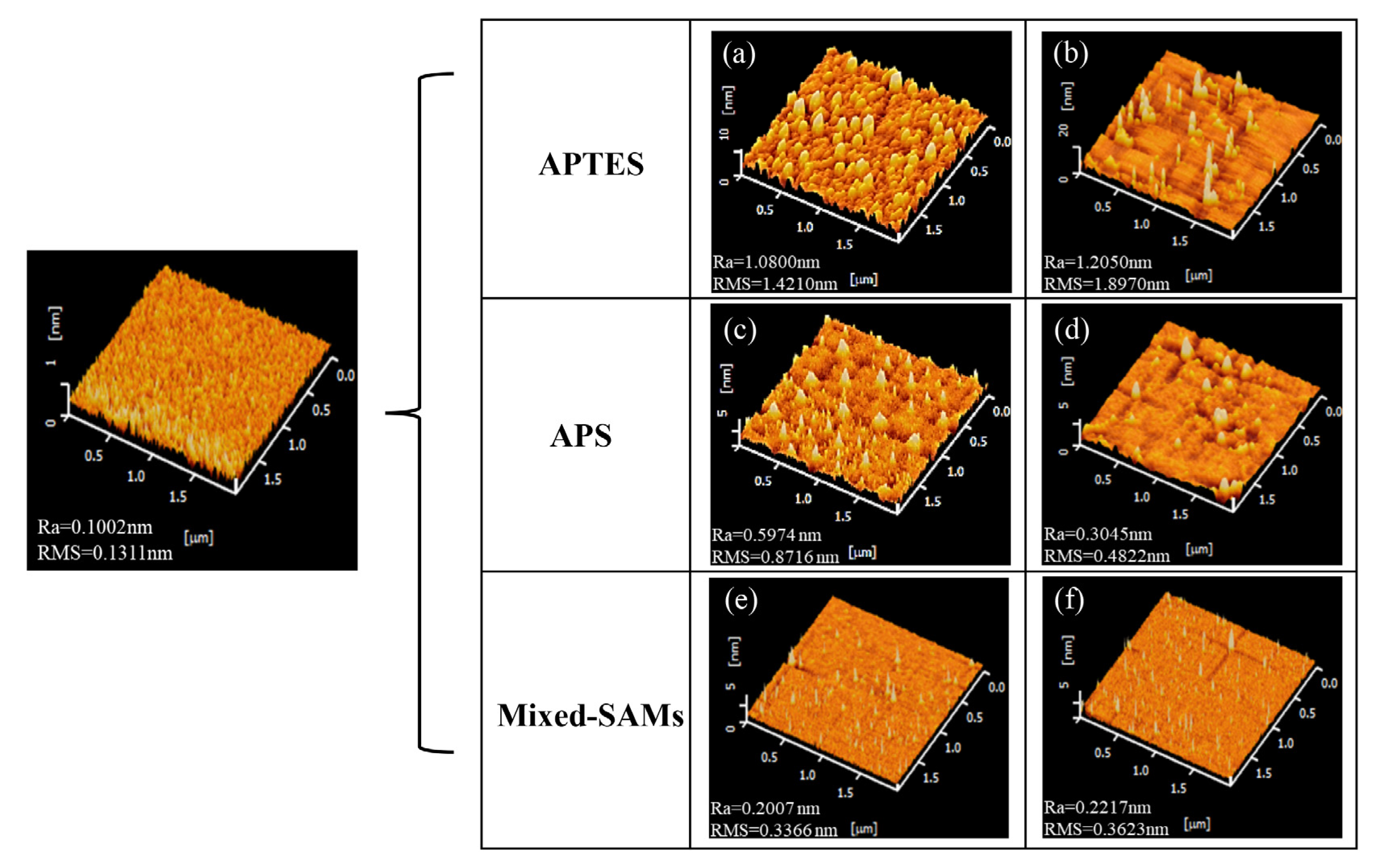
3.1. Morphology of Modified Silica Surfaces
3.2. Morphology and Characteristics of the Device Surfaces with Antibodies or Aptamers
3.3. Detection of IL-6 with the Antibody
3.4. Detection of IL-6 with the Aptamer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, L.J.; Garner, L.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi-Hafshejani, P.; Azam, N.; Wang, L.; Kuroda, M.A.; Hamilton, M.C.; Hasim, S.; Mahjouri-Samani, M. Two-Dimensional-Material-Based Field-Effect Transistor Biosensor for Detecting COVID-19 Virus (SARS-CoV-2). ACS Nano 2021, 15, 11461–11469. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT Image Visual Quantitative Evaluation and Clinical Classification of Coronavirus Disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgulchik, N.; Athanasopoulou, F.; Chu, E.; Lam, Y.; Kamaly, N. Potential Therapeutic Approaches for Targeted Inhibition of Inflammatory Cytokines Following COVID-19 Infection-Induced Cytokine Storm. Interface Focus 2022, 12, 20210006. [Google Scholar] [CrossRef] [PubMed]
- Santhamani, R.; Selvakumar, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar]
- Huang, K.-J.; Su, I.-J.; Theron, M.; Wu, Y.-C.; Lai, S.-K.; Liu, C.-C.; Lei, H.-Y. An Interferon-γ-Related Cytokine Storm in SARS Patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Gouel-Chéron, A.; Allaouchiche, B.; Guignant, C.; Davin, F.; Floccard, B.; Monneret, G.; Group, A. Early Interleukin-6 and Slope of Monocyte Human Leukocyte Antigen-DR: A Powerful Association to Predict the Development of Sepsis after Major Trauma. PLoS ONE 2012, 7, e33095. [Google Scholar] [CrossRef] [Green Version]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The Role of IL-6 and Other Mediators in the Cytokine Storm Associated with SARS-CoV-2 Infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated Levels of IL-6 and CRP Predict the Need for Mechanical Ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a Potential Biomarker of COVID-19 Progression. Med. Mal. Infect. 2020, 50, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Jafrin, S.; Aziz, M.A.; Islam, M.S. Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) Are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies. Biomark. Insights 2022, 17, 11772719221106600. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, W.; Liang, J.; Wang, L.; Yu, X.; Bao, M.; Liu, H. Association of Interleukin-6 Levels with Morbidity and Mortality in Patients with Coronavirus Disease 2019 (COVID-19). Jpn. J. Infect. Dis. 2021, 74, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Mujahid, M. Recent Advances in Electrochemical and Optical Biosensors Designed for Detection of Interleukin 6. Sensors 2020, 20, 646. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Wang, S.; Jin, H.; Bao, W.; Huang, S.; Wang, J. An Electrochemical Impedance Sensor for the Label-Free Ultrasensitive Detection of Interleukin-6 Antigen. Sens. Actuators B Chem. 2013, 178, 310–315. [Google Scholar] [CrossRef]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-Free Electrochemical Aptasensor Based on Gold and Polypyrrole Nanoparticles for Interleukin 6 Detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Li, Q.; Song, L.; Gopinath, S.C.B.; Wu, D. An Interdigitated Aptasensor to Detect Interleukin-6 for Diagnosing Rheumatoid Arthritis in Serum. Biotechnol. Appl. Biochem. 2021, 68, 1479–1485. [Google Scholar] [CrossRef]
- Spiridonova, V.A.; Novikova, T.M.; Snigirev, O. V Obtaining DNA Aptamers to Human Interleukin-6 for Biomagnetic Immunoassay Nanosensors. Mosc. Univ. Phys. Bull. 2016, 71, 135–138. [Google Scholar] [CrossRef]
- Vu, C.-A.; Chen, W.-Y.; Yang, Y.-S.; Chan, H.W.-H. Improved Biomarker Quantification of Silicon Nanowire Field-Effect Transistor Immunosensors with Signal Enhancement by RNA Aptamer: Amyloid Beta as a Case Study. Sens Actuators B Chem. 2021, 329, 129150. [Google Scholar] [CrossRef]
- Vu, C.-A.; Hu, W.-P.; Yang, Y.-S.; Chan, H.W.-H.; Chen, W.-Y. Signal Enhancement of Silicon Nanowire Field-Effect Transistor Immunosensors by RNA Aptamer. ACS Omega 2019, 4, 14765–14771. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Hu, W.P.; Yang, Y.S.; Chan, H.W.H.; Chen, W.Y. Neutralized Chimeric DNA Probe for the Improvement of GC-Rich RNA Detection Specificity on the Nanowire Field-Effect Transistor. Sci. Rep. 2019, 9, 11056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.-P.; Tsai, C.-C.; Yang, Y.-S.; Chan, H.W.-H.; Chen, W.-Y. Synergetic Improvements of Sensitivity and Specificity of Nanowire Field Effect Transistor Gene Chip by Designing Neutralized DNA as Probe. Sci. Rep. 2018, 8, 12598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.H.; Liu, M.; Nolting, B.; Go, J.G.; Gervay-Hague, J.; Liu, G. A Nanoengineering Approach for Investigation and Regulation of Protein Immobilization. ACS Nano 2008, 2, 2374–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, A.K. OligoCalc: An Online Oligonucleotide Properties Calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar]
- Javier, D.J.; Nitin, N.; Levy, M.; Ellington, A.; Richards-Kortum, R. Aptamer-Targeted Gold Nanoparticles as Molecular-Specific Contrast Agents for Reflectance Imaging. Bioconjug. Chem. 2008, 19, 1309–1312. [Google Scholar] [CrossRef] [Green Version]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Thiviyanathan, V.; Gorenstein, D.G. Aptamers and the Next Generation of Diagnostic Reagents. Proteom. Clin. Appl. 2012, 6, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Chandola, C. Aptamers for Targeted Delivery: Current Challenges and Future Opportunities. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; Tyagi, R.K., Garg, N., Shukla, R., Bisen, P.S., Eds.; IntechOpen: London, UK, 2019; pp. 1–22. [Google Scholar]
- Zhu, M.; Lerum, M.Z.; Chen, W. How to Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Nikonov, A.M.; Naumova, O.V.; Generalov, V.M.; Safatov, A.S.; Fomin, B.I. Surface Preparation as a Step in the Fabrication of Biosensors Based on Silicon Nanowire Field-Effect Transistors: Review. J. Surf. Investig. X-ray Synchrotron Neutron Technol. 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Shlyakhtenko, L.S.; Gall, A.A.; Filonov, A.; Cerovac, Z.; Lushnikov, A.; Lyubchenko, Y.L. Silatrane-Based Surface Chemistry for Immobilization of DNA, Protein-DNA Complexes and Other Biological Materials. Ultramicroscopy 2003, 97, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lyubchenko, Y.L.; Shlyakhtenko, L.S.; Ando, T. Imaging of Nucleic Acids with Atomic Force Microscopy. Methods 2011, 54, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Shlyakhtenko, L.S.; Gall, A.A.; Lyubchenko, Y.L. Mica Functionalization for Imaging of DNA and Protein-DNA Complexes with Atomic Force Microscopy. In Cell Imaging Techniques: Methods and Protocols; Taatjes, D.J., Roth, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 295–312. [Google Scholar]
- Haustein, N.; Gutiérrez-Sanz, Ó.; Tarasov, A. Analytical Model to Describe the Effect of Polyethylene Glycol on Ionic Screening of Analyte Charges in Transistor-Based Immunosensing. ACS Sens. 2019, 4, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.M. General Strategy for Biodetection in High Ionic Strength Solutions Using Transistor-Based Nanoelectronic Sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, N.; Gao, T.; Yang, X.; Dai, X.; Zhou, W.; Zhang, A.; Lieber, C.M. Specific Detection of Biomolecules in Physiological Solutions Using Graphene Transistor Biosensors. Proc. Natl. Acad. Sci. USA 2016, 113, 14633–14638. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.-P.; Wu, Y.-M.; Vu, C.-A.; Chen, W.-Y. Ultrasensitive Detection of Interleukin 6 by Using Silicon Nanowire Field-Effect Transistors. Sensors 2023, 23, 625. https://doi.org/10.3390/s23020625
Hu W-P, Wu Y-M, Vu C-A, Chen W-Y. Ultrasensitive Detection of Interleukin 6 by Using Silicon Nanowire Field-Effect Transistors. Sensors. 2023; 23(2):625. https://doi.org/10.3390/s23020625
Chicago/Turabian StyleHu, Wen-Pin, Yu-Ming Wu, Cao-An Vu, and Wen-Yih Chen. 2023. "Ultrasensitive Detection of Interleukin 6 by Using Silicon Nanowire Field-Effect Transistors" Sensors 23, no. 2: 625. https://doi.org/10.3390/s23020625
APA StyleHu, W.-P., Wu, Y.-M., Vu, C.-A., & Chen, W.-Y. (2023). Ultrasensitive Detection of Interleukin 6 by Using Silicon Nanowire Field-Effect Transistors. Sensors, 23(2), 625. https://doi.org/10.3390/s23020625







