The Dependence of the Burning Process and Ignition Temperature of a Lithium Cell on Its State of Charge
Abstract
:1. Introduction
2. Materials and Methods
- Possibly full thermal insulation from the environment of the cell subject to testing;
- Accurate measurement of cell temperature in a wide range;
- Parallel cell temperature measurements by two different measurement systems and sensors;
- Temperature measurements of cooling agents and elements of the measuring block;
- Mass measurements of the measuring block elements before and after the next test;
- Registration of all parameters measured automatically with saving to .xlsx files;
- High accuracy of measurements of both electrical parameters and temperatures, due to the use of high-quality measurement LabView modules;
- Averaging a series of measurements, e.g., 100 with a frequency of 10 kHz and accepting the result as a point measurement in order to eliminate noise;
- The ability to charge and unload the cells in any way due to the construction of one’s own virtual devices;
- High repeatability of the test run.
2.1. Test Stand
2.2. Virtual Instruments
- NI-9239—4 Channel Voltage Input Module 0–5 V resolution-24 bit.
- NI-9217—4 Channel Temperature Input Module RTD resolution-24 bit.
- NI-9227—4 Channel Current Input Module 0–5 A resolution-24 bit.
- The VI1 cell charging system, which was equipped with a 4-channel voltage and current measurement with current recording in all channels separately. The VI1 system had the ability to connect and disconnect a 4-channel power supply with current and voltage stabilization in each channel. The virtual device calculated the products of the current values of current and voltage, i.e., the instantaneous charging power. Multiplying the power by the length of the time quantum between measurements and adding them up resulted in the calculation of the amount of energy transferred to the cells during charging. The registration in the file included the time from the start of the measurements, voltage, current and energy, which allowed the charging characteristics of the cells to be found.
- The VI2 cell discharge system was designed to discharge the cell in a controlled way, so as to leave the desired part of the full charge in it. After giving up the assumed amount of energy, the cell was disconnected from the load. Another criterion for the completion of discharging was the assumed voltage value to which the cell was discharged. In this way, it was possible to precisely charge and discharge cells to the required SOC. Unlike with factory devices, it was possible to bypass the protections that are built into the automatic chargers’ software, such as by exceeding the maximum charging voltage or discharging the cell voltage to zero, i.e., below the level allowed by the manufacturer.
- Process recording system VI3. The purpose of building this system was to simultaneously register the state of the measuring module and the amount of energy transferred to the cell module. In order to reduce the number of modules in the cassette, an indirect method of current measurement was used during the measurements by using a resistor connected in series to the heater power supply system. This made it possible to eliminate the current measurement module from this virtual device. The current value was obtained by measuring the voltage across a resistor treated as a current shunt. The voltage was taken from the heater terminals. The product of the voltage determined in this way and the current was the instantaneous power transferred to the heater. The temperature module made it possible to determine the temperature in four points of the measuring block of the station.
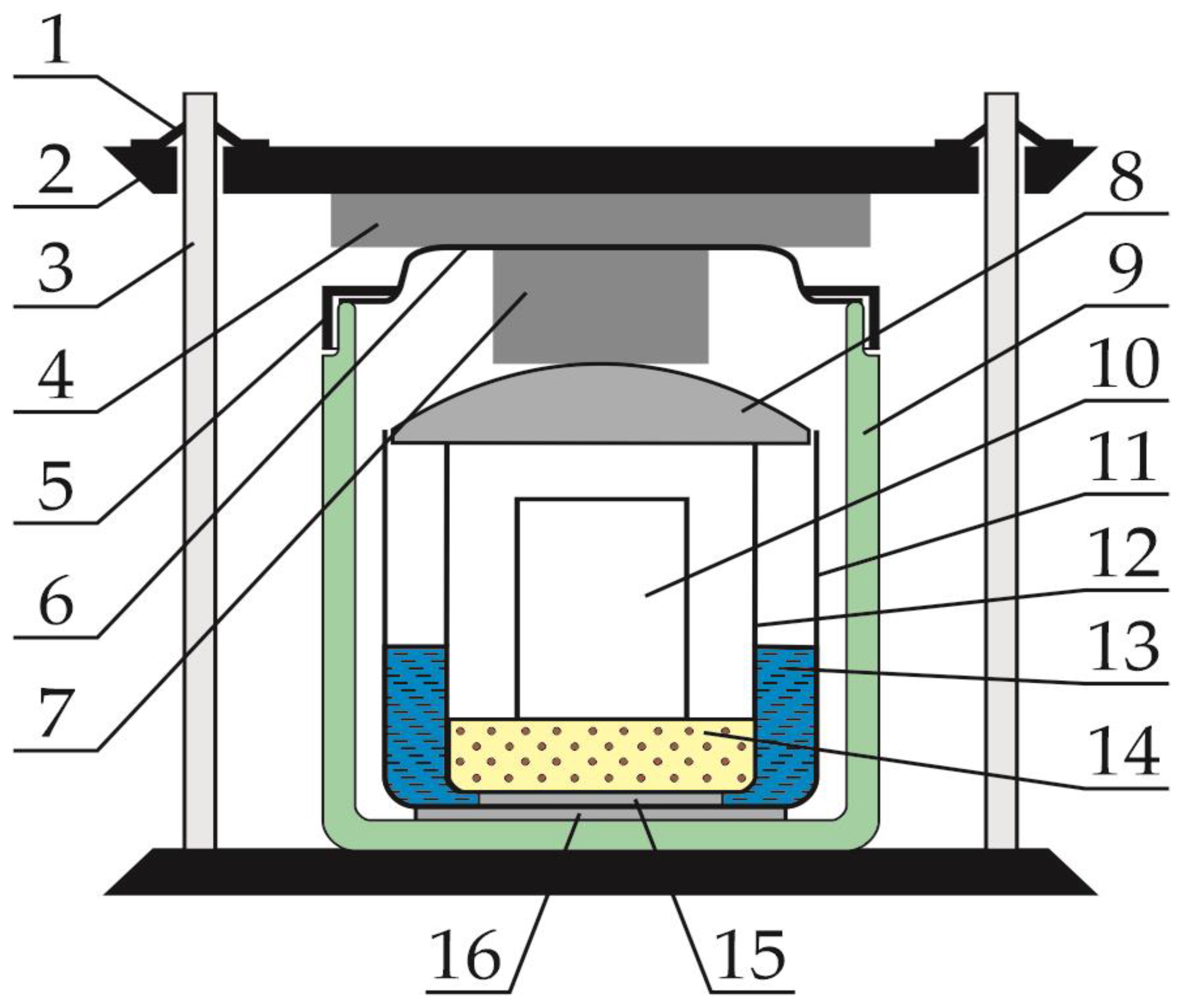
2.3. Measuring Block
2.4. Course of Measurements
3. Results
3.1. Preliminary Studies
3.2. Examinations Using the Test Stand
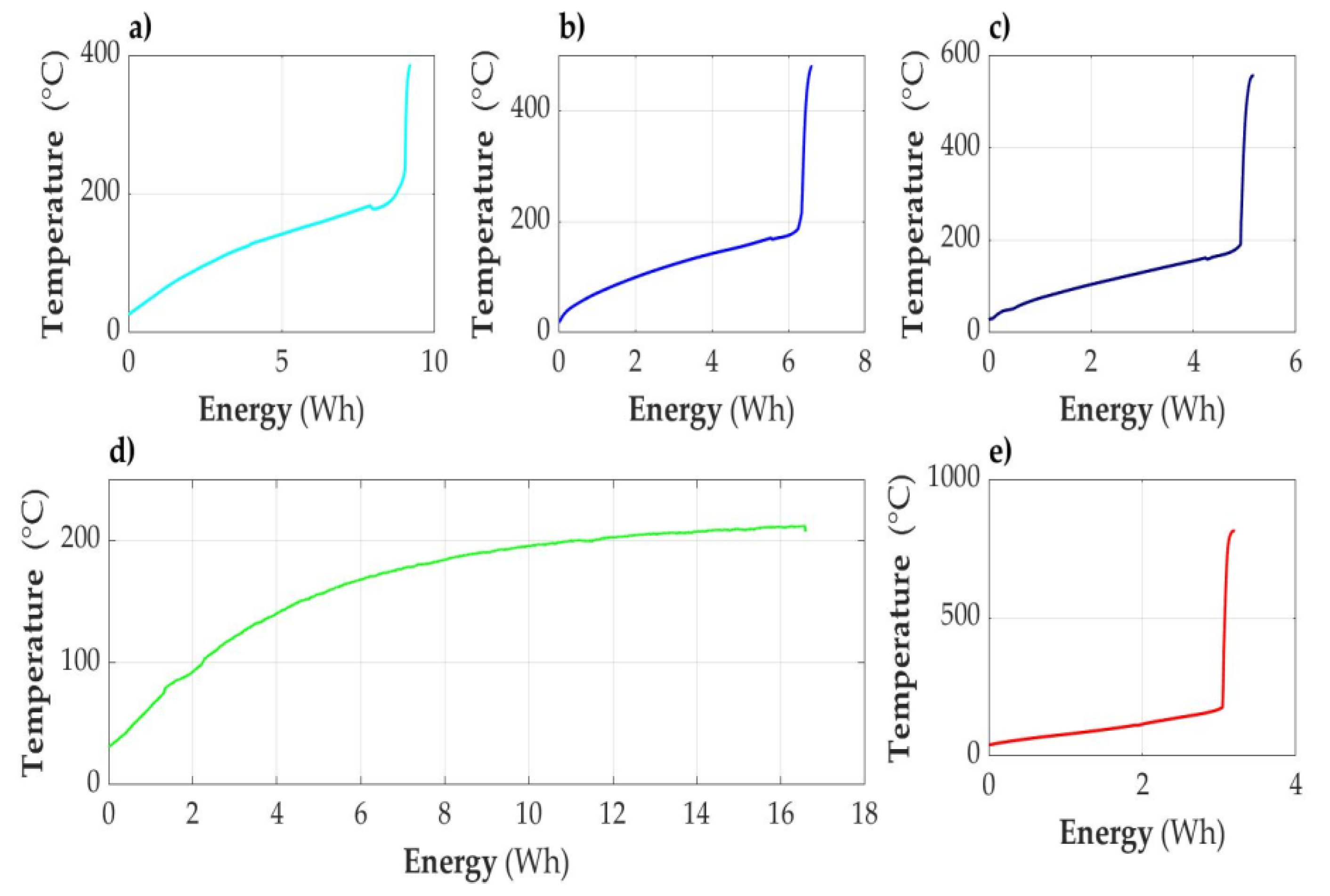
3.2.1. Acceptably Fully Discharged Cell
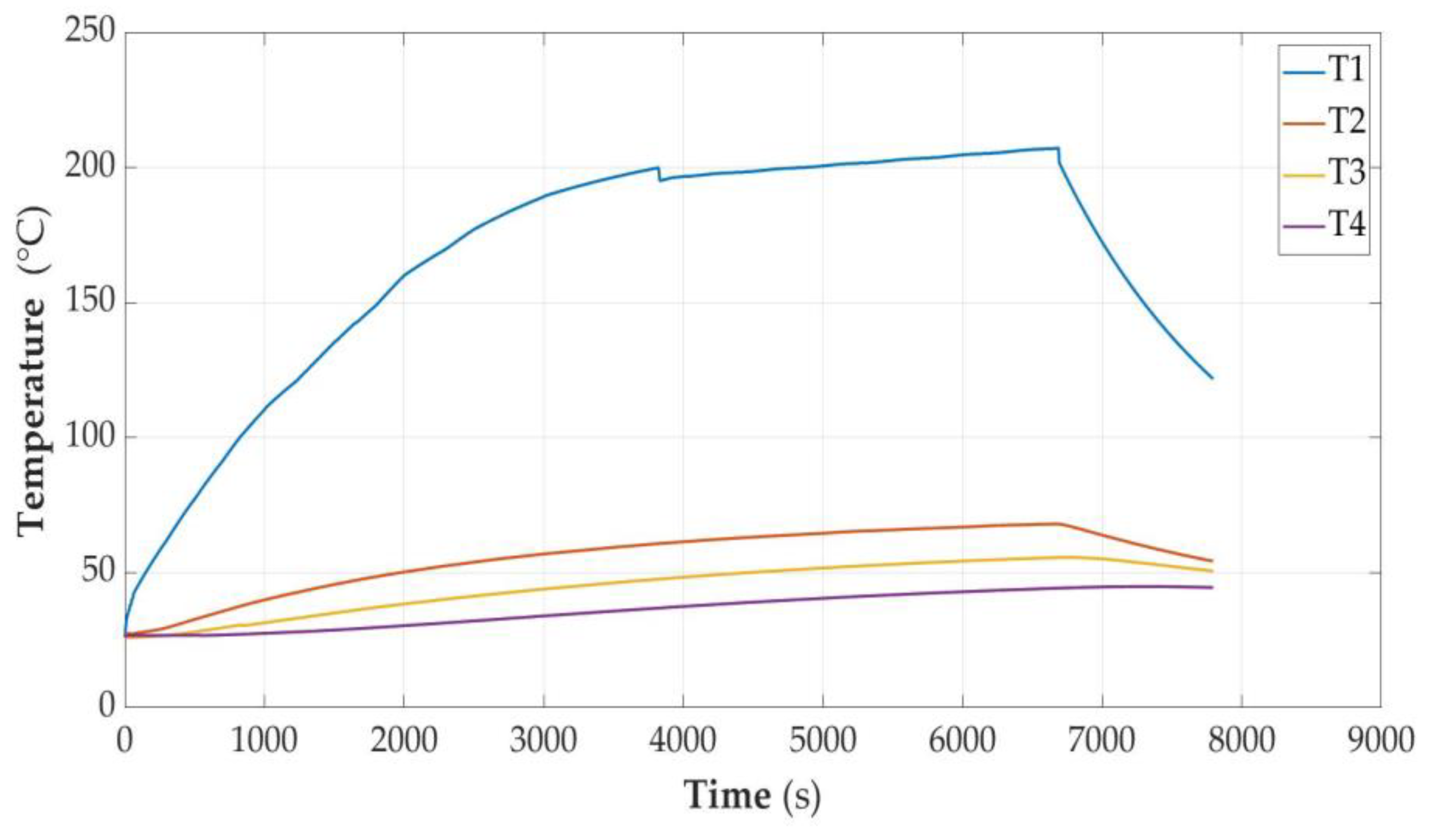
3.2.2. Cell Discharged to the State of Capacity SOC = 30%

3.2.3. Cell Partially Discharged (SOC = 50%)
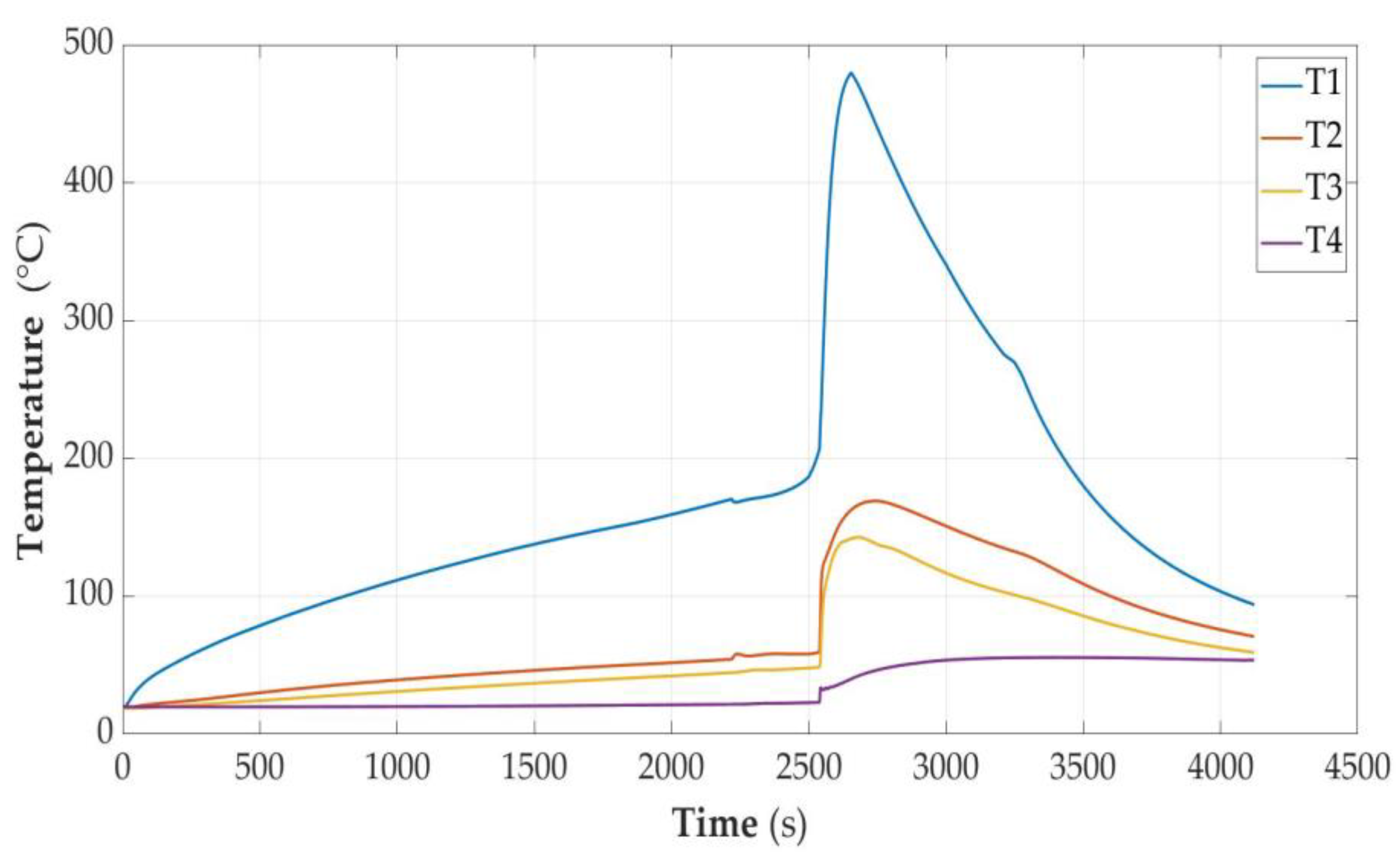
3.2.4. Cell Fully Charged (SOC = 100%)

3.2.5. Overcharged Cell (SOC > 100%)
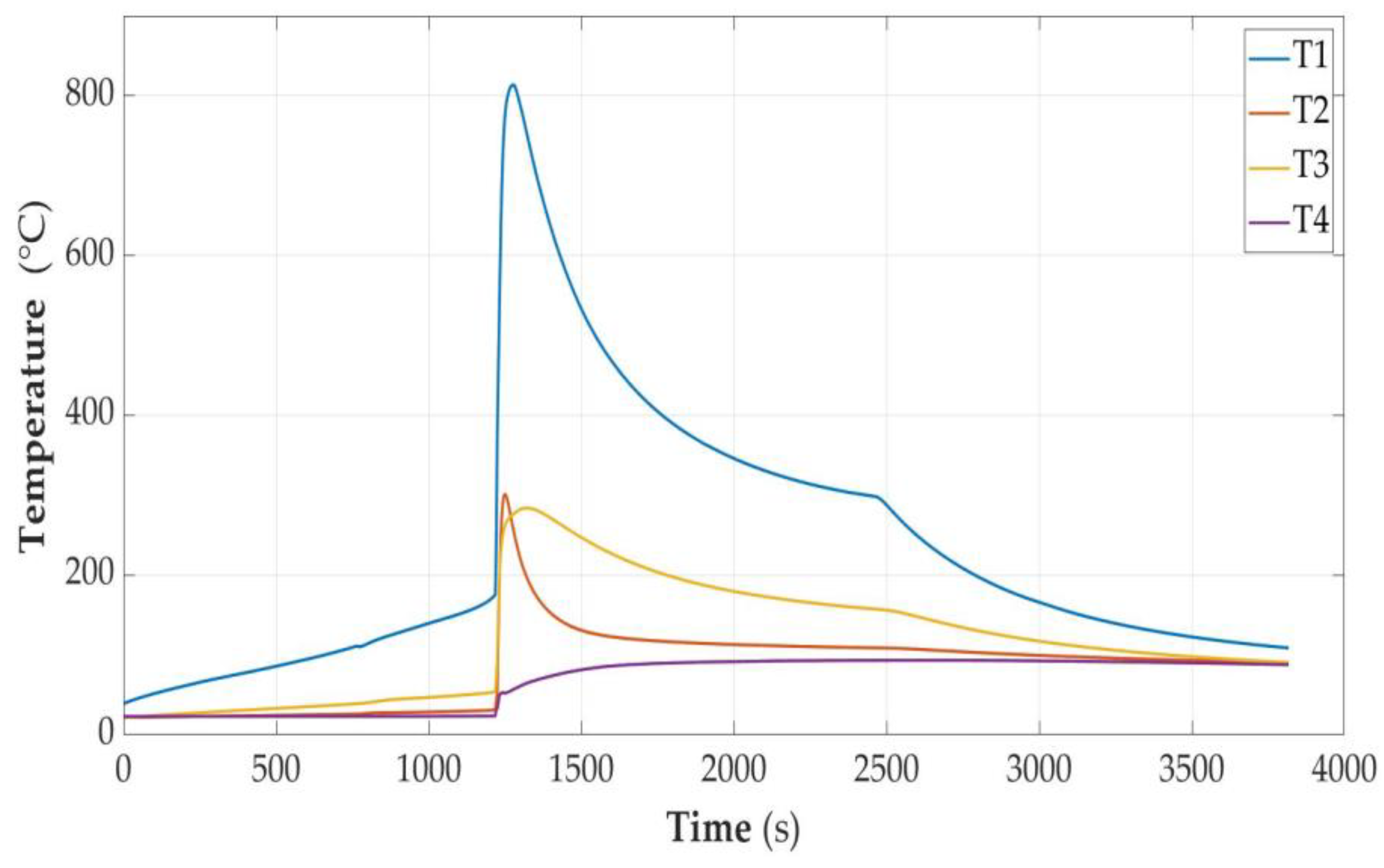
3.2.6. Cell Fully Discharged (SOC < 0%)
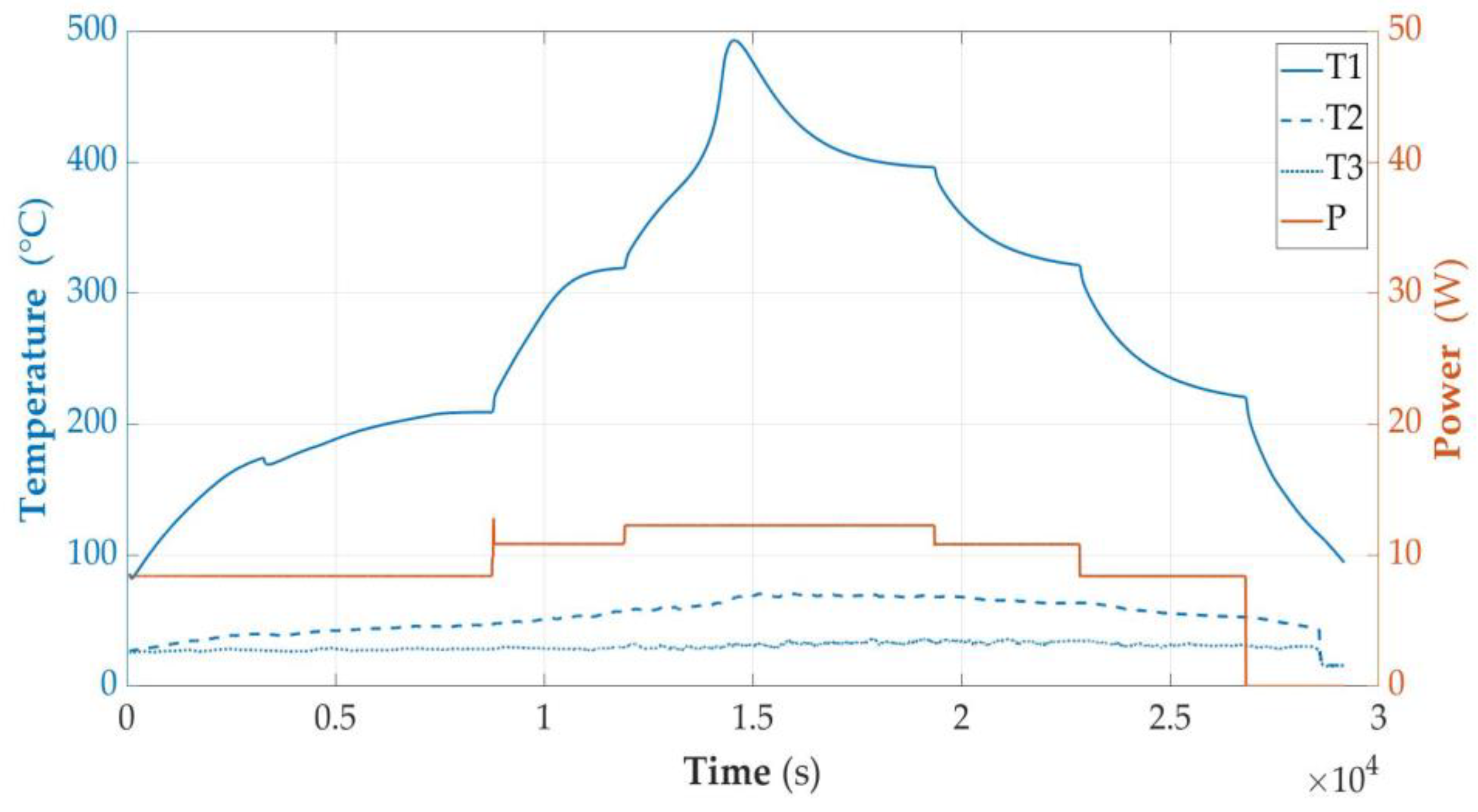
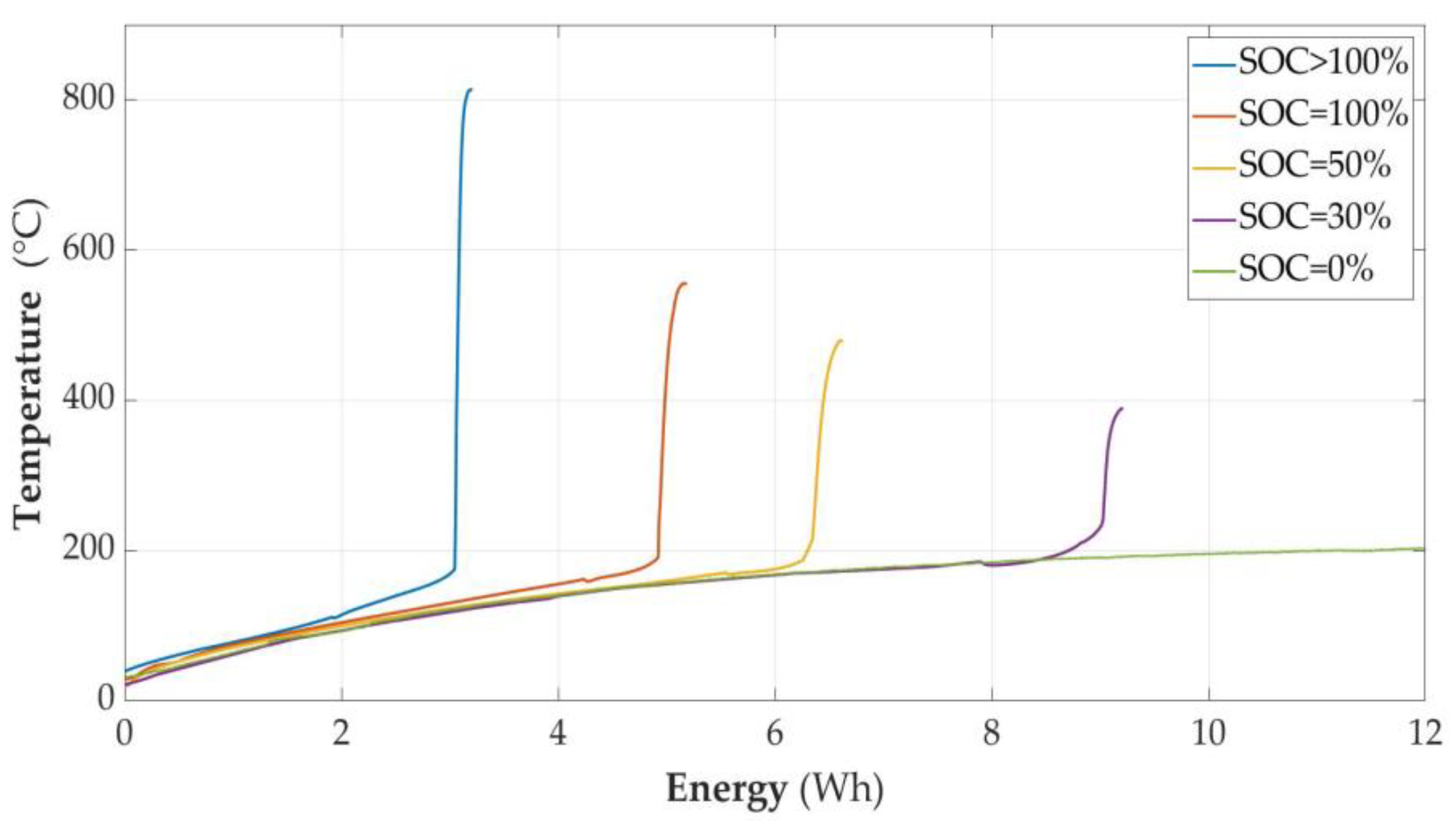
3.2.7. Temperature of Cell as a Function of Energy (E) Supplied to the Cell Module
3.2.8. Estimation of the Amount of Energy Released by the Cell in the Developed Phase of Thermal Runaway
- Qsand—in the sand;
- Qwat—in the water;
- Qair—in the air contained in the inner vessel;
- Qcov—in the cover (outer cover);
- Qinl—at the bottom of the inner vessel;
- Qinu—in the upper part of the inner vessel;
- Qimv—in an intermediate vessel;
- Qmcell—in the remains of the link.
- Qsum—Total heat equivalent to the energy released during the combustion of the cell.
- Esum -Total energy resulting from the conversion of heat Qsum in J to Wh.
- mi—is the mass of the component;
- Tli—is the temperature at the beginning of the avalanche of a given component;
- Thi—is the temperature at the end of energy transfer;
- hi—is the specific heat of component i.
4. Discussion
- It was experimentally proven that both the ignition parameters and the course of the cell fire depend on the state of charge, as shown in Table 4. The results related to the time of heating with constant power were characterized in the function delivered to the energy cell module. According to the authors, this is a more universal form of presentation. Another result is that overcharging the cell makes it much more susceptible to ignition, and the fire of this cell is much more violent. The temperature reached is of the order of 813 degrees Celsius. The measured parameters of the unsealing point as a function of Energy are also presented in Table 4.
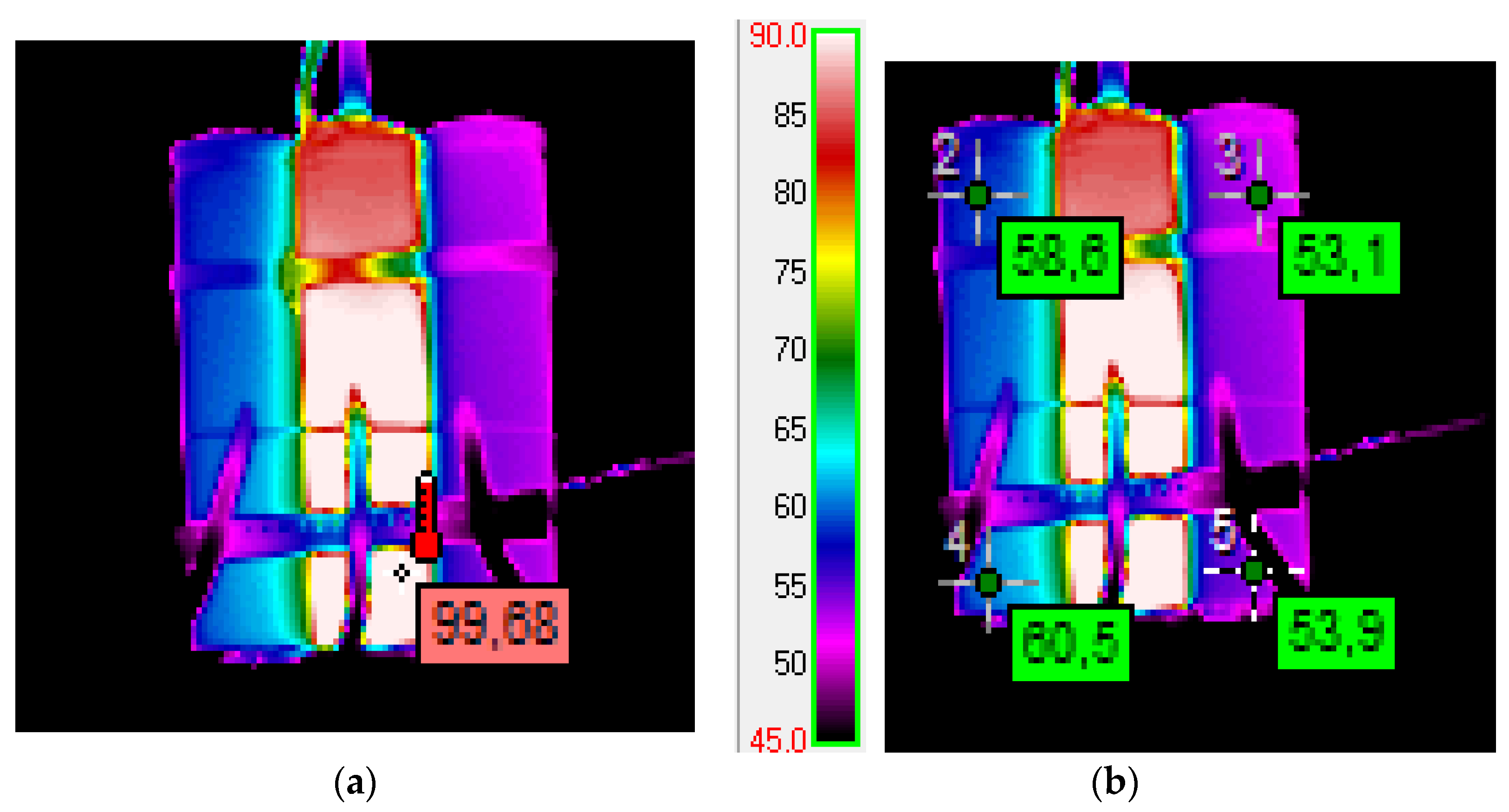
- This table also contains a summary of the maximum temperatures that occurred on the cell wall during the measurements. However, it can be assumed that the temperature that occurred at the outlet of the cell in the flue gas stream was even higher due to the traces of sputtered copper on the glass cover described in this work and the lack of copper in the remains of the burnt cell in the case where the SOC = 100% and the overcharged case.
- It was found that the higher the charge level, the less energy triggers the ignition of the cell. It is worth paying attention to the fact that the temperature differences at which the cells were unsealed were not very large, but the amount of energy causing the unsealing differed significantly. As mentioned before, the energy shown in the graphs is not exactly the value delivered to the cell, but the value delivered to the cell module. Therefore, it represents the energy supplied to the cell plus the energy losses in the elements included in the cell module. Despite this simplification, due to the repeatability of the experiments, it is possible to state that the amount of energy supplied to the cell causing its unsealing depends on the state of charge of the cell.
- Graphs of cell temperature changes over time were obtained with high accuracy and resolution, at different SOCs.
- The results of temperature measurements as a function of time were brought to dependence on the energy supplied to the cell. See Section 3.2.7.
- The amount of energy released from the cell during the thermal runaway process was also estimated at different SOCs. See Section 3.2.8 Table 3.
- A graph of temperature versus time was obtained for a model of a cell made of brass and placed in a cell module. Measurements were carried out under identical conditions to those in Section 3.2.1, Section 3.2.2, Section 3.2.3, Section 3.2.4, Section 3.2.5 and Section 3.2.6. In order to find a pattern enabling estimation of the size of external losses, a model of a brass cell was made, reflecting its shape and dimensions (the weight of the model was 176 g), and then heating was carried out as in the experiments described in Chapter 3. These calculations have not been performed, but the data may be helpful in the process of creating a simulation model. Temperature change graphs are shown in Figure A1 in Appendix A.
- Organoleptic descriptions of the cell residues at various SOC states were obtained. This is significant knowledge that is useful during post-fire tests, as it allows for a rough assessment of the state of charge of a burnt-out cell. This knowledge may be the basic premise in the event that the data recording system is also destroyed. In addition, current BMSs control the cell chains without recording parameters with an accuracy of one cell.
- The presented results were measured with a time resolution of 3 s of recorded data. However, they were measured every 0.1 ms and averaged. Measurement resolution in each channel was 24 bits, as in Section 3.2.1, Section 3.2.2, Section 3.2.3, Section 3.2.4, Section 3.2.5 and Section 3.2.6. The problem, however, was the escape of part of the flue gas in the tests for SOC = 100% and over 100%. In these cases, the estimates of the released energy are probably higher than those calculated and shown in Table 3. As has already been indicated in the text describing the method of estimating the amount of energy released, the assumption made about the temperature measured at points and that it applies to the entire volume of the medium is a significant simplification, despite even the relatively long time for temperature equalization. An acceptable solution is to create a simulation model for which the conducted tests can be input as data.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Newman, A. Global Battery Market and Future Prospects. In Proceedings of the Lithium Metal Battery Meeting, ARPA, Ionics Meeting, Dallas, TX, USA, 30–31 May 2019. [Google Scholar]
- Tarvydas, D.; Tsiropoulos, I.; Lebedeva, N. Li-ion Batteries for Mobility and Stationary Storage Applications: Scenarios for Costs and Market Growth; Publications Office of the European Union: Brussels, Belgium, 2018. [Google Scholar]
- McCoy, C.; Sriramulu, S.; Barnett, B. LI-Ion Battery Safety technologies and Their Implementation. In Proceedings of the 33rd Annual International Battery Seminar& Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Stephens, D.; Shawcross, P.; Stout, G.; Sullivan, E.; Saunders, J.; Risser, S.; Sayre, J. Lithium-Ion Battery Safety Issues for Electric and Plug-in Hybrid Vehicles; National Highway Traffic Safety Administration: Washington, DC, USA, 2017. [Google Scholar]
- Victor Chombo, P.; Laoonual, Y.; Wongwises, S. Lessons from the Electric Vehicle Crashworthiness Leading to Battery Fire. Energies 2021, 14, 4802. [Google Scholar] [CrossRef]
- Erd, A.; Stokłosa, J. Main design guidelines for battery management systems for traction purposes. In Proceedings of the 11th International Science and Technical Conference Automotive Safety, Papiernicka, Slovakia, 18–20 April 2018; pp. 1–5. [Google Scholar]
- Thomas, J.C.; Darst, J.J.; Darcy, E.C.; Finegan, D.P. Consequence Analysis of Li-Ion Battery Thermal Runaway Events with Chemical Equilibrium Analyses. In Proceedings of the NASA Aerospace Battery Workshop, Huntsville, AL, USA, 27–29 November 2018. [Google Scholar]
- Erd, A.; Stoklosa, J. Energy Dependencies in Li-Ion Cells and Their Influence on the Safety of Electric Motor vehicles and other Battery Pack. Energies 2020, 13, 6738. [Google Scholar] [CrossRef]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Zhang, M.; Zhu, L.; Chen, H.; Huang, W.; Yao, J.; Yuan, Z.; Xu, C.; Feng, X. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries 2022, 8, 201. [Google Scholar] [CrossRef]
- Erd, A.; Stoklosa, J. Failures of electronic systems and elements in electric vehicles and guidelines for reducing their intensity. In Proceedings of the 2019 Applications of Electromagnetics in Modern Engineering and Medicine (PTZE), Janów Podlaski, Poland, 9–12 June 2019; pp. 38–41. [Google Scholar]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Lee, C.; Said, A.O.; Stoliarov, S.I. Passive Mitigation of Thermal Runaway Propagation in Dense 18650 Lithium Ion Cell Assemblies. J. Electrochem. Soc. 2020, 9, 090524. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Cruceru, M.; Anghelescu, L. Phase Change Materials—Applications and Systems Designs: A Literature Review. Designs 2022, 6, 117. [Google Scholar] [CrossRef]
- Lawag, R.A.; Ali, H.M. Phase change materials for thermal management and energy storage: A review. J. Energy Storage 2022, 55, 105602. [Google Scholar] [CrossRef]
- Buidin, T.I.C.; Mariasiu, F. Battery Thermal Management Systems: Current Status and Design Approach of Cooling Technologies. Energies 2021, 14, 4879. [Google Scholar] [CrossRef]
- Zeng, W.; Niu, Y.; Li, S.; Hu, S.; Mao, B.; Zhang, Y. Cooling performance and optimization of a new hybrid thermal management system of cylindrical battery. Appl. Therm. Eng. 2022, 217, 119171. [Google Scholar] [CrossRef]
- Zahid, I.; Farooq, M.; Farhan, M.; Usman, M.; Qamar, A.; Imran, M.; Alqahtani, M.A.; Anwar, S.; Sultan, M.; Javaid, M.Y. Thermal Performance Analysis of Various Heat Sinks Based on Alumina NePCM for Passive Cooling of Electronic Components: An Experimental Study. Energies 2022, 15, 8416. [Google Scholar] [CrossRef]
- Sohel Murshed, S.M.; Nieto de Castro, C.A. A critical review of traditional and emerging techniques and fluids for electronics cooling. Renew. Sustain. Energy Rev. 2017, 78, 821–833. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, F.; Zhou, H.; Wang, Q.; Kong, J. Thermal performance of cylindrical lithium-ion battery thermal management system integrated with mini-channel liquid cooling and air cooling. Appl. Therm. Eng. 2020, 175, 115331. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Boonma, K.; Patimaporntap, N.; Mbulu, H.; Trinuruk, P.; Ruangjirakit, K.; Laoonual, Y.; Wongwises, S. A Review of the Parameters Affecting a Heat Pipe Thermal Management System for Lithium-Ion Batteries. Energies 2022, 15, 8534. [Google Scholar] [CrossRef]
- Pelin, D.; Brandis, A.; Kovačević, M.; Halak, F. Design and Testing of a Multimode Capable Passive Battery Management System. Energies 2022, 15, 4335. [Google Scholar] [CrossRef]
- Dubarry, M.; Baure, G.; Anseán, D. Perspective on State-of-Health Determination in Lithium-Ion Batteries. J. Electrochem. Energy Convers. Storage 2020, 17, 21286–21295. [Google Scholar] [CrossRef]
- Xiaosong, H.; Fei, F.; Kailong, L.; Lei, Z.; Jiale, X.; Bo, L. State estimation for advanced battery management: Key challenges and future trends. Renew. Sustain. Energy Rev. 2019, 114, 109334. [Google Scholar]
- Qian, L.; Jun, W.; Rui, X.; Weixiang, S.; Hongwen, H. Towards a smarter battery management system: A critical review on optimal charging methods of lithium ion batteries. Energy 2019, 75, 220–234. [Google Scholar]
- Behi, H.; Karimi, D.; Youssef, R.; Suresh Patil, M.; Van Mierlo, J.; Berecibar, M. Comprehensive Passive Thermal Management Systems for Electric Vehicles. Energies 2021, 14, 3881. [Google Scholar] [CrossRef]
- Special Issue Advanced Thermal Energy Conversion and Management Technologies. Available online: https://www.mdpi.com/journal/energies/special_issues/thermal_energy_conversion_management (accessed on 27 November 2022).
- Shen, H.; Zhang, Y.; Wu, Y. A comparative study on air transport safety of lithium-ion batteries with different SOCs. Appl. Therm. Eng. 2020, 179, 119–147. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar Pachauri, R.; Badoni, P.; Bharadwaj, D.; Mittal, U.; Bisht, A. Investigation on parallel hybrid electric bicycle along with issuer management system for mountainous region. J. Clean. Prod. 2022, 362, 132430. [Google Scholar] [CrossRef]
- DNK Power. Available online: https://www.dnkpower.com/wp-content/uploads/2019/02/SAMSUNG-INR21700-40T-Datasheet.pdf (accessed on 24 September 2022).
- Mizerski, W. Tablice Fizyczno-Astronomiczne; Adamantan: Warszawa, Poland, 2013. [Google Scholar]


| Model: | INR21700-40T (40T) |
|---|---|
| Size: | 21,700 |
| Style: | Flat Top |
| Nominal Capacity: | 4000 mAh |
| Discharge Current: | 35 A Maximum Continuous (1C) |
| Nominal Voltage: | 3.6 V |
| Maximum Voltage: | 4.2 V |
| Cutoff Voltage: | 2.5 V |
| Approximate Dimensions: | 21.1 mm × 70.4 mm |
| Approximate Weight: | 66.8 g |
| Detailed Specification Datasheet: | Samsung 40T Datasheet [32] |
| Sand | Water | Air | Steel | Glass | Cell Module Remains |
|---|---|---|---|---|---|
| 800 | 4145 | 1008 | 478 | 740 | 840 |
| SOC | Qsand | Qwat | Qair | Qcov | Qinl | Qinu | Qimv | Qmcell | Qsum | Esum |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (J) | (J) | (J) | (J) | (J) | (J) | (J) | (J) | (J) | (Wh) |
| 30 | 3925 | 13,866 | 39 | 3037 | 1237 | 753 | 1542 | 7138 | 31,537 | 8.76 |
| 50 | 9240 | 19,832 | 103 | 8026 | 496 | 753 | 1991 | 7356 | 47,797 | 13.27 |
| 100 | 12,283 | 19,747 | 139 | 10,850 | 1372 | 2691 | 1982 | 18,091 | 67,156 | 18.65 |
| >100 | 9482 | 49,156 | 171 | 12,497 | 1512 | 3307 | 4935 | 15,421 | 96,483 | 26.79 |
| Unsealing of the Cell | Beginning of Avalanche | Maximum Temperature | |||
|---|---|---|---|---|---|
| Energy (Wh) | Temperature (°C) | Energy (Wh) | Temperature (°C) | Temperature (°C) | |
| SOC > 100% | 1.99 | 113.2 | 3.03 | 171.2 | 813.7 |
| SOC = 100% | 4.29 | 159.1 | 4.90 | 187.2 | 552.0 |
| SOC = 50% | 5.62 | 168.9 | 6.23 | 187.9 | 478.5 |
| SOC = 30% | 7.98 | 178.9 | 8.95 | 211.1 | 387.6 |
| SOC = 0% | 9.95 | 194.2 | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erd, A.; Ciszewski, T. The Dependence of the Burning Process and Ignition Temperature of a Lithium Cell on Its State of Charge. Sensors 2023, 23, 753. https://doi.org/10.3390/s23020753
Erd A, Ciszewski T. The Dependence of the Burning Process and Ignition Temperature of a Lithium Cell on Its State of Charge. Sensors. 2023; 23(2):753. https://doi.org/10.3390/s23020753
Chicago/Turabian StyleErd, Andrzej, and Tomasz Ciszewski. 2023. "The Dependence of the Burning Process and Ignition Temperature of a Lithium Cell on Its State of Charge" Sensors 23, no. 2: 753. https://doi.org/10.3390/s23020753






