Multidimensional Biomechanics-Based Score to Assess Disease Progression in Duchenne Muscular Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Sensor and System Characteristics
2.3. Performed Tests
- Visit 1 (t0). The first visit, tagged as “t0”, was conducted to collect baseline data. During this visit, the participant performed the 6MWT once.
- Visit 2 (t0r). The objective of the second visit, which was labelled “t0r,” was to determine test–retest reliability. A subgroup of participants, made up of those who lived in the hospital area, underwent the test after a break of about 3–4 weeks.
- Visit 3 (t1). The third visit was tagged as “t1” and was planned in order to validate the test’s responsiveness. The test was performed with a time gap between t0 and t1 of approximately 6 months.
2.4. Data Analysis
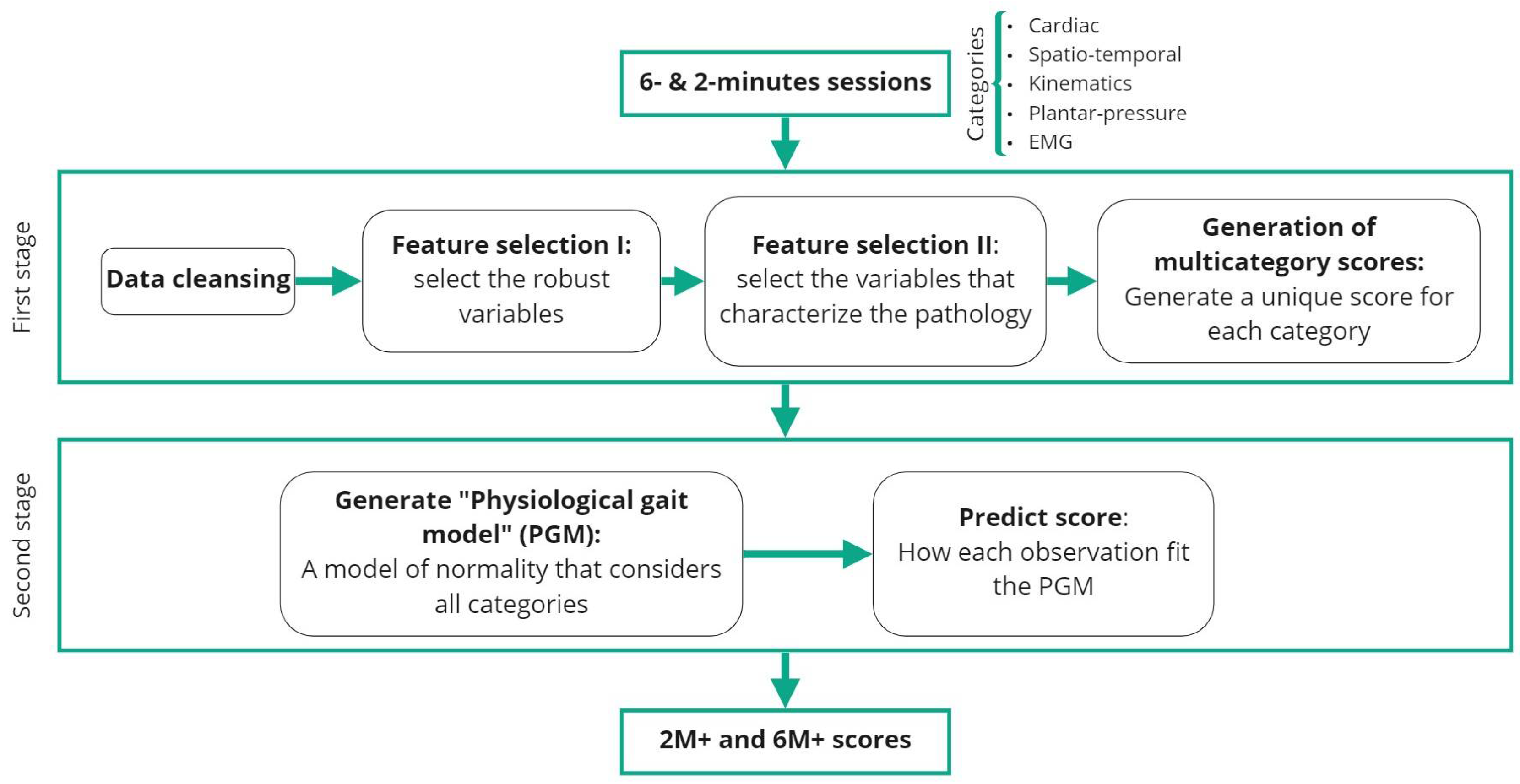
2.4.1. Pre-Processing and Feature Extraction
2.4.2. First Stage
- Data cleansing. DMD disorder is considered to affect the right and left limbs almost symmetrically [29]. Thus, the variables corresponding to the sensors placed in both right and left parts of the body were averaged into a single measure.
- Feature selection I. Robust variable selection. The most robust and repeatable variables were selected by comparing t0 and t0r sessions for all subjects. These are expected to have similar values between sessions as they were recorded in a short time-window. As the sets of data are used to compare an increase from the same individuals at different sessions, the data are paired. To determine which statistical test was the most suitable for each variable, a Shapiro–Wilk test was applied. If the variable followed a normal distribution for both sessions, a Paired Samples T-Test was applied. If not, the chosen test was the Wilcoxon Signed-Rank Test. The variables that exhibited the same underlying distribution for both sessions (p > 0.05) were considered robust and used for subsequent analysis.
- Feature selection II. Selection of the variables that best characterize the pathology. This step compared how the control and pathological distributions differ between each other. For each one of the variables, the Kruskal–Wallis test for unpaired data was used to assess if control and pathological distributions were significantly different. The values that showed a significant difference (p < 0.01) were selected for further analysis.
- Generation of a unified score for each of the categories. The selected variables, which belonged to one of the five categories, were first normalized (the minimum value was set to 0 and the maximum value to 1). The direction of each variable had to be the same. In other words, it was necessary that the pathological cases always showed the lowest numbers and the control cases the highest. Therefore, if the median value of control cases was lower than the pathological median value, the variable was inverted. Finally, a single variable for each category was computed as the mean of the normalized variables belonging to that category and adjusted between 0 and 100.
2.4.3. Second Stage
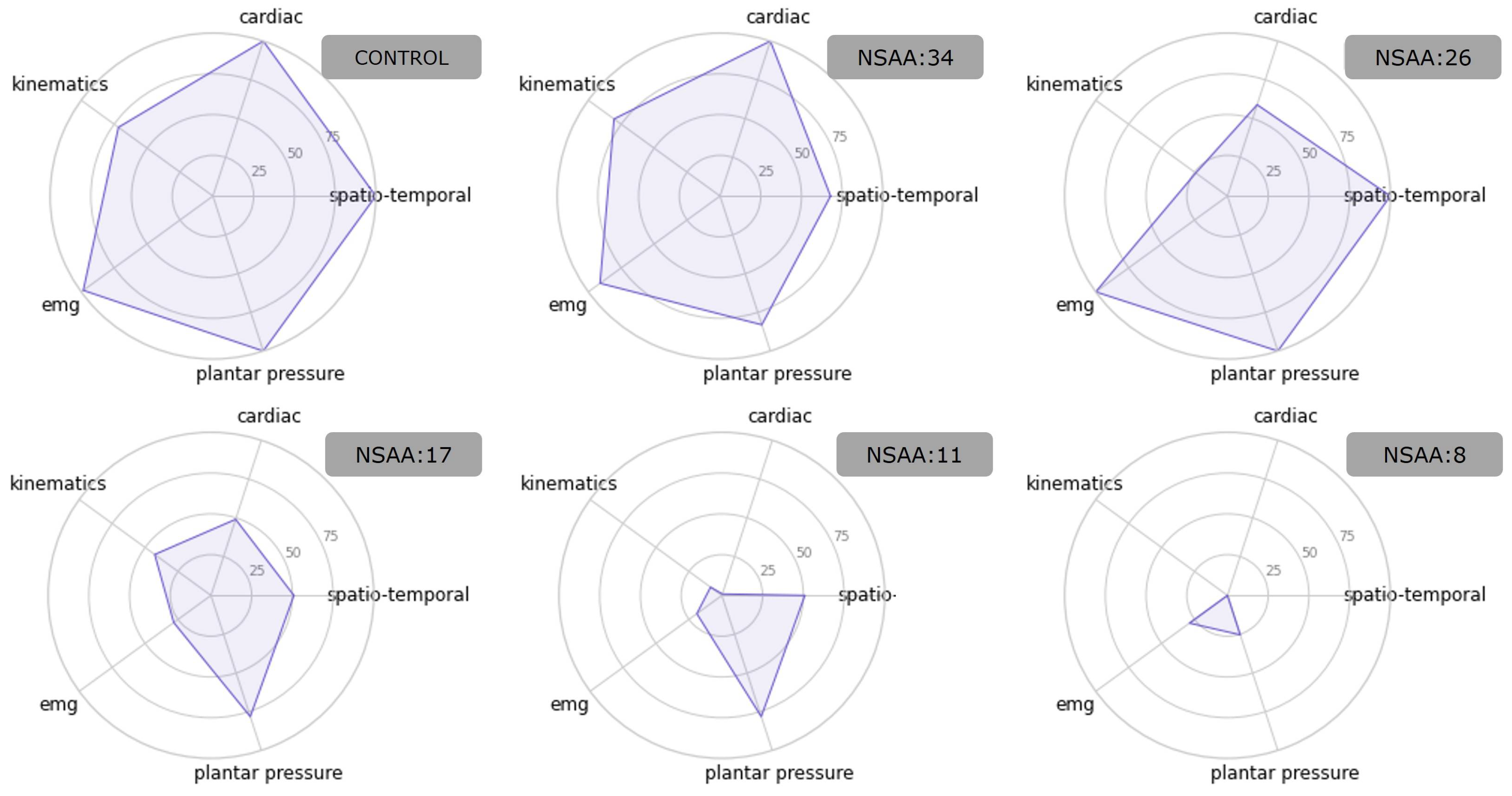
- Generation of the physiological gait model. For the control group, the computed scores for each category (Spatio-temporal, Cardiac, Kinematics, EMG, and Plantar pressure scores) were used to define a 5-dimensional gaussian-mixture model, where each dimension corresponded to one category. This model was defined as the physiological gait model (PGM). By using the Bayesian Information Criterion [30], the number of components for modelling the PGM as well as the covariance type (from spherical, diagonal, tied, and full) were determined.
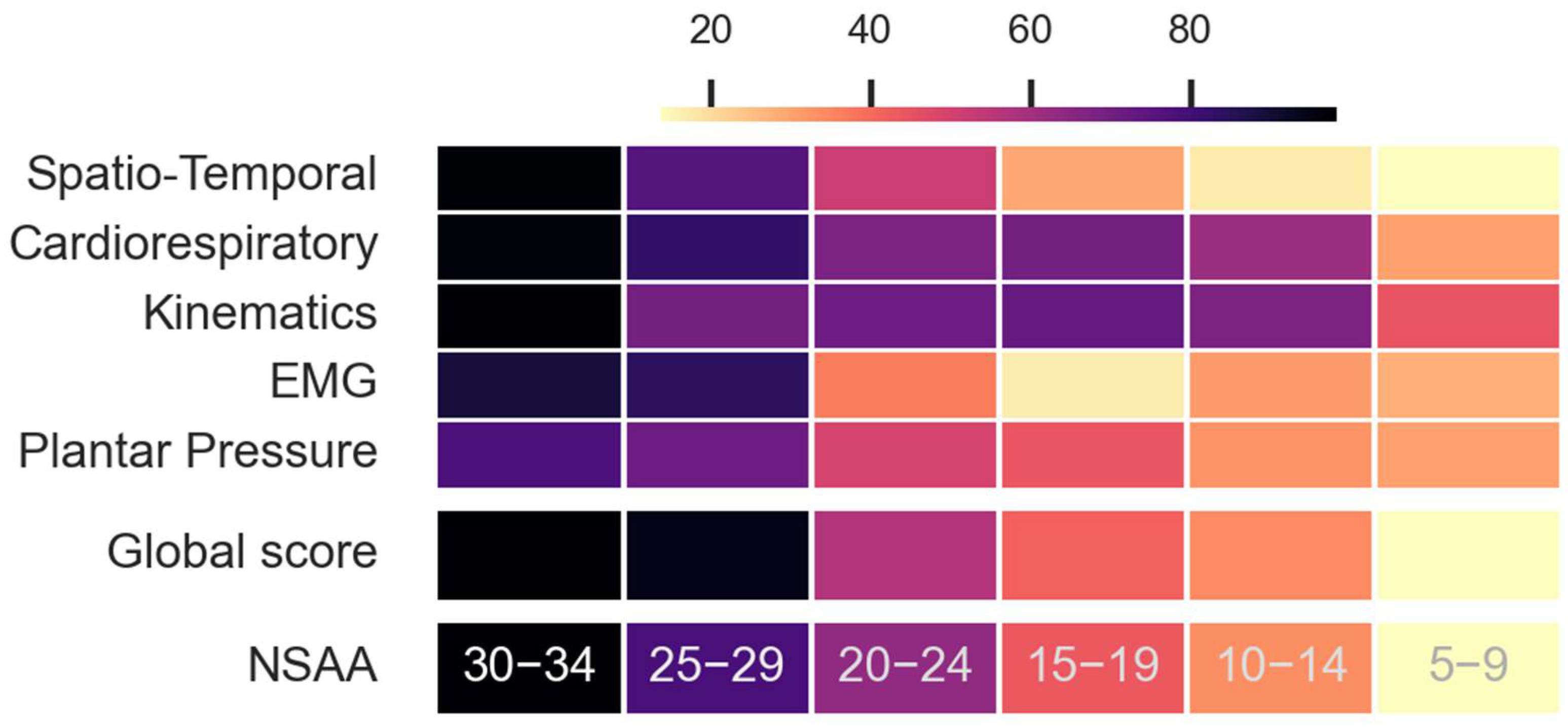
- Obtaining the global score for controls pathological observations. Both the pathological and control subjects achieved a score for both the 6 min (6M+) and 2 min (2M+) tests. These scores were calculated as the weighted log probability of belonging to the PGM and, subsequently, scaled from 0 to 100. The highest score was 100, which was expected for control subjects. The further an observation was from the model, the lower was its score. Indeed, controls would score better since they are predicted to have a higher likelihood of fitting PGM. Depending on their severity, pathological individuals were predicted to gradually stray from the PGM. Consequently, they are expected to gradually receive lower scores.
2.5. Score Validation
3. Results
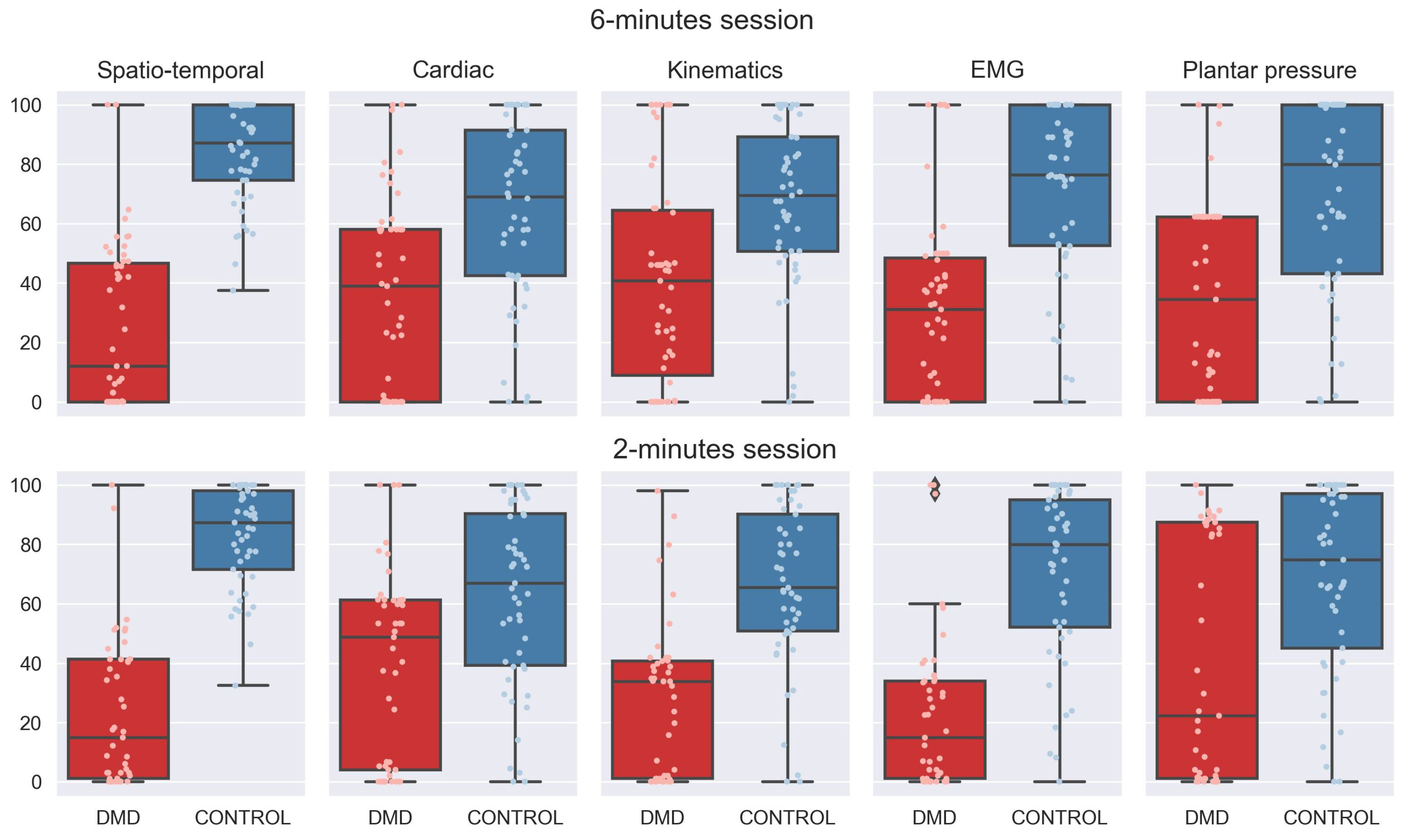
3.1. Generation of the Multidimensional Score
3.2. Generation of the 6M+ and 2M+ Scores
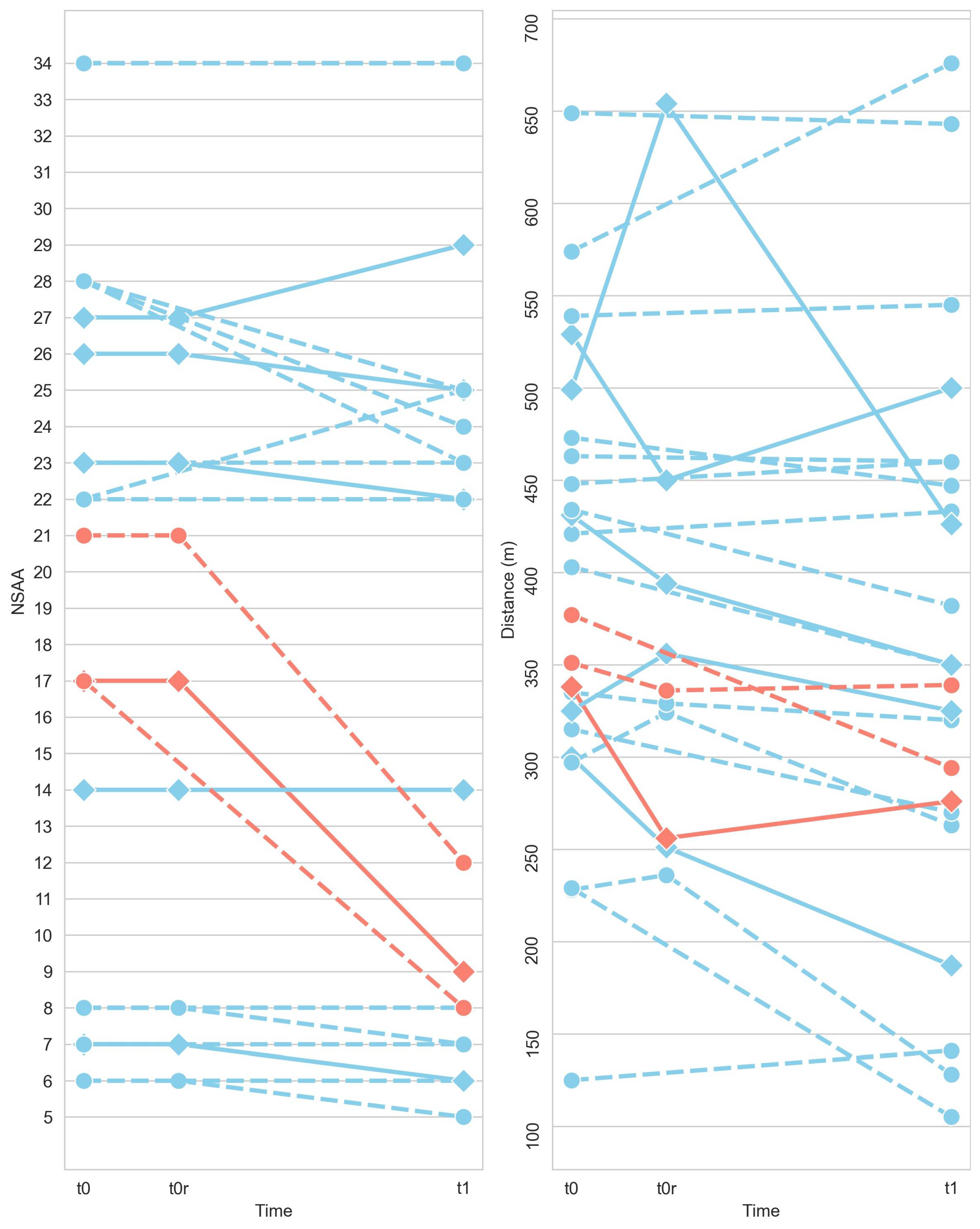
3.3. The Pathological 2M+ and 6M+ Score and Its Comparison with NSAA and Distances
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendell, J.R.; Ms, C.S.; Leslie, N.D.; Flanigan, K.; Al-Dahhak, R.; Gastier-Foster, J.; Kneile, K.; Dunn, D.M.; Duval, B.; Aoyagi, A.; et al. Evidence-based path to newborn screening for duchenne muscular dystrophy. Ann. Neurol. 2012, 71, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Bradley, D.M.; Salmon, R.; Clarke, A.; Hartley, L. Newborn bloodspot screening for Duchenne Muscular Dystrophy: 21 years experience in Wales (UK). Eur. J. Hum. Genet. 2013, 21, 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Landfeldt, E.; Thompson, R.; Sejersen, T.; McMillan, H.J.; Kirschner, J.; Lochmüller, H. Life expectancy at birth in Duchenne muscular dystrophy: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, L.; Morgenroth, L.P.; Gordish-Dressman, H.; Hoffman, E.P.; McDonald, C.M.; Cirak, S.; CINRG investigators. DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study. Neurology 2016, 87, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, I.M.; Denysyuk, H.V.; Villasana, M.V.; Sá, J.; Marques, D.L.; Morgado, J.F.; Albuquerque, C.; Zdravevski, E. Development Technologies for the Monitoring of Six-Minute Walk Test: A Systematic Review. Sensors 2022, 22, 581. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Florence, J.M.; Eagle, M.; Gappmaier, E.; Glanzman, A.M.; PTC124-GD-007-DMD Study Group; Spiegel, R.; Barth, J.; et al. THE 6-minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 2013, 48, 343–356. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Han, J.J.; Abresch, R.T.; Nicorici, A.; Elfring, G.L.; Atkinson, L.; Reha, A.; Hirawat, S.; Miller, L.L. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2009, 41, 500–510. [Google Scholar] [CrossRef]
- Prahm, K.P.; Witting, N.; Vissing, J. Decreased Variability of the 6-Minute Walk Test by Heart Rate Correction in Patients with Neuromuscular Disease. PLoS ONE 2014, 9, e114273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfano, L.N.; Lowes, L.P.; Berry, K.M.; Yin, H.; Dvorchik, I.; Flanigan, K.M.; Cripe, L.; Mendell, J.R. TP 1: Pilot study evaluating motivation on the performance of timed walking in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2014, 24, 860. [Google Scholar] [CrossRef]
- Heresi, G.A.; Dweik, R.A. Strengths and limitations of the six-minute-walk test: A model biomarker study in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1122–1124. [Google Scholar] [CrossRef]
- Bartels, B.; De Groot, J.F.; Terwee, C.B. The Six-Minute Walk Test in Chronic Pediatric Conditions: A Systematic Review of Measurement Properties. Phys. Ther. 2013, 93, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knak, K.; Andersen, L.; Witting, N.; Vissing, J. Reliability of the 2- and 6-minute walk tests in neuromuscular diseases. J. Rehabil. Med. 2017, 49, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witherspoon, J.; Vasavada, R.; Logaraj, R.; Waite, M.; Collins, J.; Shieh, C.; Meilleur, K.; Bönnemann, C.; Jain, M. Two-minute versus 6-minute walk distances during 6-minute walk test in neuromuscular disease: Is the 2-minute walk test an effective alternative to a 6-minute walk test? Eur. J. Paediatr. Neurol. 2018, 23, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vill, K.; Ille, L.; Schroeder, S.A.; Blaschek, A.; Müller-Felber, W. Six-minute walk test versus two-minute walk test in children with Duchenne muscular dystrophy: Is more time more information? Eur. J. Paediatr. Neurol. 2015, 19, 640–646. [Google Scholar] [CrossRef]
- Boehme, P.; Hansen, A.; Roubenoff, R.; Scheeren, J.; Herrmann, M.; Mondritzki, T.; Ehlers, J.; Truebel, H. How soon will digital endpoints become a cornerstone for future drug development? Drug Discov. Today 2018, 24, 16–19. [Google Scholar] [CrossRef]
- Kruizinga, M.D.; Stuurman, F.E.; Exadaktylos, V.; Doll, R.J.; Stephenson, D.T.; Groeneveld, G.J.; Driessen, G.J.A.; Cohen, A.F. Development of Novel, Value-Based, Digital Endpoints for Clinical Trials: A Structured Approach Toward Fit-for-Purpose Validation. Pharmacol. Rev. 2020, 72, 899–909. [Google Scholar] [CrossRef]
- Ephion Health. Available online: www.ephion.heatlh (accessed on 1 January 2023).
- Scott, E.; Eagle, M.; Mayhew, A.; Freeman, J.; Main, M.; Sheehan, J.; Manzur, A.; Muntoni, F.; North Star Clinical Network for Paediatric Neuromuscular Disease. Development of a Functional Assessment Scale for Ambulatory Boys with Duchenne Muscular Dystrophy. Physiother. Res. Int. 2011, 17, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Messina, S.; Vasco, G.; Main, M.; Eagle, M.; D’Amico, A.; Doglio, L.; Politano, L.; Cavallaro, F.; Frosini, S.; et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul. Disord. 2009, 19, 458–461. [Google Scholar] [CrossRef]
- Finni, T.; Hu, M.; Kettunen, P.; Vilavuo, T.; Cheng, S. Measurement of EMG activity with textile electrodes embedded into clothing. Physiol. Meas. 2007, 28, 1405–1419. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, O.; Haakana, P.; Pesola, A.J.; Häkkinen, K.; Rantalainen, T.; Havu, M.; Pullinen, T.; Finni, T. Muscle Activity and Inactivity Periods during Normal Daily Life. PLoS ONE 2013, 8, e52228. [Google Scholar] [CrossRef]
- Colyer, S.L.; McGuigan, P.M. Textile Electrodes Embedded in Clothing: A Practical Alternative to Traditional Surface Electromyography when Assessing Muscle Excitation during Functional Movements. J. Sports Sci. Med. 2018, 17, 101–109. [Google Scholar]
- Bengs, D.; Jeglinsky, I.; Surakka, J.; Hellsten, T.; Ring, J.; Kettunen, J. Reliability of Measuring Lower-Limb-Muscle Electromyography Activity Ratio in Activities of Daily Living With Electrodes Embedded in the Clothing. J. Sport Rehabil. 2017, 26. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, S.; Jeong, W. EMG Measurement with Textile-Based Electrodes in Different Electrode Sizes and Clothing Pressures for Smart Clothing Design Optimization. Polymers 2020, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Florence, J.; Eagle, M.; Gappmaier, E.; Glanzman, A.M.; PTC124-GD-007-DMD Study Group; Spiegel, R.; Barth, J.; et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve 2013, 48, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Anwary, A.R.; Yu, H.; Vassallo, M. An Automatic Gait Feature Extraction Method for Identifying Gait Asymmetry Using Wearable Sensors. Sensors 2018, 18, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, K.; Granat, M.H. A practical gait analysis system using gyroscopes. Med. Eng. Phys. 1999, 21, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Xia, D. EMD-based denoising methods in the MEMS gyroscope de-drift. In Proceedings of the 2010 IEEE 5th International Conference on Nano/Micro Engineered and Molecular Systems, Xiamen, China, 20–23 January 2010; pp. 591–594. [Google Scholar]
- Cole, W.G.; Gill, S.V.; Vereijken, B.; Adolph, K.E. Coping with asymmetry: How infants and adults walk with one elongated leg. Infant Behav. Dev. 2014, 37, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Neath, A.A.; Cavanaugh, J.E. The Bayesian information criterion: Background, derivation, and applications. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 199–203. [Google Scholar] [CrossRef]
- Thomas, S.S.; Buckon, C.E.; Nicorici, A.; Bagley, A.; McDonald, C.; Sussman, M.D. Classification of the Gait Patterns of Boys With Duchenne Muscular Dystrophy and Their Relationship to Function. J. Child Neurol. 2010, 25, 1103–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, A.; Cano, S.; Scott, E.; Eagle, M.; Bushby, K.; Muntoni, F.; North Star Clinical Network for Paediatric Neuromuscular Disease. Moving towards meaningful measurement: Rasch analysis of the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2011, 53, 535–542. [Google Scholar] [CrossRef]
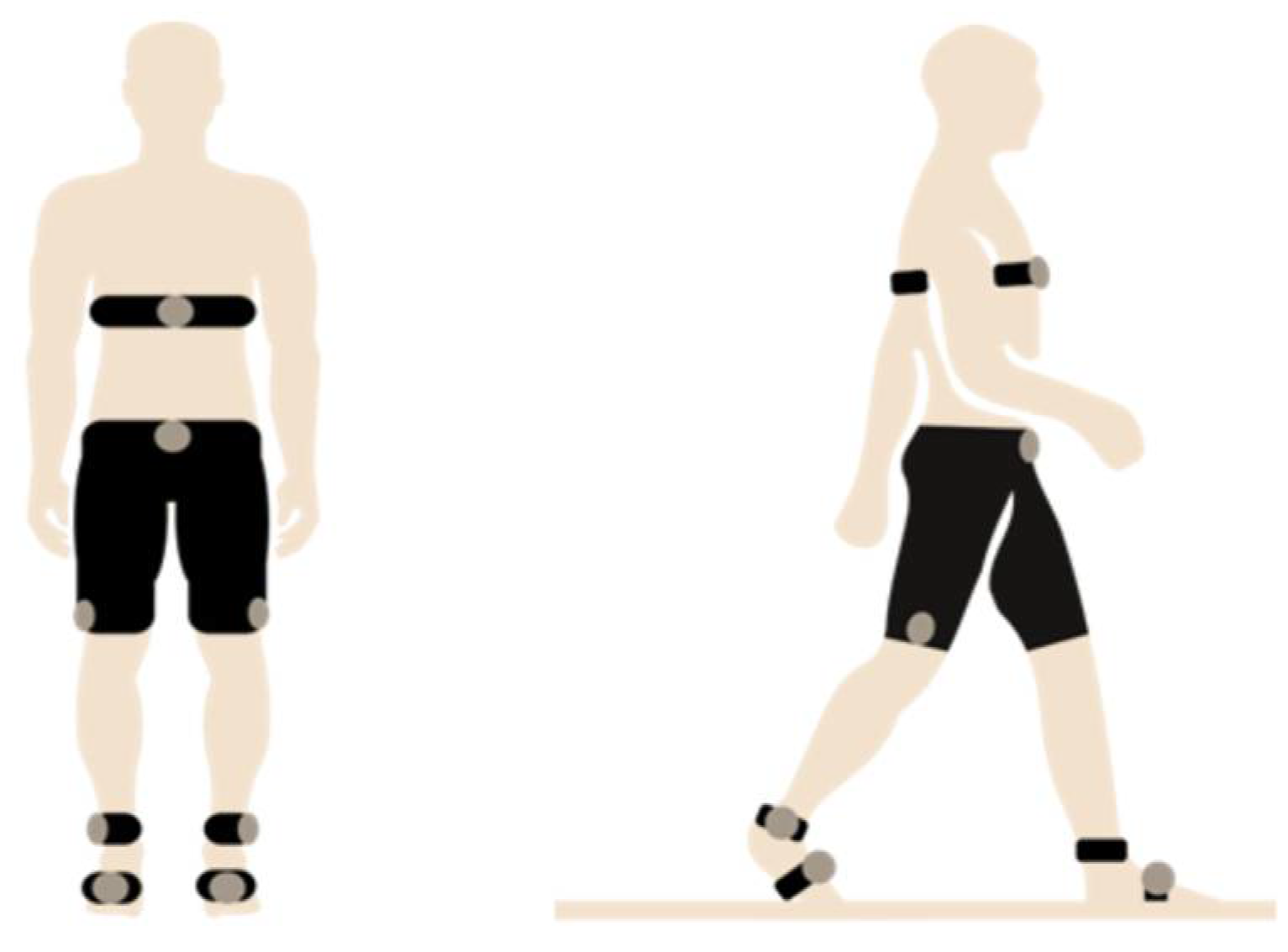



| Sensor Type | Description | Device Used | Fs | Other Specifications | Category |
|---|---|---|---|---|---|
| Surface EMGs | EMGs were integrated in custom-made, size-appropriate shorts that enabled recording of muscle activity. | Mshorts Myontec | 1000 Hz captured 25 Hz transmitted | 24-bit ADC, sampling rate of 1000 Hz, bandwidth of 40–200 Hz (−3 dB) 25 Hz (rectified and averaged from 1000 Hz Raw EMG) Electrodes: silver-coated yarn | EMG |
| Inertial sensors | Sensors were placed at the thigh, shank, and in the plantar insoles to measure joint kinematics. | Movesense inertial sensors | 104 Hz | Gyroscope: ±2000 dps Accelerometer: ±8 g | Spatio-temporal, kinematics |
| Heart rate | A strap was applied below the chest to measure cardiorespiratory parameters. | Movesense HR sensor | 1 Hz | Cardiac | |
| Plantar pressure | Pressure sensors were integrated in insoles, which were size-appropriate and used to obtain information on weight distribution during gait. | Moticon plantar insoles | 100 Hz | Gyroscope: ±2000 dps Accelerometer: ±16 g | Plantar pressure, spatio-temporal, kinematics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorelli, C.; Gómez-Martinez, M.; Subías-Beltrán, P.; Claramunt-Molet, M.; Idelsohn-Zielonka, S.; Mas-Hurtado, E.; Miralles, F.; Montolio, M.; Roselló-Ruano, M.; Medina-Cantillo, J. Multidimensional Biomechanics-Based Score to Assess Disease Progression in Duchenne Muscular Dystrophy. Sensors 2023, 23, 831. https://doi.org/10.3390/s23020831
Migliorelli C, Gómez-Martinez M, Subías-Beltrán P, Claramunt-Molet M, Idelsohn-Zielonka S, Mas-Hurtado E, Miralles F, Montolio M, Roselló-Ruano M, Medina-Cantillo J. Multidimensional Biomechanics-Based Score to Assess Disease Progression in Duchenne Muscular Dystrophy. Sensors. 2023; 23(2):831. https://doi.org/10.3390/s23020831
Chicago/Turabian StyleMigliorelli, Carolina, Meritxell Gómez-Martinez, Paula Subías-Beltrán, Mireia Claramunt-Molet, Sebastian Idelsohn-Zielonka, Eudald Mas-Hurtado, Felip Miralles, Marisol Montolio, Marina Roselló-Ruano, and Julita Medina-Cantillo. 2023. "Multidimensional Biomechanics-Based Score to Assess Disease Progression in Duchenne Muscular Dystrophy" Sensors 23, no. 2: 831. https://doi.org/10.3390/s23020831





