Organic Electronics—Microfluidics/Lab on a Chip Integration in Analytical Applications
Abstract
:1. Background
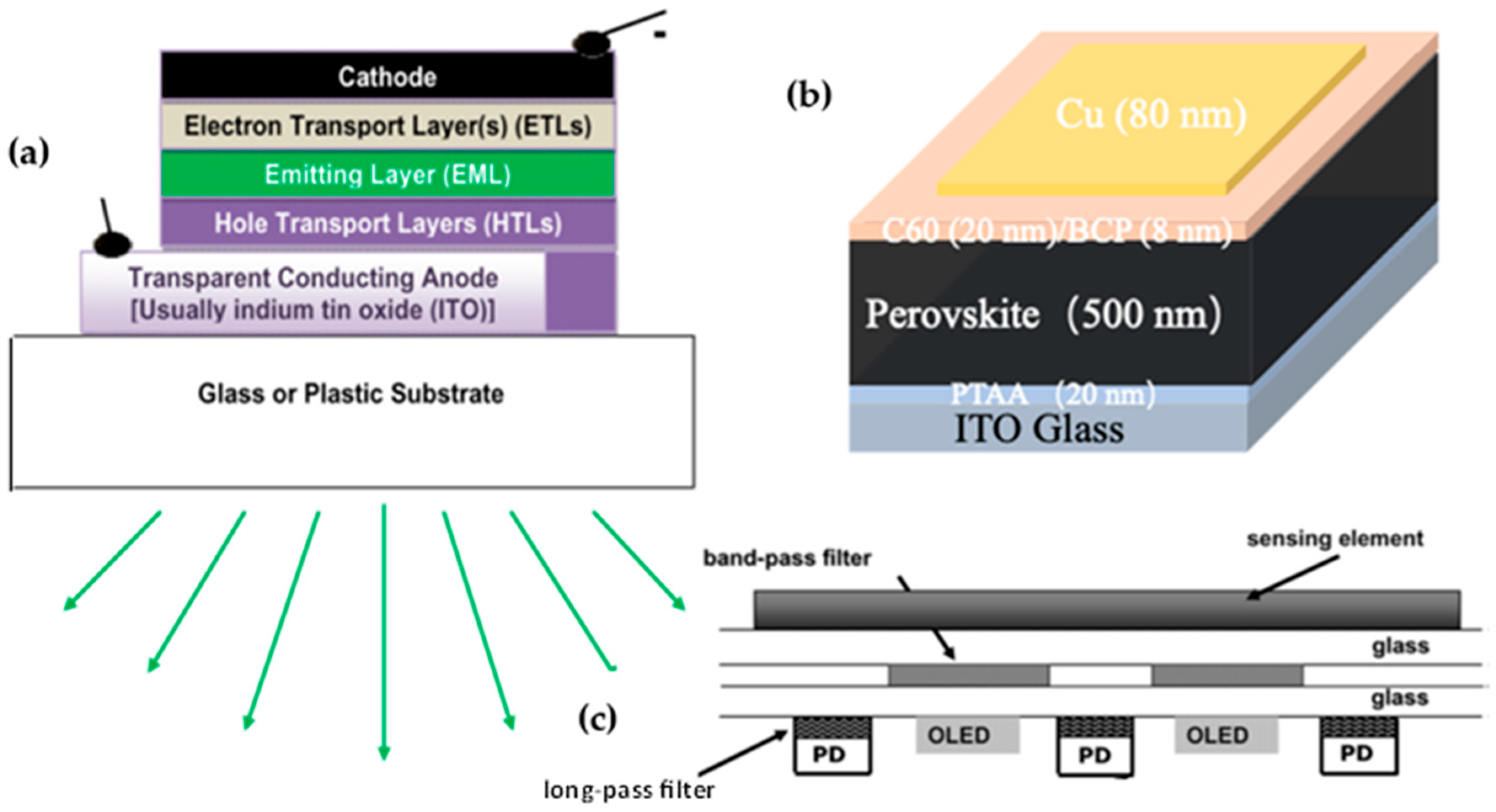
1.1. OLEDs
1.2. OPDs, PPDs, and OLED–OPD Combinations
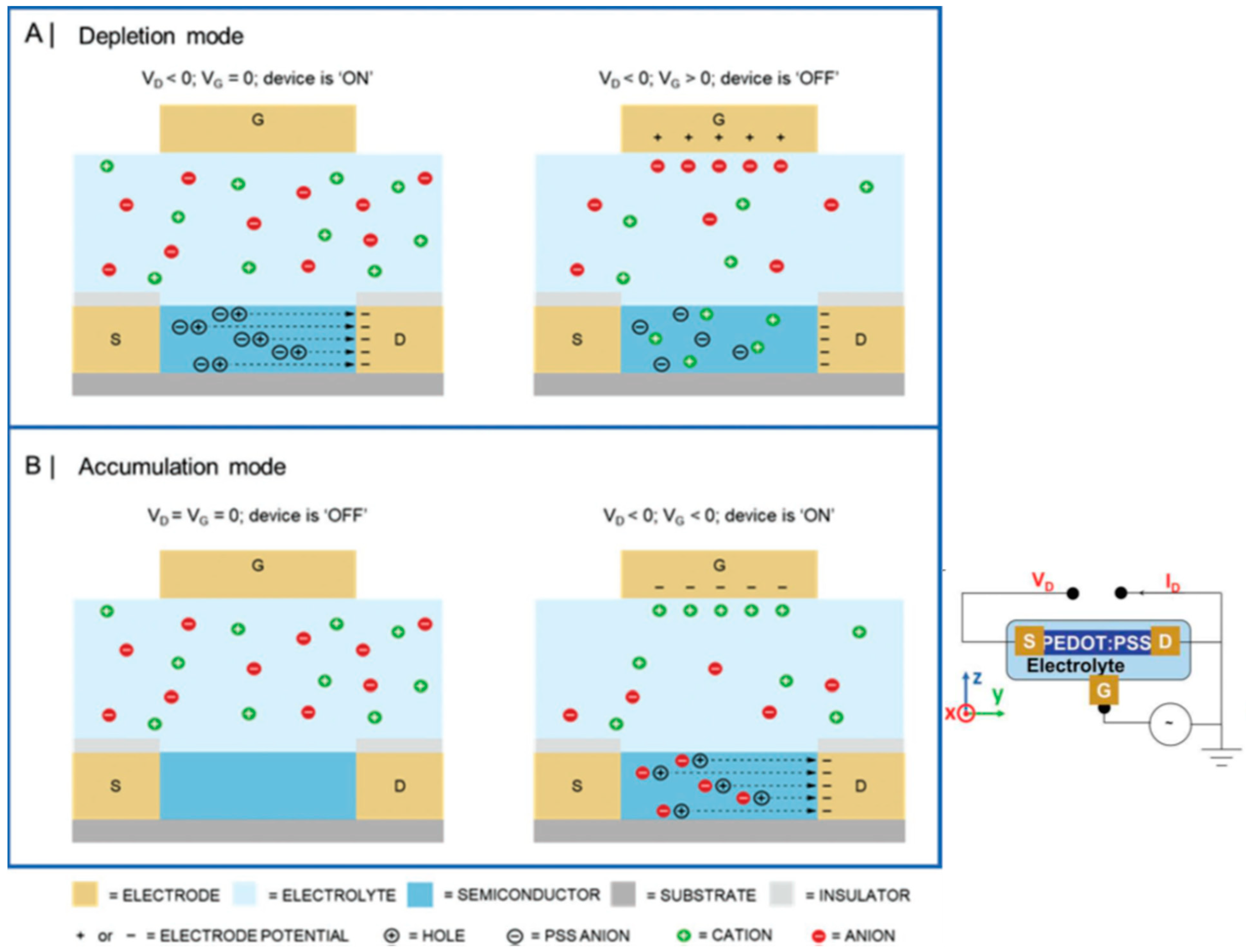
1.3. OFETs
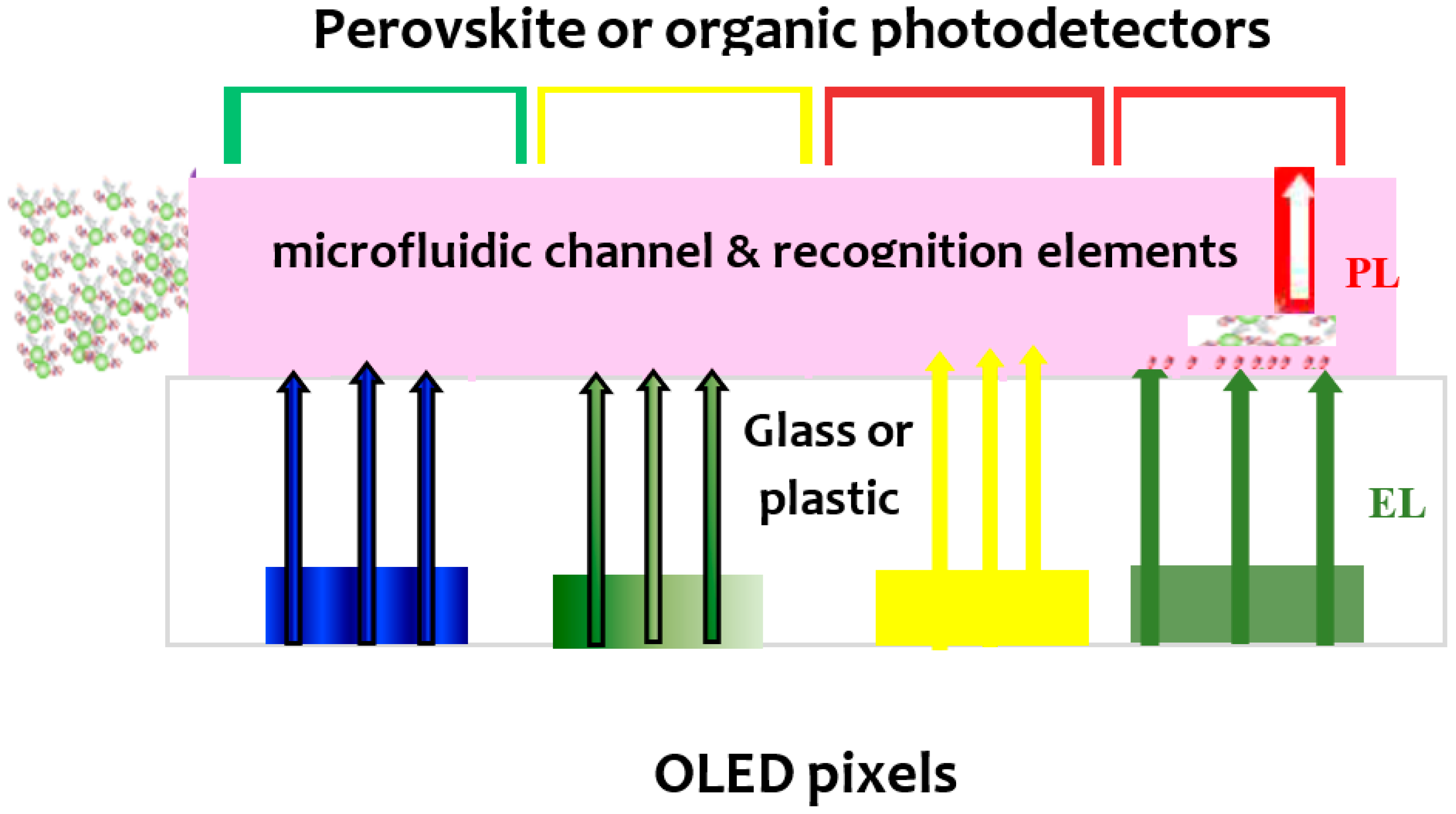
2. OE-Microfluidics/LOC Integration
2.1. Optical Applications
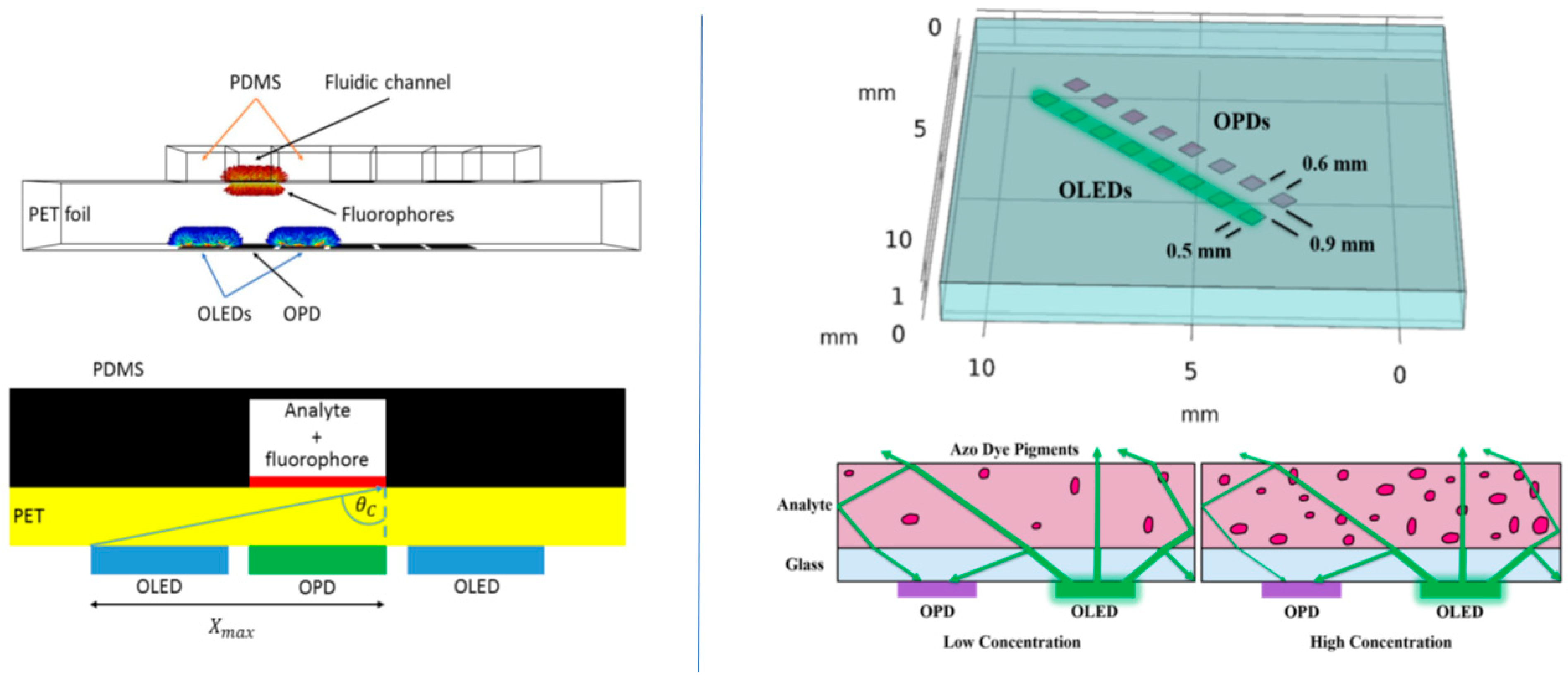
2.1.1. OLEDs-Microfluidic Integration
2.1.2. OPDs-Microfluidic Integration
2.1.3. Organic Electronic Light Source-OPD/LOC Integration
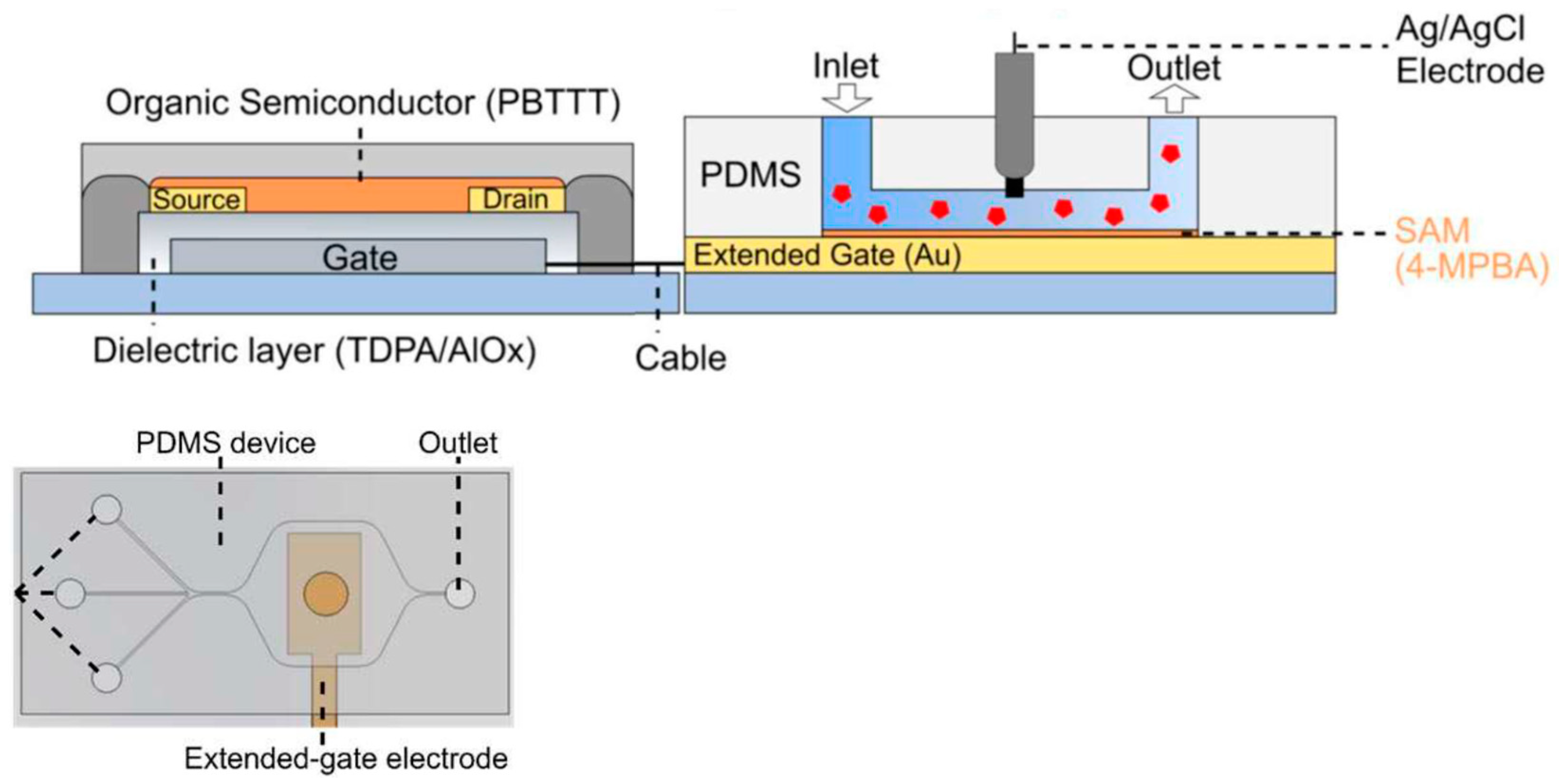
2.2. OFET—Microfluidics Integration
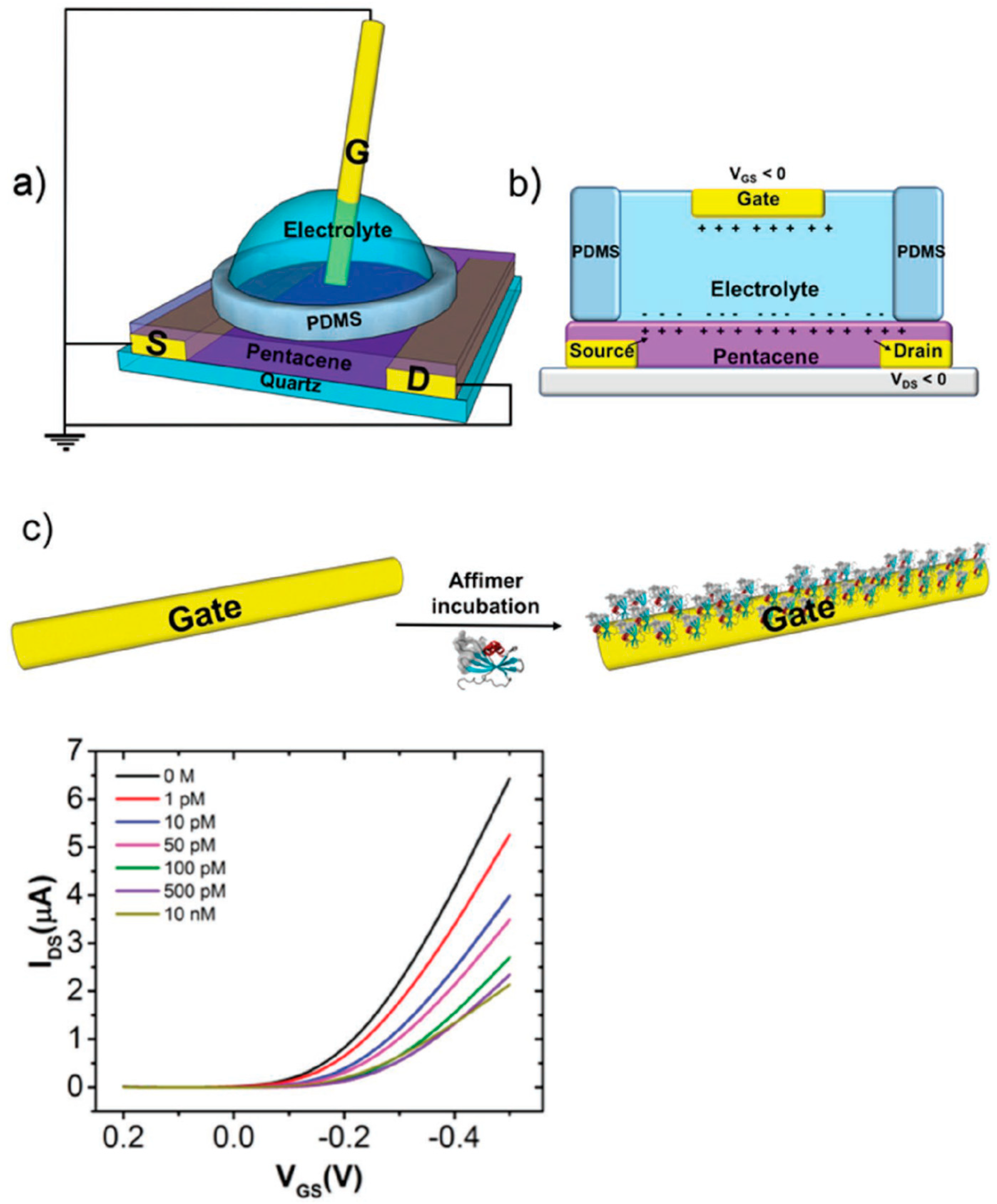
2.2.1. Examples of EGOFETs
2.2.2. Examples of OECTs
3. Summary and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluid. 2021, 25, 99. [Google Scholar] [CrossRef]
- Xie, Y.; Dai, L.; Yang, Y. Microfluidic technology and its application in the point-of-care testing field. Biosens. Bioelectron. X 2022, 10, 100109. [Google Scholar] [CrossRef]
- Pal, A.; Kaswan, K.; Barman, S.R.; Lin, Y.-Z.; Chung, J.-H.; Sharma, M.K.; Liu, K.-L.; Chen, B.-H.; Wu, C.-C.; Lee, S.; et al. Microfluidic nanodevices for drug sensing and screening applications. Biosens. Bioelectron. 2023, 219, 114783. [Google Scholar] [CrossRef]
- Shinar, R.; Wolanyk, J.; Kaudel, R.; Park, J.M.; Shinar, J. Organic and Hybrid Electronics in Optical Analytical Applications. Mater. Matt. 2019, 14, 19. [Google Scholar]
- Williams, G.; Backhouse, C.; Aziz, H. Integration of Organic Light Emitting Diodes and Organic Photodetectors for Lab-on-a-Chip Bio-Detection Systems. Electronics 2014, 3, 43–75. [Google Scholar] [CrossRef]
- Murawski, C.; Gather, M.C. Emerging Biomedical Applications of Organic Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2100269. [Google Scholar] [CrossRef]
- Ohayon, D.; Druet, V.; Inal, S. A guide for the characterization of organic electrochemical transistors and channel materials. Chem. Soc. Rev. 2023, 52, 1001–1023. [Google Scholar] [CrossRef]
- Shinar, J.; Shinar, R. Organic Light-Emitting Devices (OLEDs) and OLED-Based Chemical and Biological Sensors: Ashinn Overview. J. Phys. D Appl. Phys. 2008, 41, 133001. [Google Scholar] [CrossRef]
- Cai, Y.; Shinar, R.; Zhou, Z.; Shinar, J. Multianalyte sensor array based on an organic light emitting diode platform. Sens. Actuators B 2008, 134, 727–735. [Google Scholar] [CrossRef]
- Cai, Y.; Smith, A.; Shinar, J.; Shinar, R. Data analysis and aging in phosphorescent oxygen-based sensors. Sens. Actuators B Chem. 2010, 146, 14–22. [Google Scholar] [CrossRef]
- Smith, A.; Cai, Y.; Vengasandra, S.; Shinar, R.; Shinar, J. Advances toward commercialization of a new generation of low-cost (O)LED-based dissolved oxygen and bioanalyte monitors. In Organic Semiconductors in Sensors and Bioelectronics III; Shinar, R., Kymissis, I., Eds.; SPIE Conference Proceedings; SPIE: Bellingham, WA, USA, 2010; Volume 7779, p. 77790P-1. [Google Scholar]
- Vengasandra, S.; Cai, Y.; Grewell, D.; Shinar, J.; Shinar, R. Polypropylene CD-organic light-emitting diode biosensing platform. Lab Chip 2010, 10, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cai, Y.; Park, J.-M.; Ho, K.-M.; Shinar, J.; Shinar, R. Organic Light-Emitting Diode Sensing Platform: Challenges and Solutions. Adv. Funct. Mater. 2011, 21, 4744–4753. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, T.; Cui, W.; Shinar, J.; Shinar, R. Multiple approaches for enhancing all-organic electronics photoluminescent sensors: Simultaneous oxygen and pH monitoring. Anal. Chim. Acta 2013, 778, 70–78. [Google Scholar] [CrossRef] [PubMed]
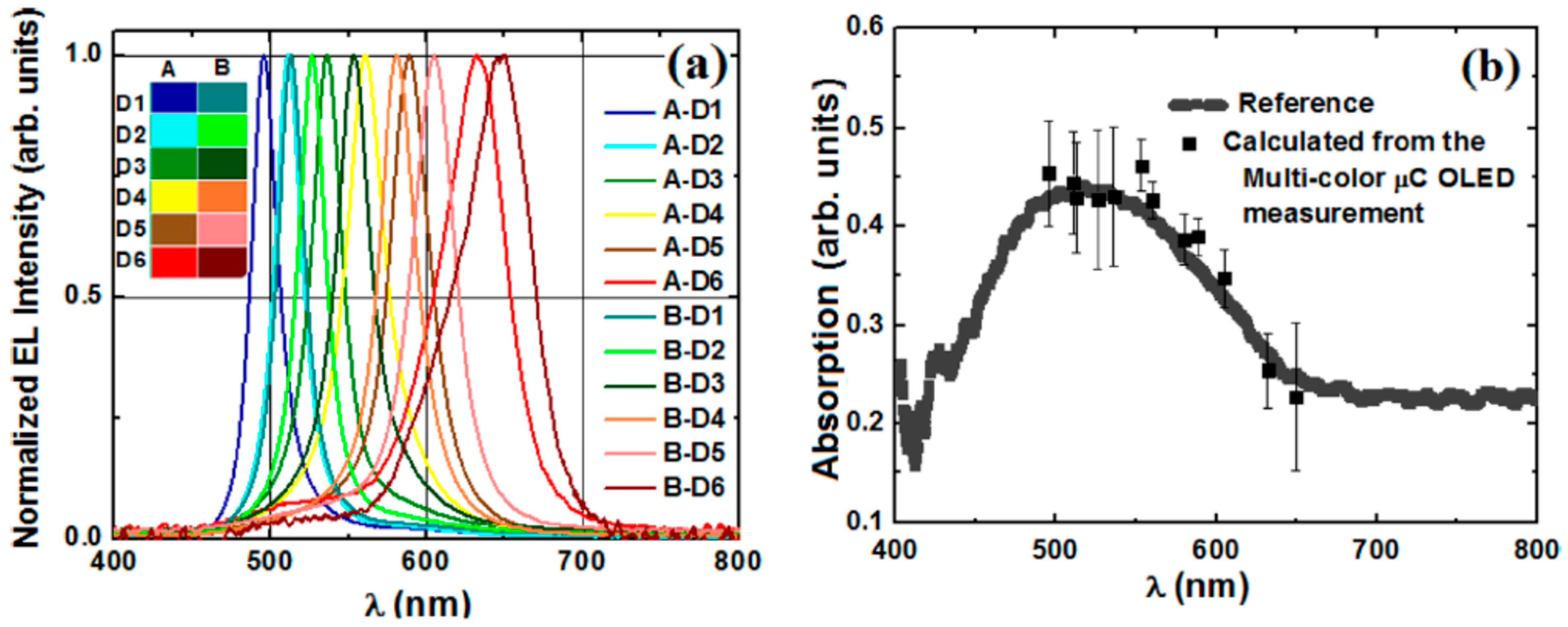
- Manna, E.; Fungura, F.; Biswas, R.; Shinar, J.; Shinar, R. Tunable Near UV Microcavity OLED Arrays: Characterization and Analytical Applications. Adv. Funct. Mater. 2015, 25, 1226–1232. [Google Scholar] [CrossRef]
- Wolanyk, J.; Xiao, X.; Fralaide, M.; Lauersdorf, N.J.; Kaudal, R.; Dykstra, E.; Huang, J.; Shinar, J.; Shinar, R. Tunable perovskite-based photodetectors in optical sensing. Sens. Actuators B 2020, 321, 128462. [Google Scholar] [CrossRef]
- Attili, S.; Lesar, A.; McNeill, A.; Camacho-Lopez, M.; Moseley, H.; Ibbotson, S.; Samuel, I.; Ferguson, J. An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br. J. Dermatol. 2009, 161, 170–173. [Google Scholar] [CrossRef]
- Melendez-Celis, U.; Spezzia-Mazzocco, T.; Persheyev, S.; Lian, C.; Samuel, I.; Ramirez-San-Juan, J.C.; Ramos-Garcia, R. Organic light emitting diode for in vitro antimicrobial photodynamic therapy of Candida strains. Photodiagn. Photodyn. Ther. 2021, 36, 102567. [Google Scholar] [CrossRef]
- Bansal, A.K.; Hou, S.; Kulyk, O.; Bowman, E.M.; Samuel, I.D.W. Wearable Organic Optoelectronic Sensors for Medicine. Adv. Mater. 2015, 27, 7638–7644. [Google Scholar] [CrossRef]
- Cabral, F.V.; Lian, C.; Persheyev, S.; Smith, T.K.; Ribeiro, M.S.; Samuel, I.D.W. Organic Light-Emitting Diodes as an Innovative Approach for Treating Cutaneous Leishmaniasis. Adv. Mater. Technol. 2021, 6, 2100395. [Google Scholar] [CrossRef]
- Lian, C.; Yoshida, K.; Nogues, C.; Samuel, I.D.W. Organic Light-Emitting Diode Based Fluorescence Sensing System for DNA Detection. Adv. Mater. Technol. 2022, 7, 2100806. [Google Scholar] [CrossRef]
- Lian, C.; Young, D.; Randall, R.E.; Samuel, I.D.W. Organic Light-Emitting Diode Based Fluorescence-Linked Immunosorbent Assay for SARS-CoV-2 Antibody Detection. Biosensors 2022, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
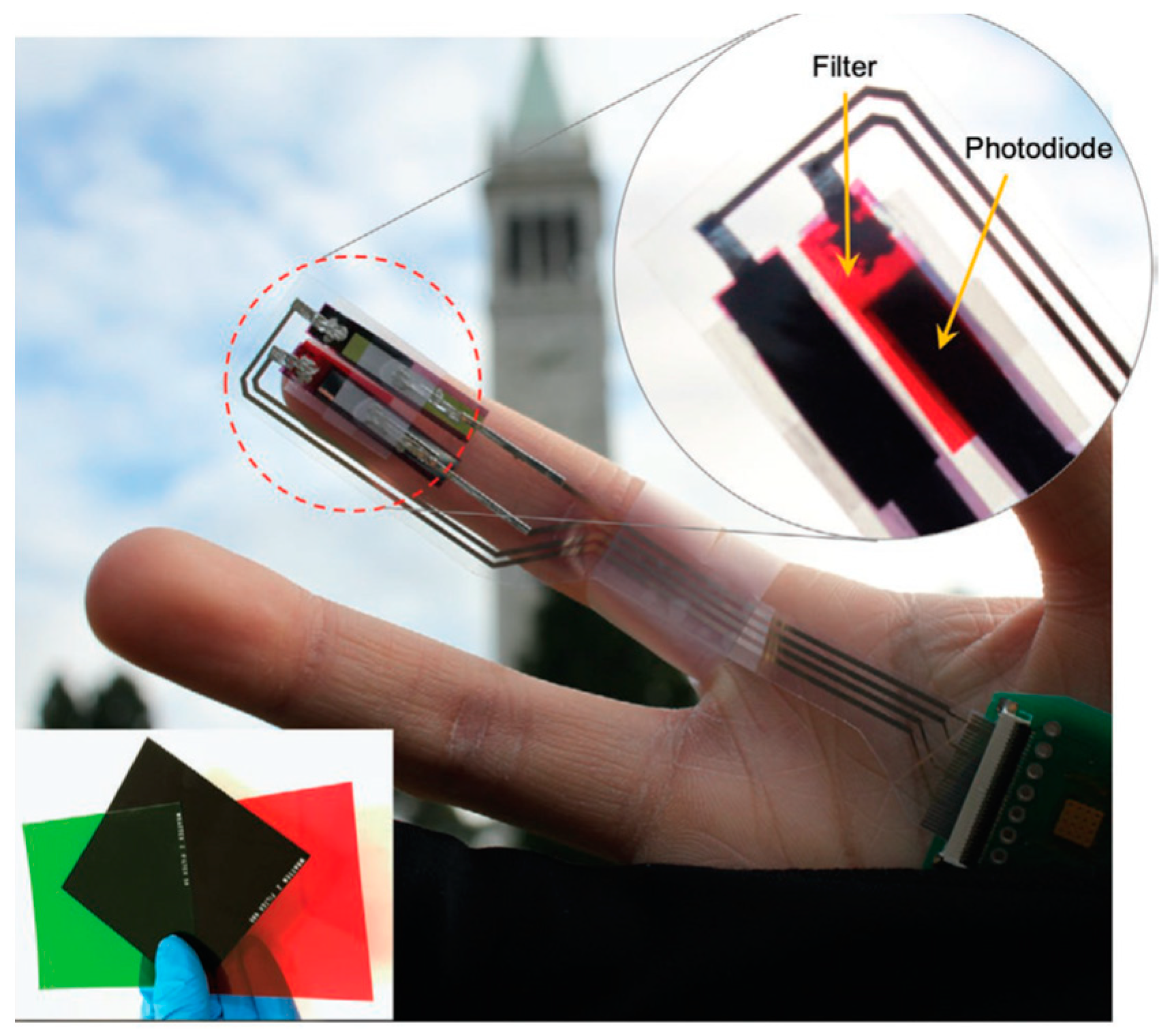
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Khan, Y.; Han, D.; Pierre, A.; Ting, J.; Wang, X.; Lochner, C.M.; Bovo, G.; Yaacobi-Gross, N.; Newsome, C.; Wilson, R.; et al. A flexible organic reflectance oximeter array. Proc. Natl. Acad. Sci. USA 2018, 115, E11015–E11024. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Adachi, C. Organic light-emitting diode with liquid emitting layer. Appl. Phys. Lett. 2009, 95, 053304. [Google Scholar] [CrossRef]
- Hirata, S.; Kubota, K.; Jung, H.H.; Hirata, O.; Goushi, K.; Yahiro, M.; Adachi, C. Improvement of Electroluminescence Performance of Organic Light-Emitting Diodes with a Liquid-Emitting Layer by Introduction of Electrolyte and a Hole-Blocking Layer. Adv. Mater. 2011, 23, 889–893. [Google Scholar] [CrossRef]
- Nalwa, K.S.; Cai, Y.; Thoeming, A.L.; Shinar, J.; Shinar, R.; Chaudhary, S. Polythiophene-Fullerene Based Photodetectors: Tuning of Spectral Response and Application in Photoluminescence Based (Bio)Chemical Sensors. Adv. Mater. 2010, 22, 4157–4161. [Google Scholar] [CrossRef]
- Sagmeister, M.; Lamprecht, B.; Kraker, E.; Haase, A.; Jakopic, G.; Köstler, S.; Ditlbacher, H.; Galler, N.; Abel, T.; Mayr, T. Integrated Organic Optical Sensor Arrays Based on Ring-Shaped Organic Photodiodes. In Organic Semiconductors in Sensors and Bioelectronics IV, Proceedings of the SPIE 8118, San Diego, CA, USA, 21 August 2011; Shinar, R., Kymissis, I., Eds.; SPIE International Society for Optical Engineering: Bellingham, WA, USA, 2011; Volume 8118, p. 811805. [Google Scholar]
- Fang, Y.; Huang, J. Resolving Weak Light of Sub-picowatt per Square Centimeter by Hybrid Perovskite Photodetectors Enabled by Noise Reduction. Adv. Mater. 2015, 27, 2804–2810. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, D.; Zhang, F.; Li, L.; Du, M.; Wang, C.; Yu, Y.; Huang, Q.; Zhang, M.; Li, L.; et al. Highly Sensitive Low-Bandgap Perovskite Photodetectors with Response from Ultraviolet to the Near-Infrared Region. Adv. Funct. Mater. 2017, 27, 1703953. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Bao, C.; Fang, Y.; Wei, H.; Tang, S.; Zhang, F.; Huang, J. Self-Filtered Narrowband Perovskite Photodetectors with Ultrafast and Tuned Spectral Response. Adv. Opt. Mater. 2017, 5, 1700672. [Google Scholar] [CrossRef]
- Xiao, X.; Bao, C.; Fang, Y.; Dai, J.; Ecker, B.R.; Wang, C.; Lin, Y.; Tang, S.; Liu, Y.; Deng, Y.; et al. Argon Plasma Treatment to Tune Perovskite Surface Composition for High Efficiency Solar Cells and Fast Photodetectors. Adv. Mater. 2018, 30, 1705176. [Google Scholar] [CrossRef]
- Han, D.; Khan, Y.; Ting, J.; Zhu, J.; Combe, C.; Wadsworth, A.; McCulloch, I.; Arias, A.C. Pulse Oximetry Using Organic Optoelectronics under Ambient Light. Adv. Mater. Technol. 2020, 5, 1901122. [Google Scholar] [CrossRef]
- Chow, P.C.Y.; Someya, T. Organic Photodetectors for Next-Generation Wearable Electronics. Adv. Mater. 2020, 32, 1902045. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Chen, J.; Li, Y.; Tang, J. Recent Progress in Organic Photodetectors and their Applications. Adv. Sci. 2020, 8, 2002418. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, E.; Lee, Y.; Kim, H.; Lee, J.; Kim, M.; Yoo, H.J.; Yoo, S. Toward all-day wearable health monitoring: An ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 2018, 4, eaas9530. [Google Scholar] [CrossRef]
- Bilgaiyan, A.; Elsamnah, F.; Ishidai, H.; Shim, C.-H.; Bin Misran, M.A.; Adachi, C.; Hattori, R. Enhancing Small-Molecule Organic Photodetector Performance for Reflectance-Mode Photoplethysmography Sensor Applications. ACS Appl. Electron. Mater. 2020, 2, 1280–1288. [Google Scholar] [CrossRef]
- Alamouti, S.F.; Jan, J.; Yalcin, C.; Ting, J.; Arias, A.C.; Muller, R. A Sparse Sampling Sensor Front-End IC for Low Power Continuous SpO2 & HR Monitoring. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 997–1007. [Google Scholar]
- Pandey, R.K.; Chao, P.C.-P. A Dual-Channel PPG Readout System with Motion-Tolerant Adaptability for OLED-OPD Sensors. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 36–51. [Google Scholar] [CrossRef]
- Liu, R.; Xu, C.; Biswas, R.; Shinar, J.; Shinar, R. MoO3 as combined hole injection layer and tapered spacer in combinatorial multicolor microcavity organic light emitting diodes. Appl. Phys. Lett. 2011, 99, 093305. [Google Scholar] [CrossRef]
- Manna, E.; Xiao, T.; Shinar, J.; Shinar, R. Organic Photodetectors in Analytical Applications. Electronics 2015, 4, 688–722. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Chen, J.; Wang, F.; Han, R.; Zhang, C.; Kong, J.; Li, L.; Yang, J. A Review of Perovskite-Based Photodetectors and Their Applications. Nanomaterials 2022, 12, 4390. [Google Scholar] [CrossRef] [PubMed]
- Harwell, J.R.; Glackin, J.M.E.; Davis, N.J.L.K.; Gillanders, R.N.; Credgington, D.; Turnbull, G.A.; Samuel, I.D.W. Sensing of explosive vapor by hybrid perovskites: Effect of dimensionality. APL Mater. 2020, 8, 071106. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Torricelli, F.; Adrahtas, D.Z.; Bao, Z.; Berggren, M.; Biscarini, F.; Bonfiglio, A.; Bortolotti, C.A.; Frisbie, C.D.; Macchia, E.; Malliaras, G.G.; et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. 2021, 1, 66. [Google Scholar]
- Luo, L.; Liu, Z. Recent progress in organic field-effect transistor-based chem/bio-sensors. View 2022, 3, 20200115. [Google Scholar] [CrossRef]
- Zhang, X.; Pu, Z.; Su, X.; Li, C.; Zheng, H.; Li, D. Flexible organic field-effect transistors-based biosensors: Progress and perspectives. Anal. Bioanal. Chem. 2023, 415, 1607–1625. [Google Scholar] [CrossRef]
- Wang, D.; Noël, V.; Piro, B. Electrolytic Gated Organic Field-Effect Transistors for Application in Biosensors—A Review. Electronics 2016, 5, 9. [Google Scholar] [CrossRef]
- Doumbia, A.; Webb, M.; Behrendt, J.M.; Wilson, R.J.; Turner, M.L. Robust Microfluidic Integrated Electrolyte-Gated Organic Field-Effect Transistor Sensors for Rapid, In Situ and Label-Free Monitoring of DNA Hybridization. Adv. Electron. Mater. 2022, 8, 2200142. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. New trends in single-molecule bioanalytical detection. Anal. Bioanal. Chem. 2020, 412, 5005–5014. [Google Scholar] [CrossRef]
- Curto, V.F.; Marchiori, B.; Hama, A.; Pappa, A.-M.; Ferro, M.P.; Braendlein, M.; Rivnay, J.; Fiocchi, M.; Malliaras, G.G.; Ramuz, M.; et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring. Microsyst. Nanoeng. 2017, 3, 17028. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Marquez, A.V.; McEvoy, N.; Pakdel, A. Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics. Molecules 2020, 25, 5288. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Wang, J.; Lee, S.; Yokota, T.; Someya, T. Gas-Permeable Organic Electrochemical Transistor Embedded with a Porous Solid-State Polymer Electrolyte as an on-Skin Active Electrode for Electrophysiological Signal Acquisition. Adv. Funct. Mater. 2022, 32, 2200458. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; LeLeux, P.; Ghestem, A.; Ismailova, E.; Hervé, T.; Sanaur, S.; Bernard, C. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef]
- Lee, W.; Kim, D.; Matsuhisa, N.; Nagase, M.; Sekino, M.; Malliaras, G.G.; Yokota, T.; Someya, T. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl. Acad. Sci. USA 2017, 114, 10554–10559. [Google Scholar] [CrossRef]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef]
- Possanzini, L.; Decataldo, F.; Mariani, F.; Gualandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Textile sensors platform for the selective and simultaneous detection of chloride ion and pH in sweat. Sci. Rep. 2020, 10, 17180. [Google Scholar] [CrossRef]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Tang, G.; Cao, J.; Liu, H.; Zhao, Z.; Griggs, S.; Yang, A.; Wang, N.; Cheng, H.; Liu, C.; et al. Perovskite Solar Cell-Gated Organic Electrochemical Transistors for Flexible Photodetectors with Ultrahigh Sensitivity and Fast Response. Adv. Mater. 2023, 35, 2207763. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Qin, Z.; Zhang, Y.; Chen, C.; Liu, S.; Chen, H. Expanding the potential of biosensors: A review on organic field effect transistor (OFET) and organic electrochemical transistor (OECT) biosensors. Mater. Futures 2023, 2, 042401. [Google Scholar] [CrossRef]
- Camou, S.; Kitamura, M.; Gouy, J.-P.; Fujita, H.; Arakawa, Y.; Fujii, T. Applications of Photonic Technology 5; SPIE: Bellingham, WA, USA, 2003; Volume 4833. [Google Scholar] [CrossRef]
- Schöler, L.; Seibel, K.; Panczyk, K.; Böhm, M. An integrated PLED—A light source for application specific lab-on-microchips (ALM). Microelectron. Eng. 2009, 86, 1502–1504. [Google Scholar] [CrossRef]
- Edel, J.B.; Beard, N.P.; Hofmann, O.; Demello, J.C.; Bradley, D.D.C.; Demello, A.J. Thin-film polymer light emitting diodes as integrated excitation sources for microscale capillary electrophoresis. Lab Chip 2004, 4, 136–140. [Google Scholar] [CrossRef]
- Yao, B.; Yang, H.; Liang, Q.; Luo, G.; Wang, L.; Ren, K.; Gao, Y.; Wang, Y.; Qiu, Y. High-Speed, Whole-Column Fluorescence Imaging Detection for Isoelectric Focusing on a Microchip Using an Organic Light Emitting Diode as Light Source. Anal. Chem. 2006, 78, 5845–5850. [Google Scholar] [CrossRef]
- Vannahme, C.; Klinkhammer, S.; Lemmer, U.; Mappes, T. Plastic lab-on-a-chip for fluorescence excitation with integrated organic semiconductor lasers. Opt. Express 2011, 19, 8179–8186. [Google Scholar] [CrossRef]
- Marcello, A.; Sblattero, D.; Cioarec, C.; Maiuri, P.; Melpignano, P. A deep-blue OLED-based biochip for protein microarray fluorescence detection. Biosens. Bioelectron. 2013, 46, 44–47. [Google Scholar] [CrossRef]
- Acharya, A.; Packirisamy, M.; Izquierdo, R. LED Hybrid Integrated Polymer Microfluidic Biosensing for Point of Care Testing. Micromachines 2015, 6, 1406–1420. [Google Scholar] [CrossRef]
- Song, Y.J.; Kim, J.-W.; Cho, H.-E.; Son, Y.H.; Lee, M.H.; Lee, J.; Choi, K.C.; Lee, S.-M. Fibertronic Organic Light-Emitting Diodes toward Fully Addressable, Environmentally Robust, Wearable Displays. ACS Nano 2020, 14, 1133–1140. [Google Scholar] [CrossRef]
- Shinar, R.; Shinar, J. Light extraction from organic light emitting diodes (OLEDs). J. Phys. Photonics 2022, 4, 032002. [Google Scholar] [CrossRef]
- Dykstra, E.; Fralaide, M.; Zhang, Y.; Biswas, R.; Slafer, W.D.; Shinar, J.; Shinar, R. OLEDs on planarized light outcoupling-enhancing structures in plastic. Org. Electron. 2022, 111, 106648. [Google Scholar] [CrossRef]
- Kasahara, T.; Matsunami, S.; Edura, T.; Oshima, J.; Adachi, C.; Shoji, S.; Mizuno, J. Fabrication and performance evaluation of microfluidic organic light emitting diode. Sens. Actuators A Phys. 2013, 195, 219–223. [Google Scholar] [CrossRef]
- Tsuwaki, M.; Kasahara, T.; Edura, T.; Matsunami, S.; Oshima, J.; Shoji, S.; Adachi, C.; Mizuno, J. Fabrication and characterization of large-area flexible microfluidic organic light-emitting diode with liquid organic semiconductor. Sens. Actuators A Phys. 2014, 216, 231–236. [Google Scholar] [CrossRef]
- Kasahara, T.; Matsunami, S.; Edura, T.; Ishimatsu, R.; Oshima, J.; Tsuwaki, M.; Imato, T.; Shoji, S.; Adachi, C.; Mizuno, J. Multi-color microfluidic organic light-emitting diodes based on on-demand emitting layers of pyrene-based liquid organic semiconductors with fluorescent guest dopants. Sens. Actuators B Chem. 2015, 207, 481–489. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kasahara, T.; Edura, T.; Oshima, J.; Ishimatsu, R.; Tsuwaki, M.; Imato, T.; Shoji, S.; Mizuno, J. Microfluidic White Organic Light-Emitting Diode Based on Integrated Patterns of Greenish-Blue and Yellow Solvent-Free Liquid Emitters. Sci. Rep. 2015, 5, 14822. [Google Scholar] [CrossRef]
- Kasahara, T.; Kuwae, H.; Kobayashi, N.; Nobori, A.; Oshima, J.; Shoji, S.; Mizuno, J. Recent Advances in Research and Development of Microfluidic Organic Light-Emitting Devices. J. Photopolym. Sci. Technol. 2017, 30, 467–474. [Google Scholar] [CrossRef]
- Kawamura, M.; Kuwae, H.; Kamibayashi, T.; Oshima, J.; Kasahara, T.; Shoji, S.; Mizuno, J. Liquid/solution-based microfluidic quantum dots light-emitting diodes for high-colour-purity light emission. Sci. Rep. 2020, 10, 14528. [Google Scholar] [CrossRef]
- Muramatsu, J.; Onoe, H. Microfluidic multicolor display by juxtapositional color mixing with a pattern of primary color pixels. J. Micromech. Microeng. 2022, 32, 025002. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.-J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.-Y.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef]
- Yuan, X.; Zhao, Y.; Xie, D.; Pan, L.; Liu, X.; Duan, C.; Huang, F.; Cao, Y. Polythiophenes for organic solar cells with efficiency surpassing 17%. Joule 2022, 6, 647–661. [Google Scholar] [CrossRef]
- Hofmann, O.; Miller, P.; Sullivan, P.; Jones, T.S.; Demello, J.C.; Bradley, D.D.; Demello, A.J. Thin-film organic photodiodes as integrated detectors for microscale chemiluminescence assays. Sens. Actuators B 2005, 106, 878–884. [Google Scholar] [CrossRef]
- Wang, X.; Hofmann, O.; Das, R.; Barrett, E.M.; Demello, A.J.; Demello, J.C.; Bradley, D.D.C. Integrated thin-film polymer/fullerene photodetectors for on-chip microfluidic chemiluminescence detection. Lab Chip 2007, 7, 58–63. [Google Scholar] [CrossRef]
- Wang, X.; Amatatongchai, M.; Nacapricha, D.; Hofmann, O.; de Mello, J.C.; Bradley, D.D.; de Mello, A.J. Thin-film organic photodiodes for integrated on-chip chemiluminescence detection—Application to antioxidant capacity screening. Sens. Actuators B 2009, 140, 643–648. [Google Scholar] [CrossRef]
- Wojciechowski, J.R.; Shriver-Lake, L.C.; Yamaguchi, M.Y.; Fureder, E.; Pieler, R.; Schamesberger, M.; Winder, C.; Prall, H.J.; Sonnleitner, M.; Ligler, F.S. Organic Photodiodes for Biosensor Miniaturization. Anal. Chem. 2009, 81, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Dong, T. Microfluidic Biosensor Array with Integrated Poly(2,7-Carbazole)/Fullerene-Based Photodiodes for Rapid Multiplexed Detection of Pathogens. Sensors 2013, 13, 15898–15911. [Google Scholar] [CrossRef]
- Charwat, V.; Purtscher, M.; Tedde, S.F.; Hayden, O.; Ertl, P. Standardization of microfluidic cell cultures using integrated organic photodiodes and a miniaturized electrode array. Lab Chip 2013, 13, 785. [Google Scholar] [CrossRef]
- Abel, T.; Sagmeister, M.; Lamprecht, B.; Kraker, E.; Köstler, S.; Ungerböck, B.; Mayr, T. Filter-free integrated sensor array based on luminescence and absorbance measurements using ring-shaped organic photodiodes. Anal. Bioanal. Chem. 2012, 404, 2841–2849. [Google Scholar] [CrossRef]
- Titov, I.; Kopke, M.; Schneidewind, N.C.; Buhl, J.; Murat, Y.; Gerken, M. OLED-OPD Matrix for Sensing on a Single Flexible Substrate. IEEE Sens. J. 2020, 20, 7540–7547. [Google Scholar] [CrossRef]
- Titov, I.; Köpke, M.; Gerken, M. Monolithic Integrated OLED–OPD Unit for Point-of-Need Nitrite Sensing. Sensors 2022, 22, 910. [Google Scholar] [CrossRef]
- Pais, A.; Banerjee, A.; Klotzkin, D.; Papautsky, I. High-sensitivity, disposable lab-on-a-chip with thin-film organic electronics for fluorescence detection. Lab Chip 2008, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, F.; Chalifour, A.; Yu, L.; Chodavarapu, V.; Juneau, P.; Izquierdo, R. Algal fluorescence sensor integrated into a microfluidic chip for water pollutant detection. Lab Chip 2012, 12, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Exner, A.T.; Pavlichenko, I.; Baierl, D.; Schmidt, M.; Derondeau, G.; Lotsch, B.V.; Lugli, P.; Scarpa, G. A step towards the electrophotonic nose: Integrating 1D photonic crystals with organic light-emitting diodes and photodetectors. Laser Photon. Rev. 2014, 8, 726–733. [Google Scholar] [CrossRef]
- Liu, R.; Ishimatsu, R.; Yahiro, M.; Adachi, C.; Nakano, K.; Imato, T. Fluorometric flow-immunoassay for alkylphenol polyethoxylates on amicrochip containing a fluorescence detector comprised of an organic light emitting diode and an organic photodiode. Talanta 2015, 134, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Kemper, F.; Beckert, E.; Eberhardt, R.; Tünnermann, A. Highly sensitive on-chip fluorescence sensor with integrated fully solution processed organic light sources and detectors. RSC Adv. 2017, 7, 26384–26391. [Google Scholar] [CrossRef]
- Someya, T.; Dodabalapur, A.; Gelperin, A.; Katz, H.E.; Bao, Z. Integration and Response of Organic Electronics with Aqueous Microfluidics. Langmuir 2002, 18, 5299–5302. [Google Scholar] [CrossRef]
- White, S.P.; Sreevatsan, S.; Frisbie, C.D.; Dorfman, K.D. Rapid, Selective, Label-Free Aptameric Capture and Detection of Ricin in Potable Liquids Using a Printed Floating Gate Transistor. ACS Sens. 2016, 1, 1213–1216. [Google Scholar] [CrossRef]
- de Brito Oliveira, D.C.; Costa, F.H.M.; da Silva, J.A.F. The Integration of Field Effect Transistors to Microfluidic Devices. Micromachines 2023, 14, 791. [Google Scholar] [CrossRef]
- Didier, P.; Lobato-Dauzier, N.; Clément, N.; Genot, A.J.; Sasaki, Y.; Leclerc, É.; Minamiki, T.; Sakai, Y.; Fujii, T.; Minami, T. Microfluidic System with Extended-Gate-Type Organic Transistor for Real-Time Glucose Monitoring. ChemElectroChem 2020, 7, 1332–1336. [Google Scholar] [CrossRef]
- Ohshiro, K.; Sasaki, Y.; Zhou, Q.; Didier, P.; Nezaki, T.; Yasuike, T.; Kamiko, M.; Minami, T. A microfluidic organic transistor for reversible and real-time monitoring of H2O2 at ppb/ppt levels in ultrapure water. Chem. Commun. 2022, 58, 5721. [Google Scholar] [CrossRef]
- Dorfman, K.D.; Adrahtas, D.Z.; Thomas, M.S.; Frisbie, C.D. Microfluidic opportunities in printed electrolyte-gated transistor biosensors. Biomicrofluidics 2020, 14, 011301. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Martines, L.; Bonfiglio, A. Interfacing cells with organic transistors: A review of in vitro and in vivo applications. Lab Chip 2021, 21, 795. [Google Scholar] [CrossRef] [PubMed]
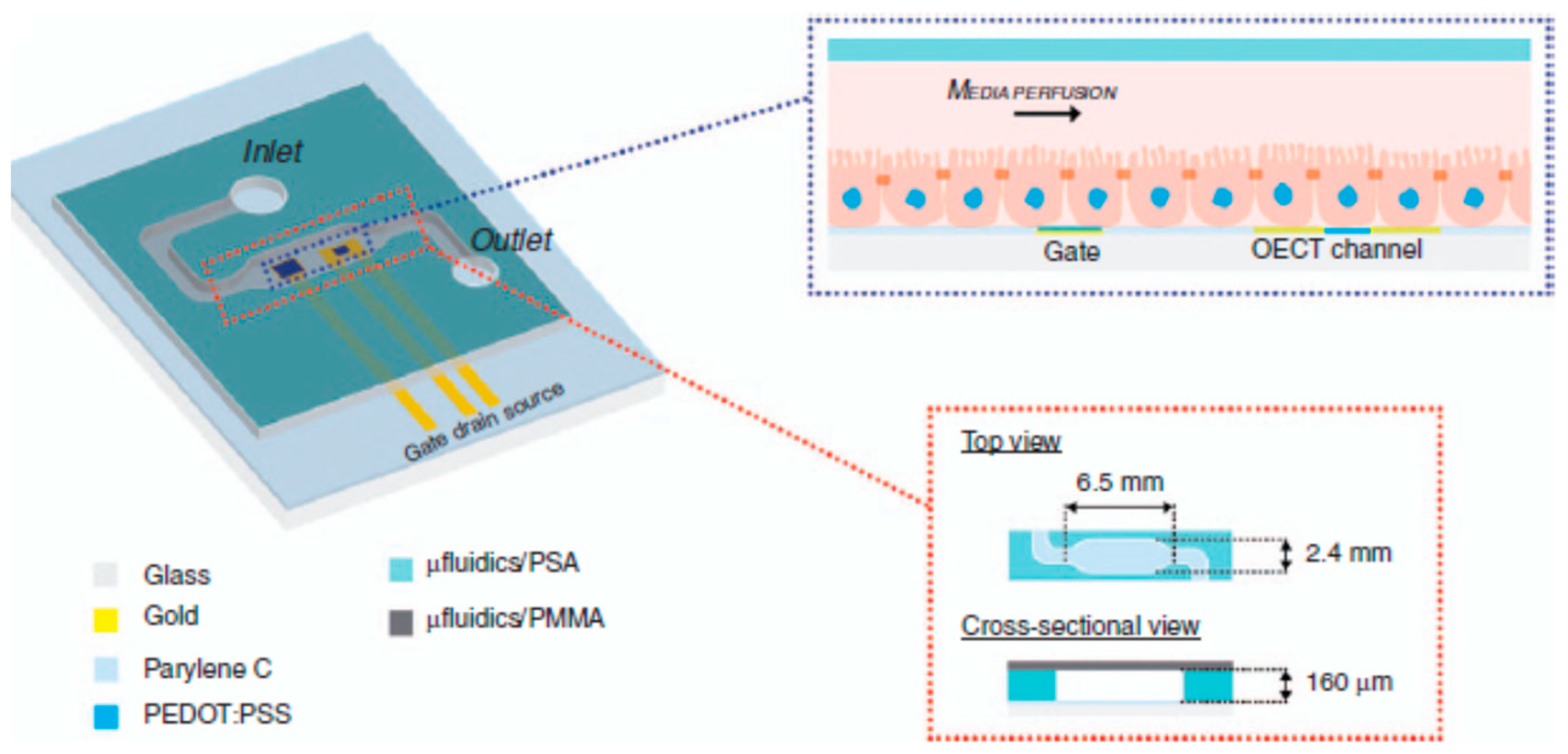
- Berto, M.; Diacci, C.; D’Agata, R.; Pinti, M.; Bianchini, E.; Di Lauro, M.; Casalini, S.; Cossarizza, A.; Berggren, M.; Simon, D.; et al. EGOFET Peptide Aptasensor for Label-Free Detection of Inflammatory Cytokines in Complex Fluids. Adv. Biosyst. 2018, 2, 1700072. [Google Scholar] [CrossRef]
- Parkula, V.; Berto, M.; Diacci, C.; Patrahau, B.; Di Lauro, M.; Kovtun, A.; Liscio, A.; Sensi, M.; Samorì, P.; Greco, P.; et al. Harnessing Selectivity and Sensitivity in Electronic Biosensing: A Novel Lab-on-Chip Multigate Organic Transistor. Anal. Chem. 2020, 92, 9330–9337. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Casalini, S.; Parkula, V.; Selvaraj, M.; Saygin, G.D.; Greco, P.; Biscarini, F.; Mas-Torrent, M. Label-free immunodetection of α-synuclein by using a microfluidics coplanar electrolyte-gated organic field-effect transistor. Biosens. Bioelectron. 2020, 167, 112433. [Google Scholar] [CrossRef]
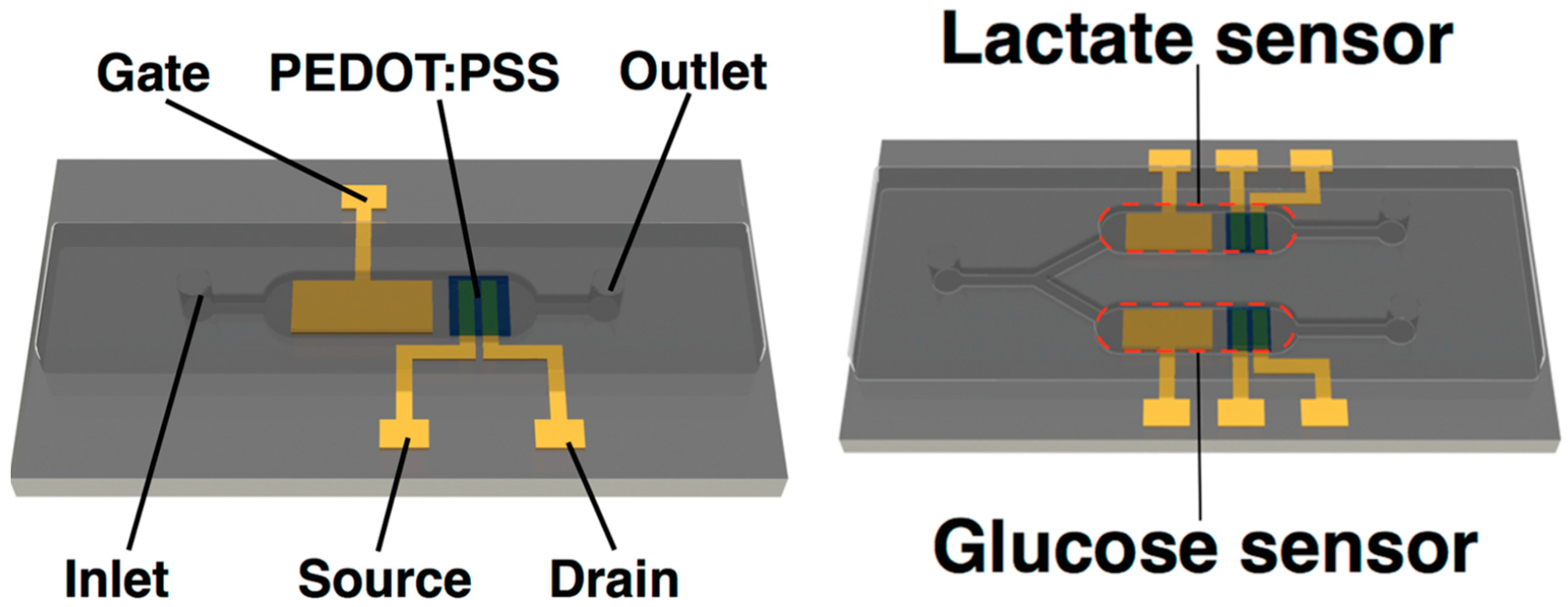
- Ji, X.; Lau, H.Y.; Ren, X.; Peng, B.; Zhai, P.; Feng, S.-P.; Paddy, K.; Chan, L. Highly Sensitive Metabolite Biosensor Based on Organic Electrochemical Transistor Integrated with Microfluidic Channel and Poly(N-vinyl-2-pyrrolidone)-Capped Platinum Nanoparticles. Adv. Mater. Technol. 2016, 1, 1600042. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Hama, A.; Chen, X.; McCulloch, I.; Inal, S. Microfluidics integrated n-type organic electrochemical transistor for metabolite sensing. Sens. Actuators B Chem. 2021, 329, 129251. [Google Scholar] [CrossRef]
- Koklu, A.; Wustoni, S.; Musteata, V.-E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic Integrated Organic Electrochemical Transistor with a Nanoporous Membrane for Amyloid-β Detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef]
- Peruzzi, C.; Battistoni, S.; Montesarchio, D.; Cocuzza, M.; Marasso, S.L.; Verna, A.; Pasquardini, L.; Verucchi, R.; Aversa, L.; Erokhin, V.; et al. Interfacing aptamers, nanoparticles and graphene in a hierarchical structure for highly selective detection of biomolecules in OECT devices. Sci. Rep. 2021, 11, 9380. [Google Scholar] [CrossRef]
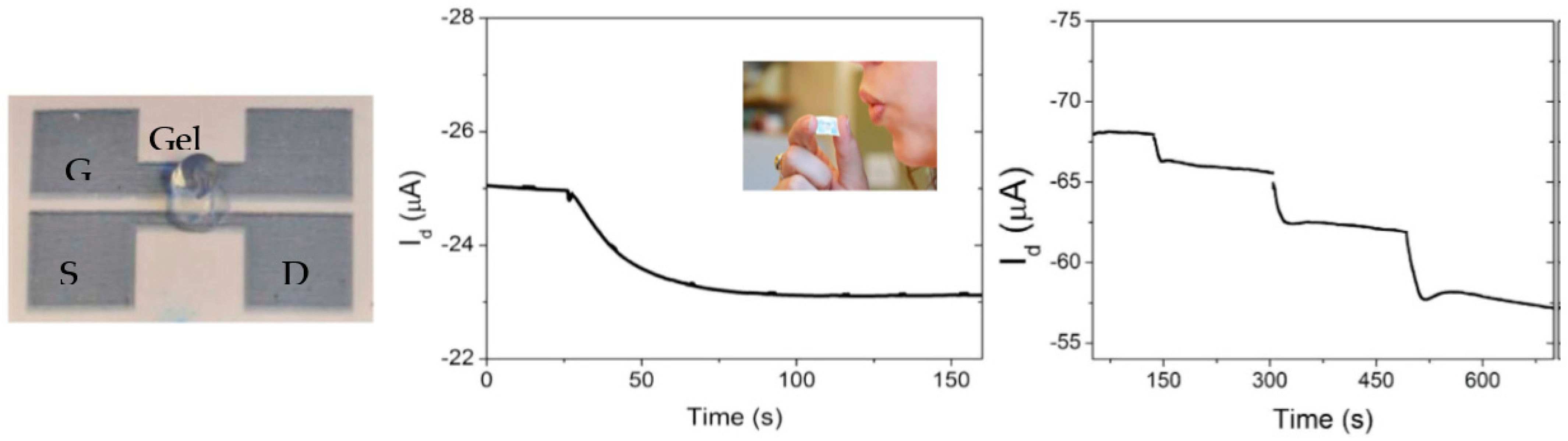
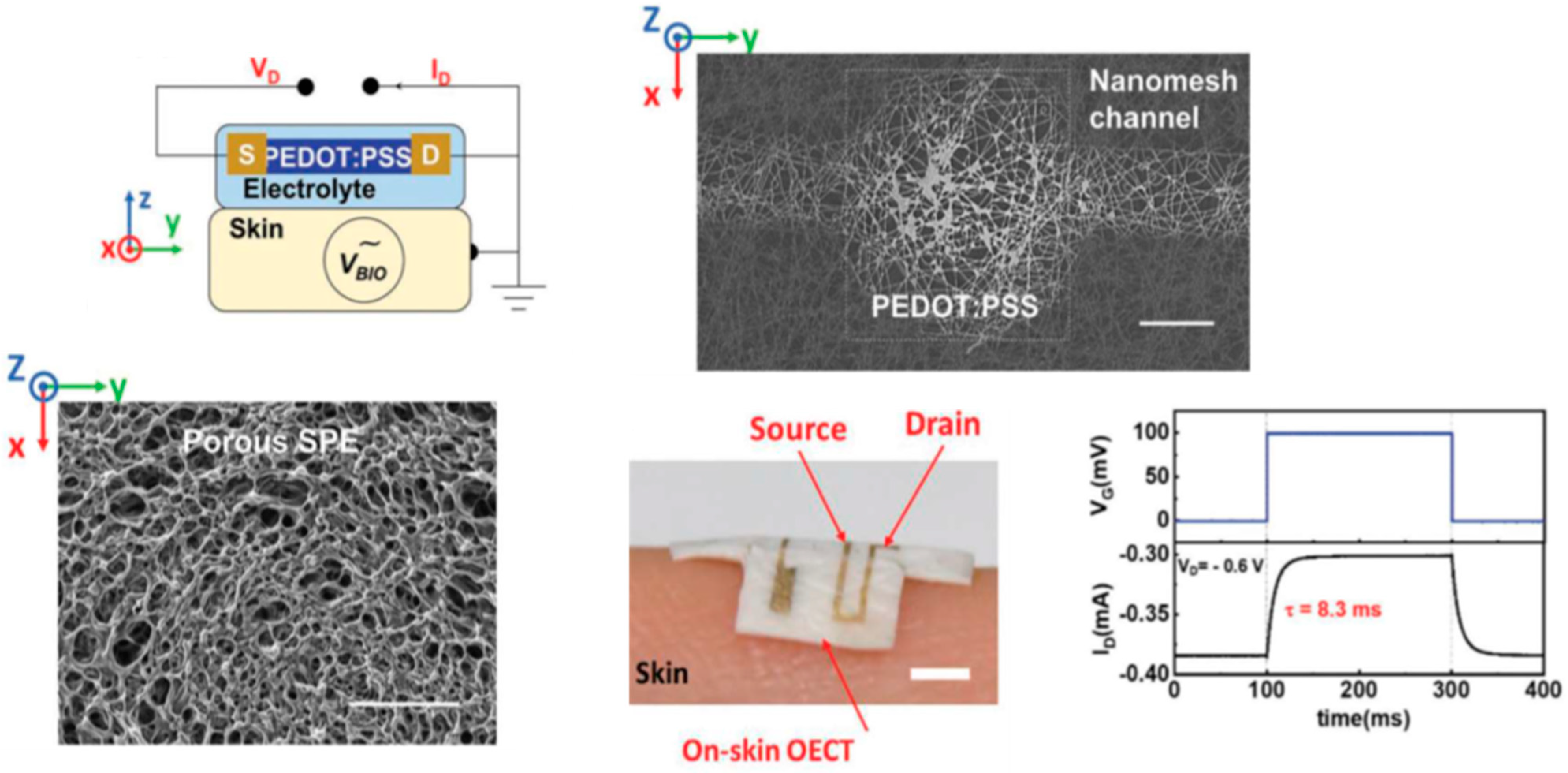
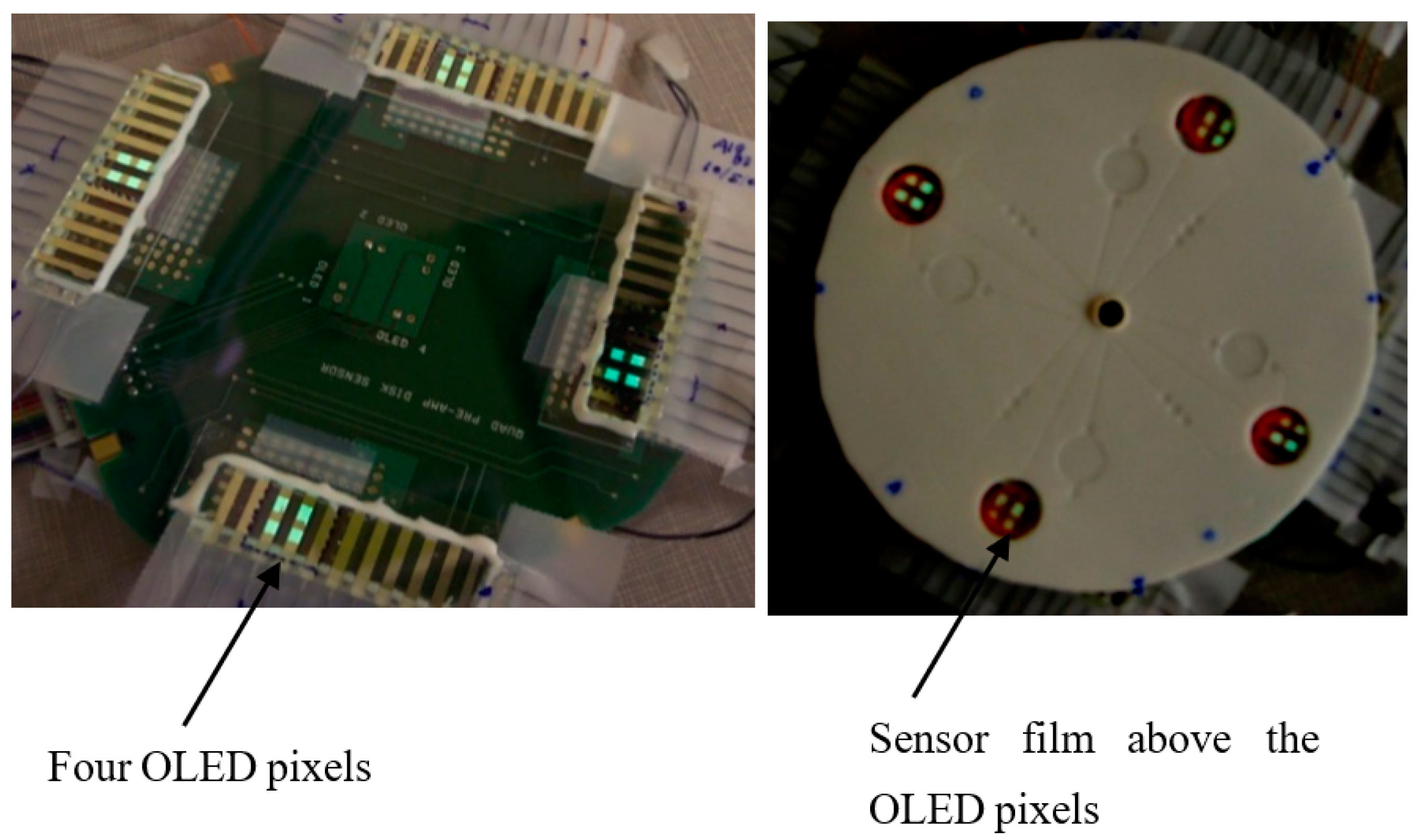



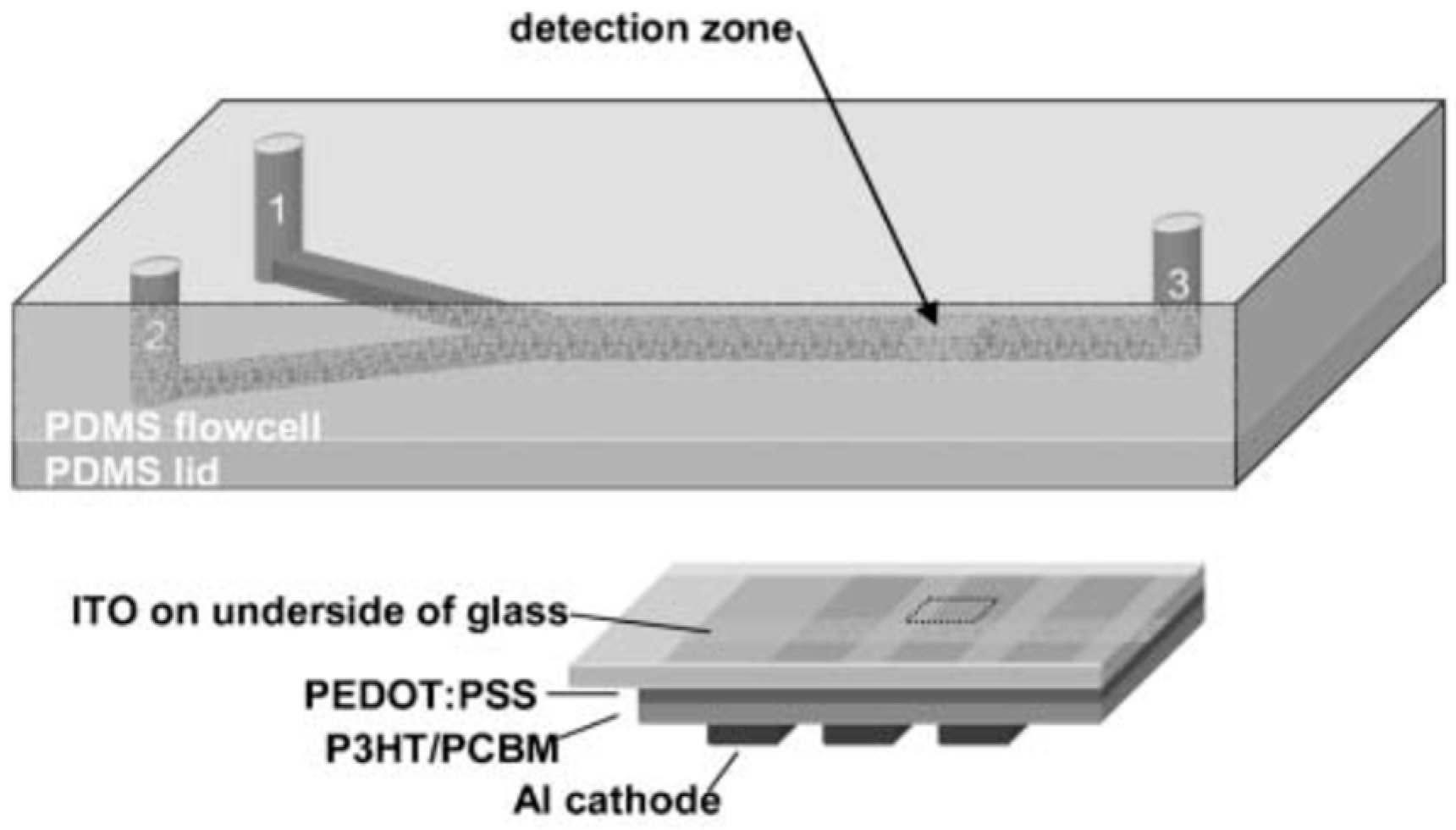


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinar, R.; Shinar, J. Organic Electronics—Microfluidics/Lab on a Chip Integration in Analytical Applications. Sensors 2023, 23, 8488. https://doi.org/10.3390/s23208488
Shinar R, Shinar J. Organic Electronics—Microfluidics/Lab on a Chip Integration in Analytical Applications. Sensors. 2023; 23(20):8488. https://doi.org/10.3390/s23208488
Chicago/Turabian StyleShinar, Ruth, and Joseph Shinar. 2023. "Organic Electronics—Microfluidics/Lab on a Chip Integration in Analytical Applications" Sensors 23, no. 20: 8488. https://doi.org/10.3390/s23208488




