Quantification of hCG Hormone Using Tapered Optical Fiber Decorated with Gold Nanoparticles
Abstract
:1. Introduction
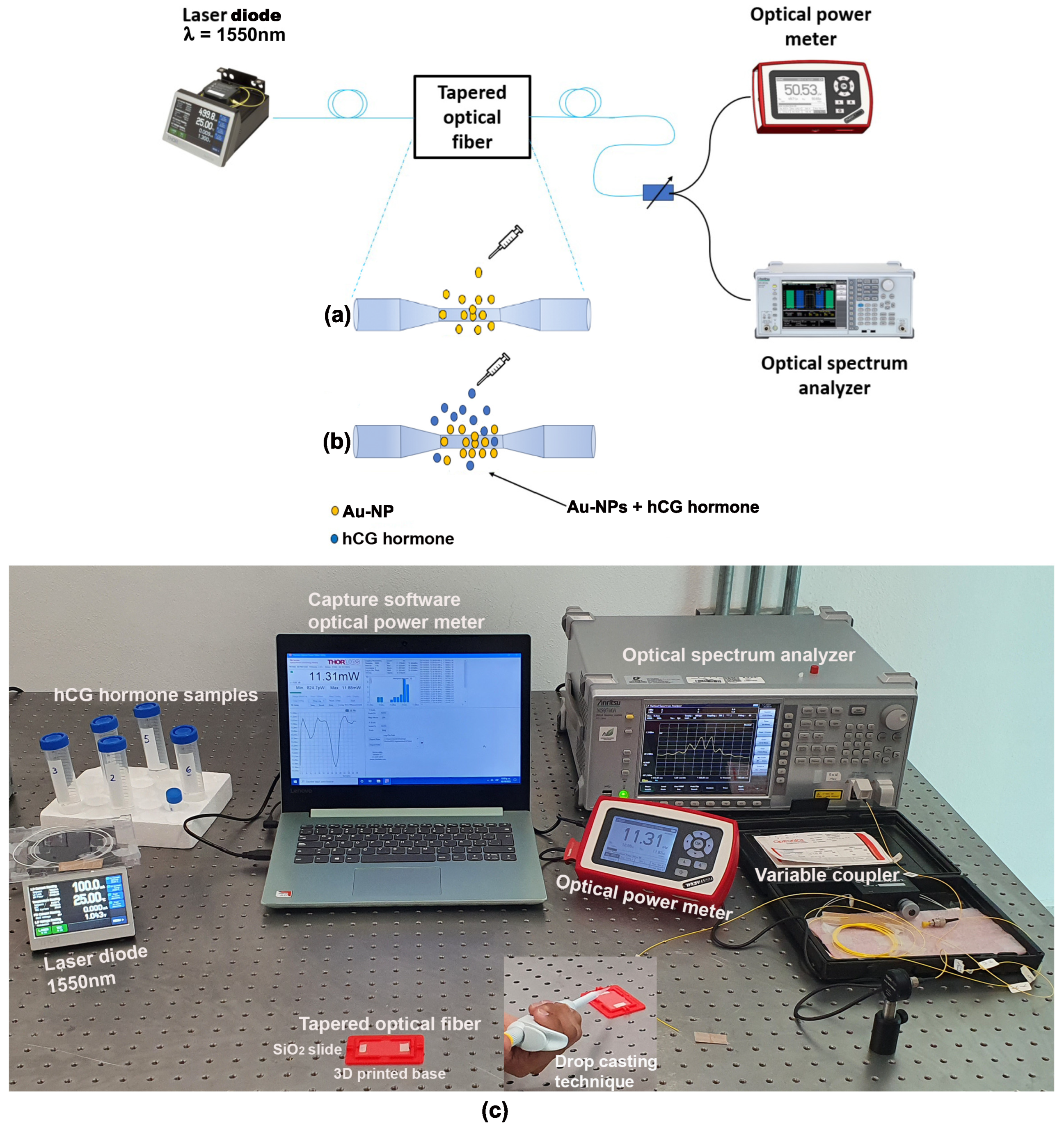
2. Experimental Setup
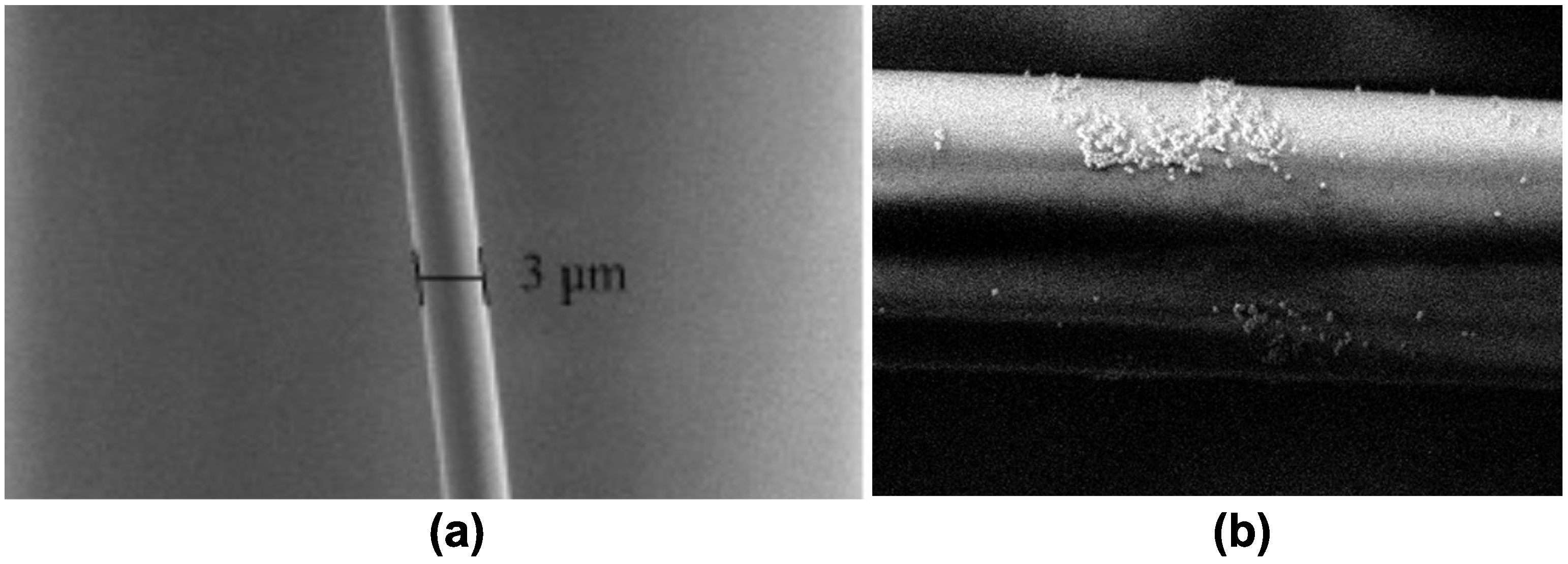
2.1. Tapered Optical Fiber Fabrication
2.2. Tapered Optical Fiber Decoration
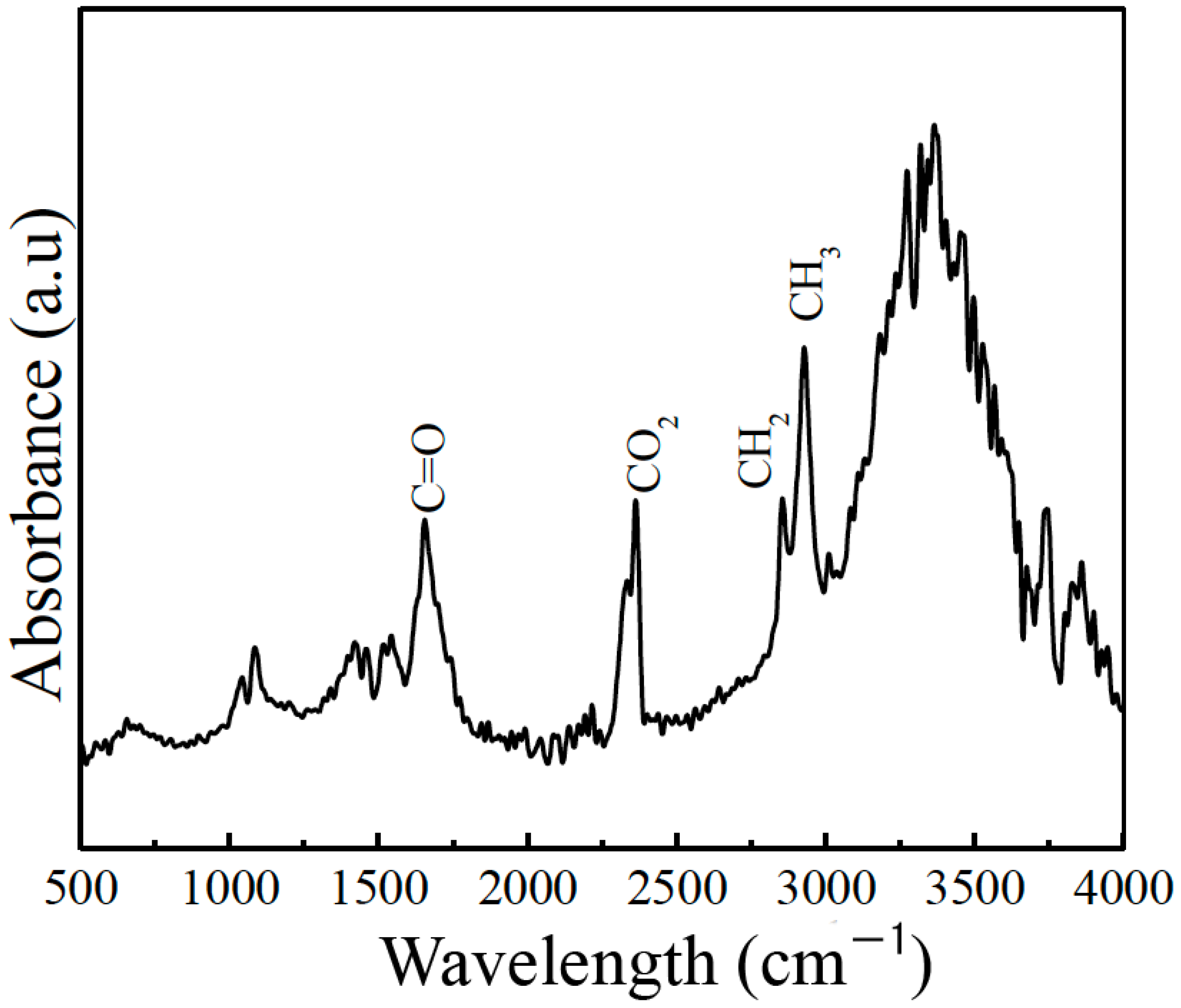
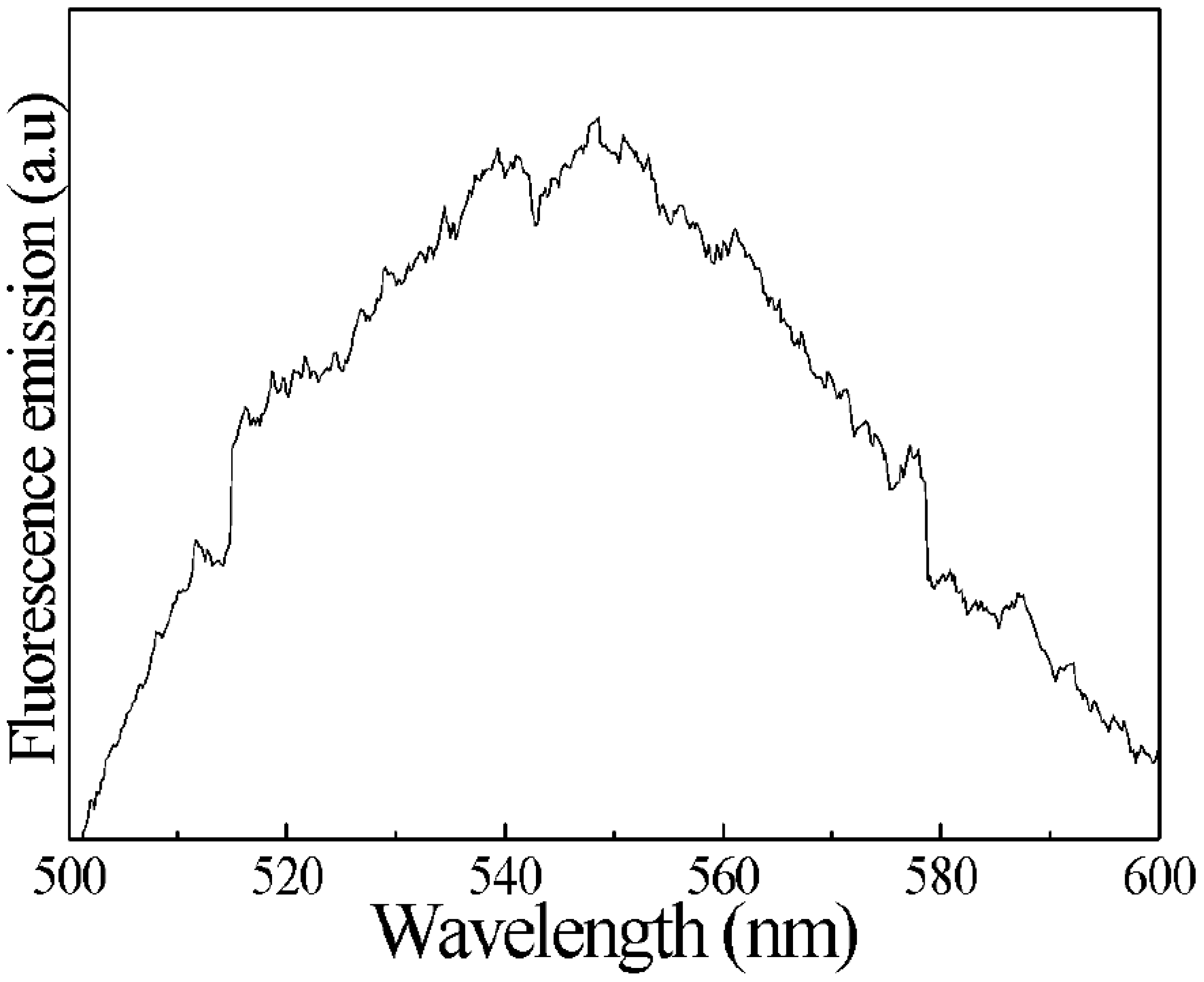
2.3. Characterization of the hCG Hormone
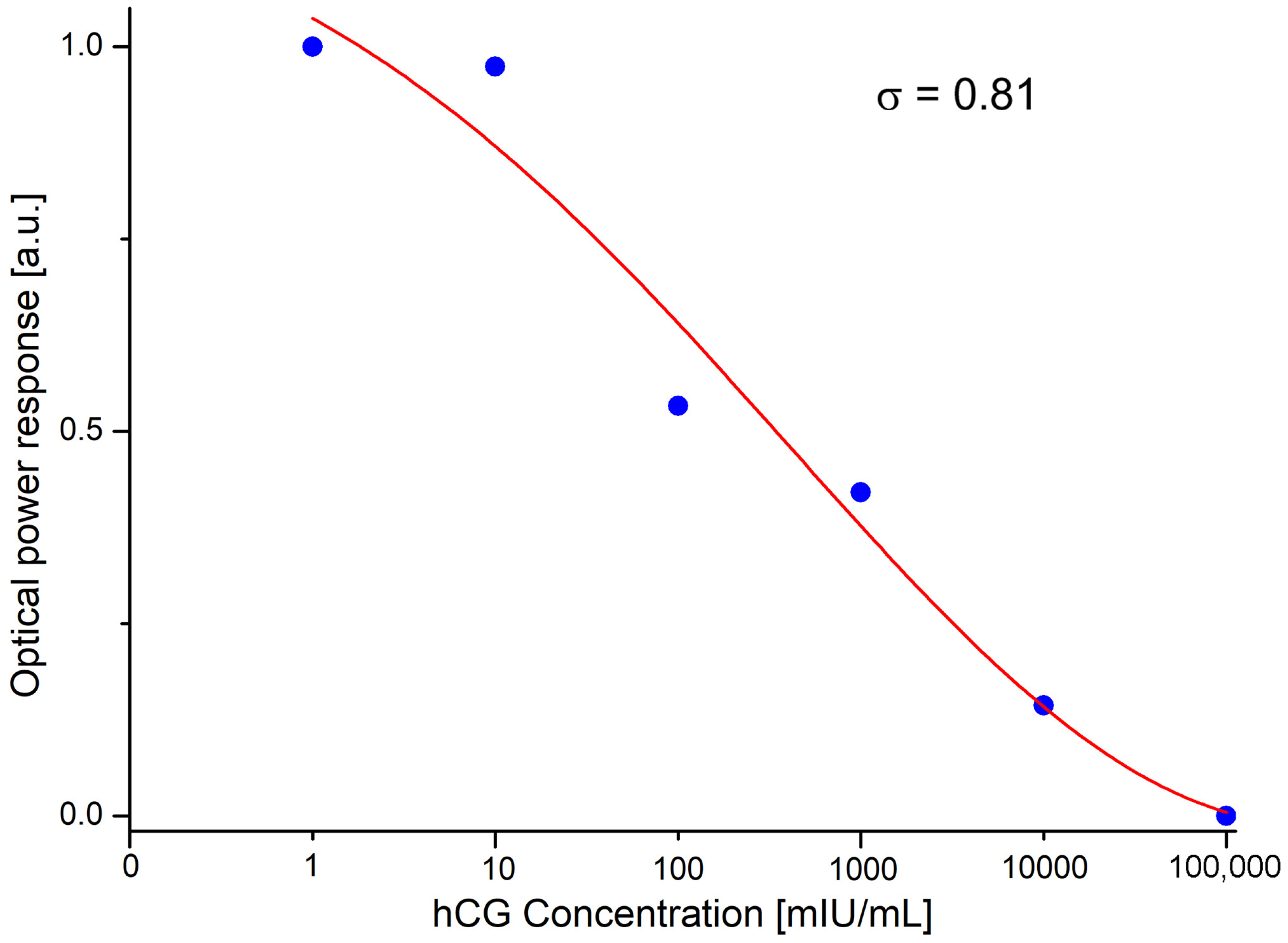
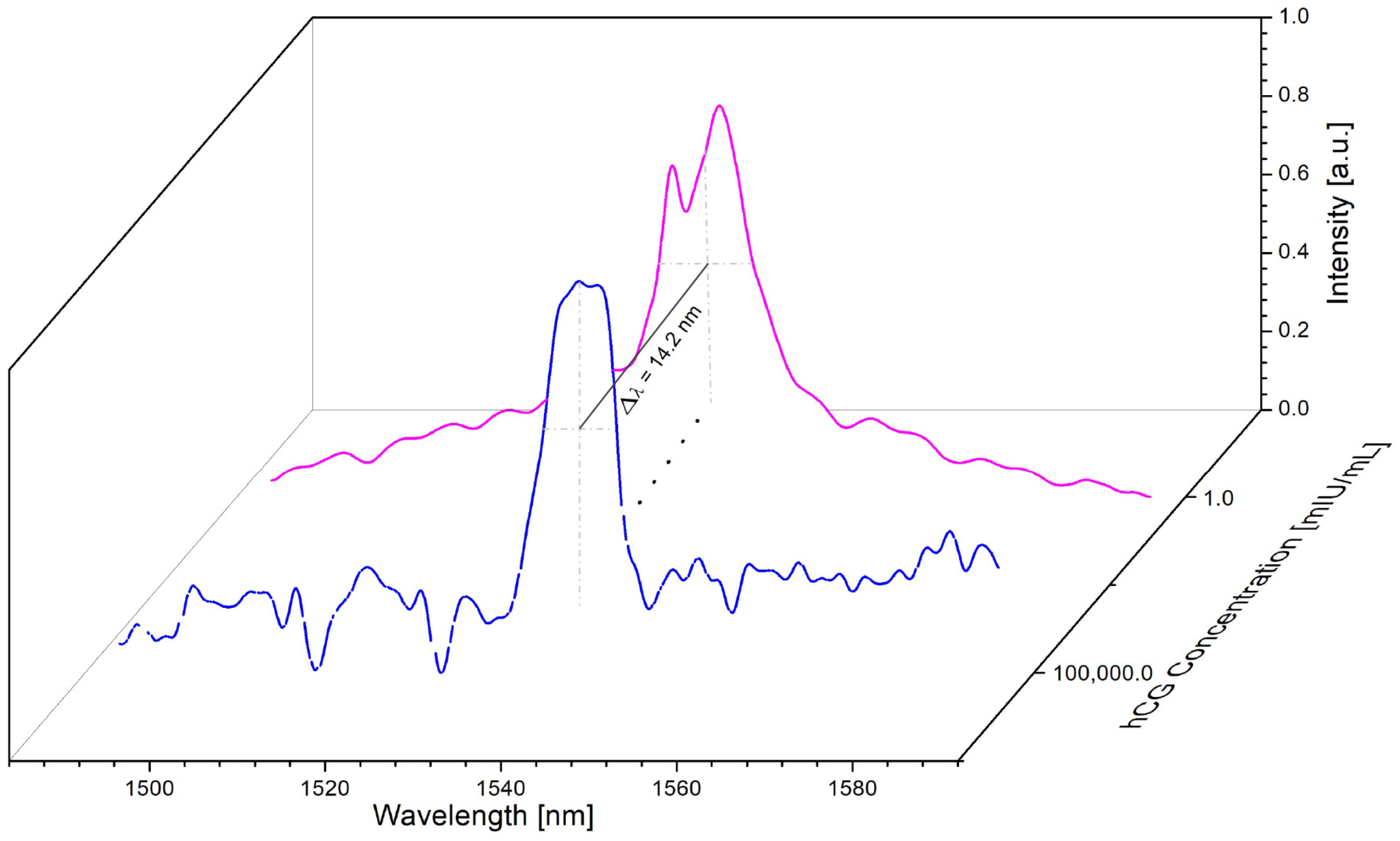
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenman, U.H.; Alfthan, H.; Hotakainen, K. Human chorionic gonadotropin in cancer. Clin. Biochem. 2004, 37, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Dando, I.; Carmona-Carmona, C.A.; Zampieri, N. Human chorionic gonadotropin-mediated induction of breast cancer cell proliferation and differentiation. Cells 2021, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Treeck, O.; Ortmann, O.O. Human chorionic gonadotropin and breast cancer. Int. J. Mol. Sci. 2017, 18, 1587. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, M.T.; Martin, J.E.; Badenoch, D.F.; Baithun, S.I. Beta hCG as a prognostic marker in adenocarcinoma of the prostate. Clin. Biochem. 1996, 49, 329–332. [Google Scholar] [CrossRef]
- Venyo, A.K.; Herring, D.; Greenwood, H.; Maloney, D.J. The expression of beta human chorionic gonadotrophin (β-HCG) in human urothelial carcinoma. Pan. Afr. Med. J. 2010, 7, 20. [Google Scholar] [CrossRef]
- Hoshi, S.; Numahata, K.; Morozumi, K.; Katumata, Y.; Kuromoto, A.; Takai, Y.; Hoshi, K.; Bilim, V.; Sasagawa, I. Bladder cancer metastasis producing beta-human chorionic gonadotropin, squamous cell carcinoma antigen, granulocyte-colony stimulating factor, and parathyroid hormone-related protein. IJU Case Rep. 2018, 2, 47–50. [Google Scholar] [CrossRef]
- Syrigos, K.N.; Fyssas, I.; Konstandoulakis, M.M.; Harrington, K.J.; Papadopoulos, S.; Milingos, N.; Peveretos, P.; Golematis, B.C. Beta human chorionic gonadotropin concentrations in serum of patients with pancreatic adenocarcinoma. Gut 1998, 42, 88–91. [Google Scholar] [CrossRef]
- Uesato, Y.; Kawamata, F.; Ishino, S.; Ono, S.; Tamashiro, K.; Koyama, H.; Takatsuki, M. Human chorionic gonadotropin-β promotes pancreatic cancer progression via the epithelial mesenchymal transition signaling pathway. J. Gastrointest. Oncol. 2022, 13, 1384–1394. [Google Scholar] [CrossRef]
- Liu, F.; Du, F.; Chen, X. Multiple tumor marker protein chip detection system in diagnosis of pancreatic cancer. World J. Surg. Oncol. 2014, 12, 333. [Google Scholar] [CrossRef]
- Manohar, A.; Werner, E.; Murphy, J. Elevated Human Chorionic Gonadotrophin Due to Pancreatic Cancer. Open J. Anesthesiol. 2012, 2, 178–182. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, W.; Su, J.; Xue, Y.; Wei, W.; Liu, S. The enzyme linked immunosorbent and chemiluminescence assay for the detection of human chorionic gonadotrophin using soybean peroxidase as label enzyme. Curr. Anal. Chem. 2015, 11, 80–87. [Google Scholar] [CrossRef]
- Chu, C.; Li, L.; Li, S.; Li, M.; Ge, S.; Yu, J.; Yan, M.; Song, X. Fluorescence-based immunoassay for human chorionic gonadotropin based on polyfluorene-coated silica nanoparticles and polyaniline-coated Fe3O4 nanoparticles. Microchim. Acta 2013, 180, 1509–1516. [Google Scholar] [CrossRef]
- Lund, H.; Paus, E.; Berger, P.; Stenman, U.H.; Torcellini, T.; Halvorsen, T.G.; Reubsaet, L. Epitope analysis and detection of human chorionic gonadotropin (hCG) variants by monoclonal antibodies and mass spectrometry. Tumour Biol. 2014, 35, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Koo, K.M.; Kim, T.H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2020, 9, 15. [Google Scholar] [CrossRef]
- Zhang, B.; Mao, Q.; Zhang, X.; Jiang, T.; Chen, M.; Yu, F.; Fu, W. A novel piezoelectric quartz micro-array immunosensor based on self-assembled monolayer for determination of human chorionic gonadotropin. Curr. Anal. Chem. 2015, 11, 711–720. [Google Scholar] [CrossRef]
- Chen, L.; Leng, Y.; Liu, B.; Liu, J.; Wan, S.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-singlemode coupler for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128283. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Liu, J.; Wan, S.; Wu, T.; Yuan, J.; Zhou, X.; Long, K.; Shao, L.; Fu, Y.Q.; et al. Novel Microfiber Sensor and Its Biosensing Application for Detection of hCG Based on a Singlemode-Tapered Hollow Core-Singlemode Fiber Structure. IEEE Sens. J. 2020, 20, 9071–9078. [Google Scholar] [CrossRef]
- Belal, S.J.; Alameri, L.M. Rashid, F.F.; Mansour, T.S. Laser biosensor as for pregnancy test by using photonic crystal fiber. Int. J. Med. Res. Health Sci. 2019, 8, 41–49. [Google Scholar]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, N.; Barthwal, H.; Arya, B.; Singh, T. Application of Fiber Optics in Bio-Sensing. In Fiber Optics; Huerta-Cuellar, G., Ed.; IntechOpen: London, UK, 2021; pp. 125–142. [Google Scholar]
- Lee, B. Review of the present status of optical fiber sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar] [CrossRef]
- Lyu, S.; Wu, Z.; Shi, X.; Wu, Q. Optical Fiber Biosensors for Protein Detection: A Review. Photonics 2022, 9, 987. [Google Scholar] [CrossRef]
- Zeni, L.; Perri, C.; Cennamo, N.; Arcadio, F.; D’Agostino, G.; Salmona, M.; Beeg, M.; Gobbi, M. A portable optical-fibre-based surface plasmon resonance biosensor for the detection of therapeutic antibodies in human serum. Sci. Rep. 2020, 10, 11154. [Google Scholar] [CrossRef] [PubMed]
- De Acha, N.; Socorro-Leránoz, A.B.; Elosúa, C.; Matías, I.R. Trends in the Design of Intensity-Based Optical Fiber Biosensors 2010–2020. Biosensors 2021, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, W.; Wu, N.; Zou, X.; Wang, X. Tapered Optical Fiber Sensor for Label-Free Detection of Biomolecules. Sensors 2011, 11, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Hawamdeh, G.; Jarrad, N.A.; Yasin, O.; Al-Gharabli, S.I.; Shadfan, R. Detection of Human Chorionic Gonadotropin (hCG) Hormone using Digital Lateral Flow Immunoassay. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3845–3848. [Google Scholar]
- Salim, E.T.; Fakhri, M.A.; Tariq, S.M.; Azzahrani, A.S.; Ibrahim, R.K.; Alwahib, A.A.; Alhasan, S.F.H.; Ramizy, A.; Salih, E.Y.; Salim, Z.T. The Unclad Single-Mode Fiber-Optic Sensor Simulation for Localized Surface Plasmon Resonance Sensing Based on Silver Nanoparticles Embedded Coating. Plasmonics 2023, 18. [Google Scholar] [CrossRef]
- Huo, Z.; Li, Y.; Chen, B.; Zhang, W.; Yang, X.; Yang, X. Recent advances in surface plasmon resonance imaging and biological applications. Talanta 2023, 255, 124213. [Google Scholar] [CrossRef]
- Nasirifar, R.; Danaie, M.; Dideban, A. Highly sensitive surface plasmon resonance sensor using perforated optical fiber for biomedical applications. Optik 2022, 250, 168051. [Google Scholar] [CrossRef]
- Esmailidastjerdipour, P.; Shahshahani, F. Numerical Simulation of Surface Plasmon Resonance Optical Fiber Biosensor Enhanced by Using Alloys for Medical Application. Sens. Imaging 2023, 24, 4. [Google Scholar] [CrossRef]
- Gómez-Pavón, L.C.; Luis-Ramos, A.; González-Sierra, N.E.; Zaca-Morán, P.; Muñoz-Pacheco, J.M.; Coxca-Gutiérrez, R.G. Design and fabrication of subwavelength optical fibers. In Proceedings of the Latin America Optics and Photonics Conference, Medellin, Colombia, 22–26 August 2016. [Google Scholar]
- González-Sierra, N.E.; Gómez-Pavón, L.C.; Pérez-Sánchez, G.F.; Luis-Ramos, A.; Zaca-Morán, P.; Muñoz-Pacheco, J.M.; Chávez-Ramírez, F. Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes. Sensors 2017, 17, 2039. [Google Scholar] [CrossRef]
- Zandbaaf, S.; Khanmohammadi, M.; Garmarudi, A.; Rashidi, B. Diagnosis of pregnancy based classification of embryo culture medium samples by infrared spectrometry and chemometrics. Infrared Phys. Technol. 2020, 104, 103069. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Liu, L. A Graphene Oxide-Based Fluorescent Method for the Detection of Human Chorionic Gonadotropin. Sensors 2016, 16, 1699. [Google Scholar] [CrossRef] [PubMed]
- Taha, B.A.; Ali, N.; Sapiee, N.M.; Fadhel, M.M.; Mat Yeh, R.M.; Bachok, N.N.; Al Mashhadany, Y.; Arsad, N. Comprehensive Review Tapered Optical Fiber Configurations for Sensing Application: Trend and Challenges. Biosensors 2021, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Leng, Y.; Liu, B.; Zhou, J.; Shao, L.; Yuan, J.; Fan, X.; Wan, S.; Wu, T.; Liu, J.; et al. Ultrasensitive biosensor based on magnetic microspheres enhanced microfiber interferometer. Biosens. Bioelectron. 2019, 145, 111563. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, S.; Li, J.; Guo, Y.; Han, Y.; Wang, Y.; Gong, L.; Yuan, J.; Qiu, S.; Shen, J. Refractive index sensing of twin-core fiber based on Mach Zehnder interference and SPR effect. J. Phys. D Appl. Phys. 2022, 55, 185103. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Cantoran, D.S.; Gómez, C.L.; Gómez-Pavón, L.d.C.; Zaca-Morán, P.; Castillo-López, D.N.; Luis-Ramos, A.; Muñoz-Pacheco, J.M. Quantification of hCG Hormone Using Tapered Optical Fiber Decorated with Gold Nanoparticles. Sensors 2023, 23, 8538. https://doi.org/10.3390/s23208538
Villegas-Cantoran DS, Gómez CL, Gómez-Pavón LdC, Zaca-Morán P, Castillo-López DN, Luis-Ramos A, Muñoz-Pacheco JM. Quantification of hCG Hormone Using Tapered Optical Fiber Decorated with Gold Nanoparticles. Sensors. 2023; 23(20):8538. https://doi.org/10.3390/s23208538
Chicago/Turabian StyleVillegas-Cantoran, David Saúl, Celia Lizeth Gómez, Luz del Carmen Gómez-Pavón, Placido Zaca-Morán, Dulce Natalia Castillo-López, Arnulfo Luis-Ramos, and Jesús Manuel Muñoz-Pacheco. 2023. "Quantification of hCG Hormone Using Tapered Optical Fiber Decorated with Gold Nanoparticles" Sensors 23, no. 20: 8538. https://doi.org/10.3390/s23208538






