A Cross-Day Analysis of EMG Features, Classifiers, and Regressors for Swallowing Events Detection and Fluid Intake Volume Estimation
Abstract
:1. Introduction
2. Methodology
2.1. Subjects
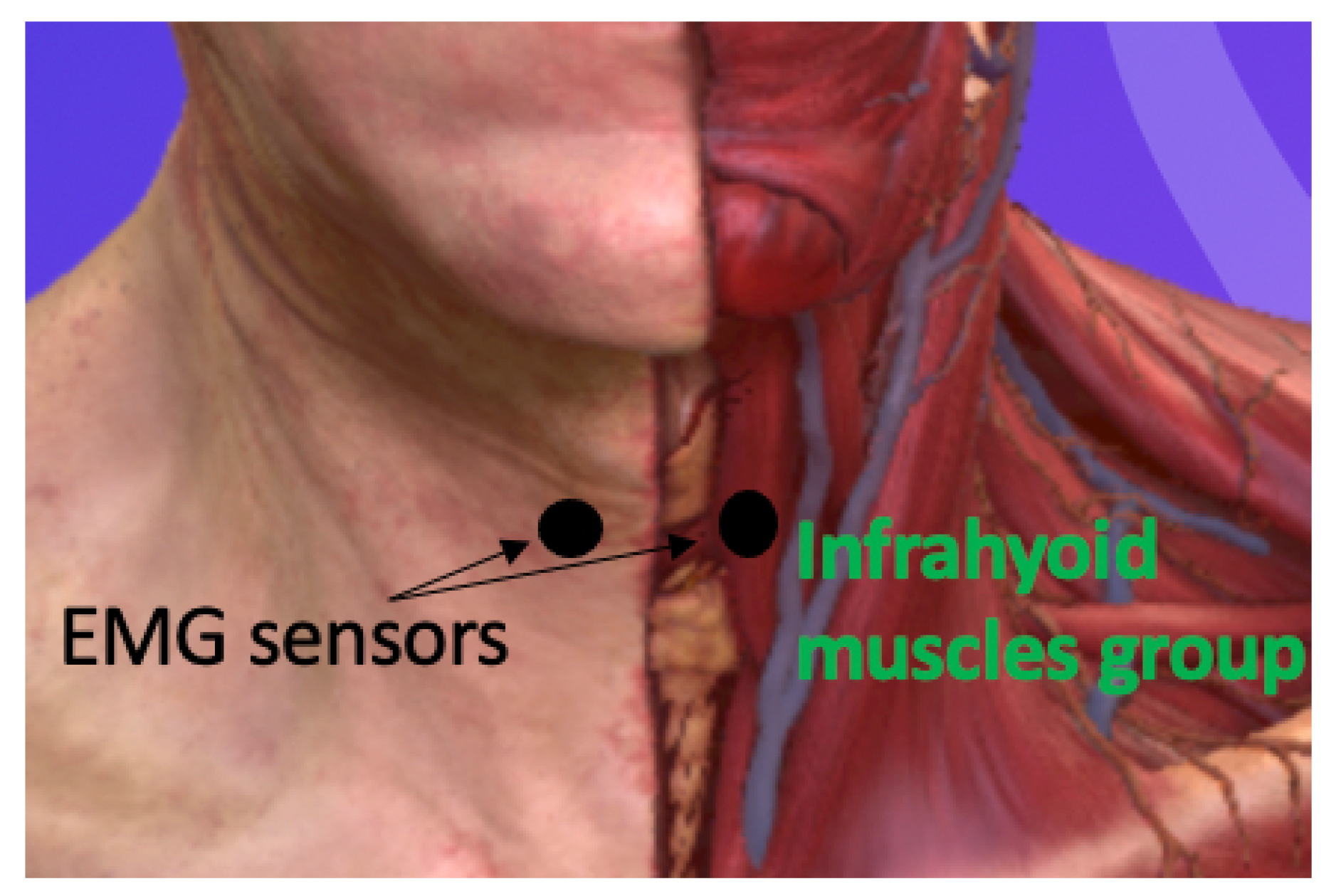
2.2. Experimental Procedure
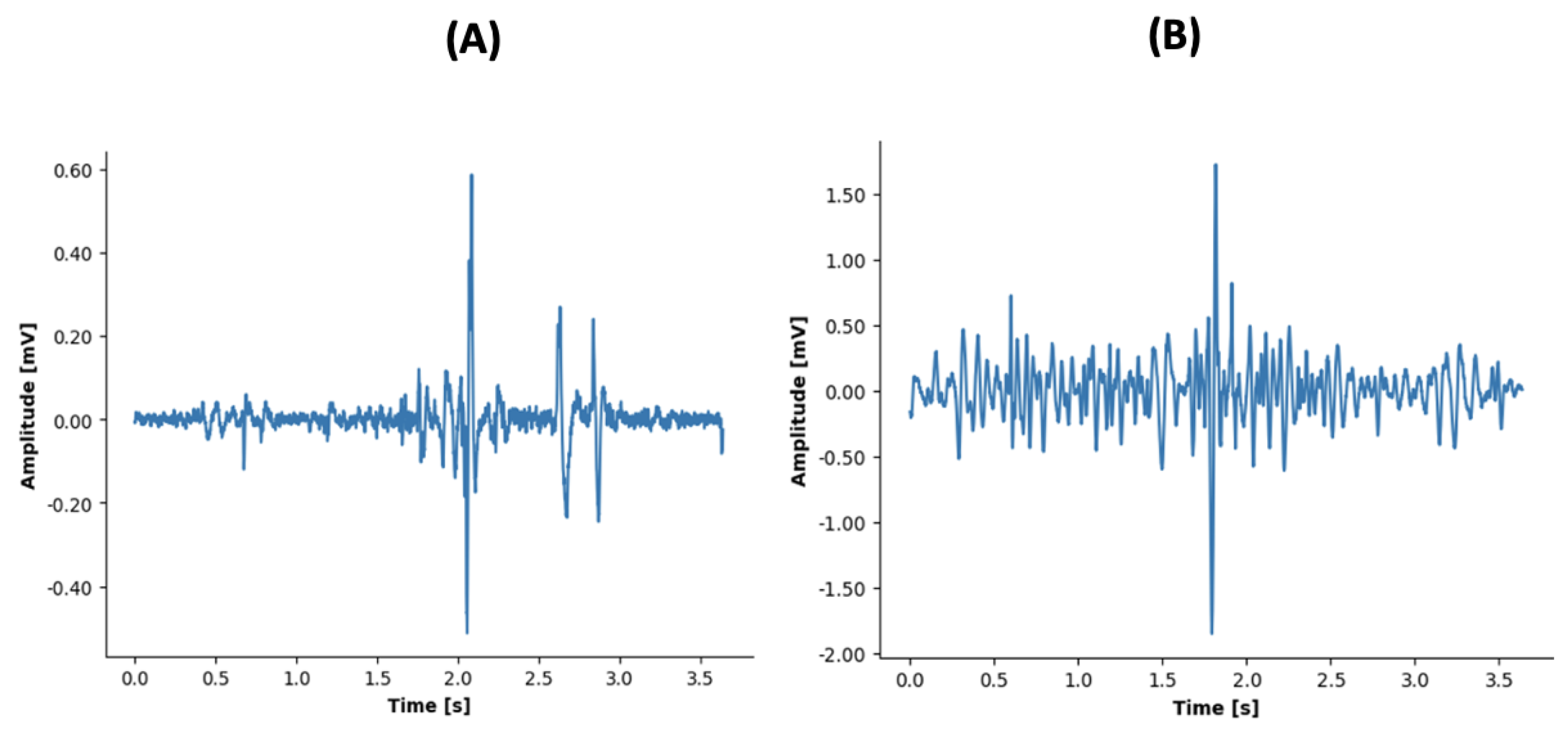
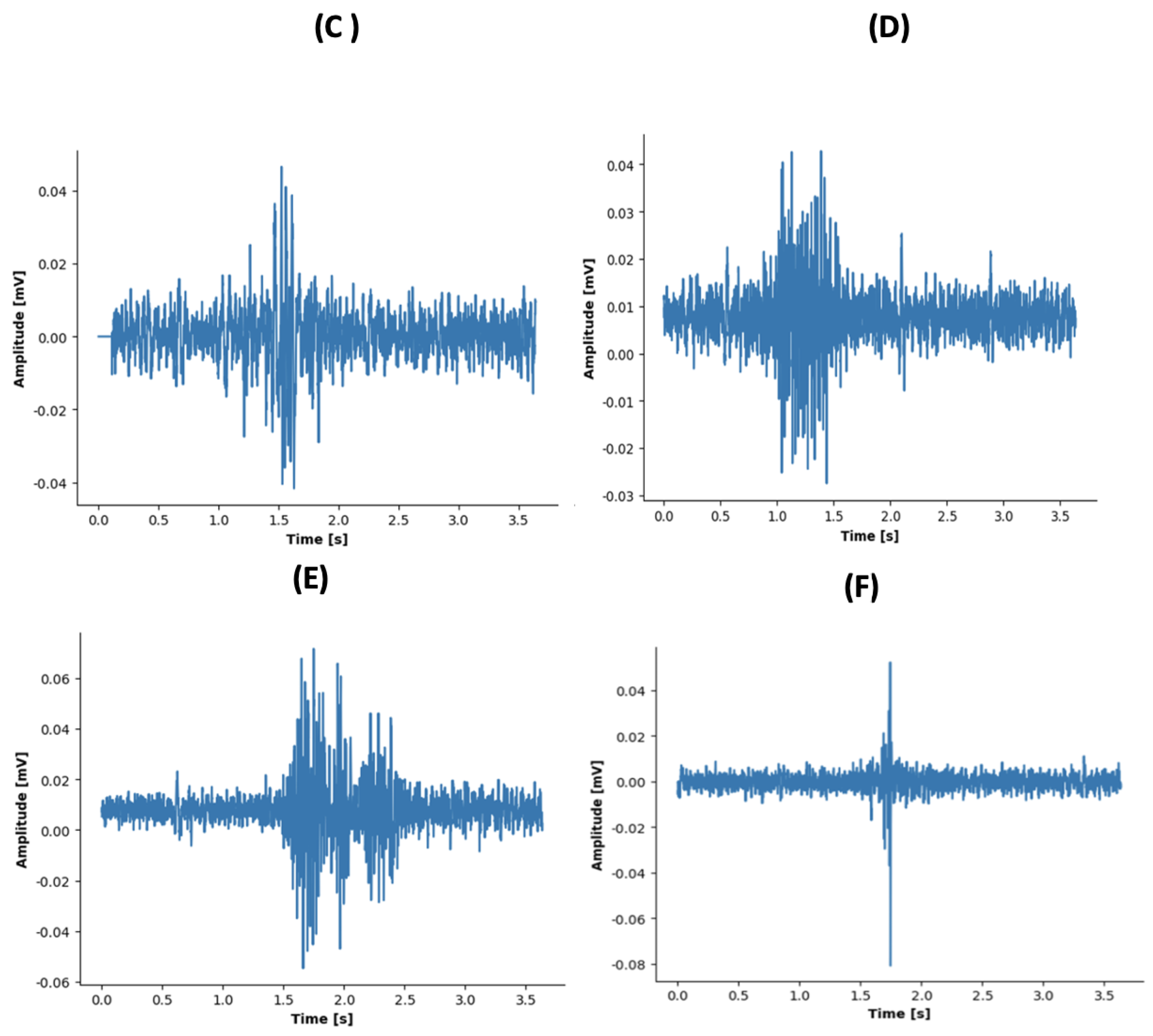
2.3. Data Analysis
3. Results
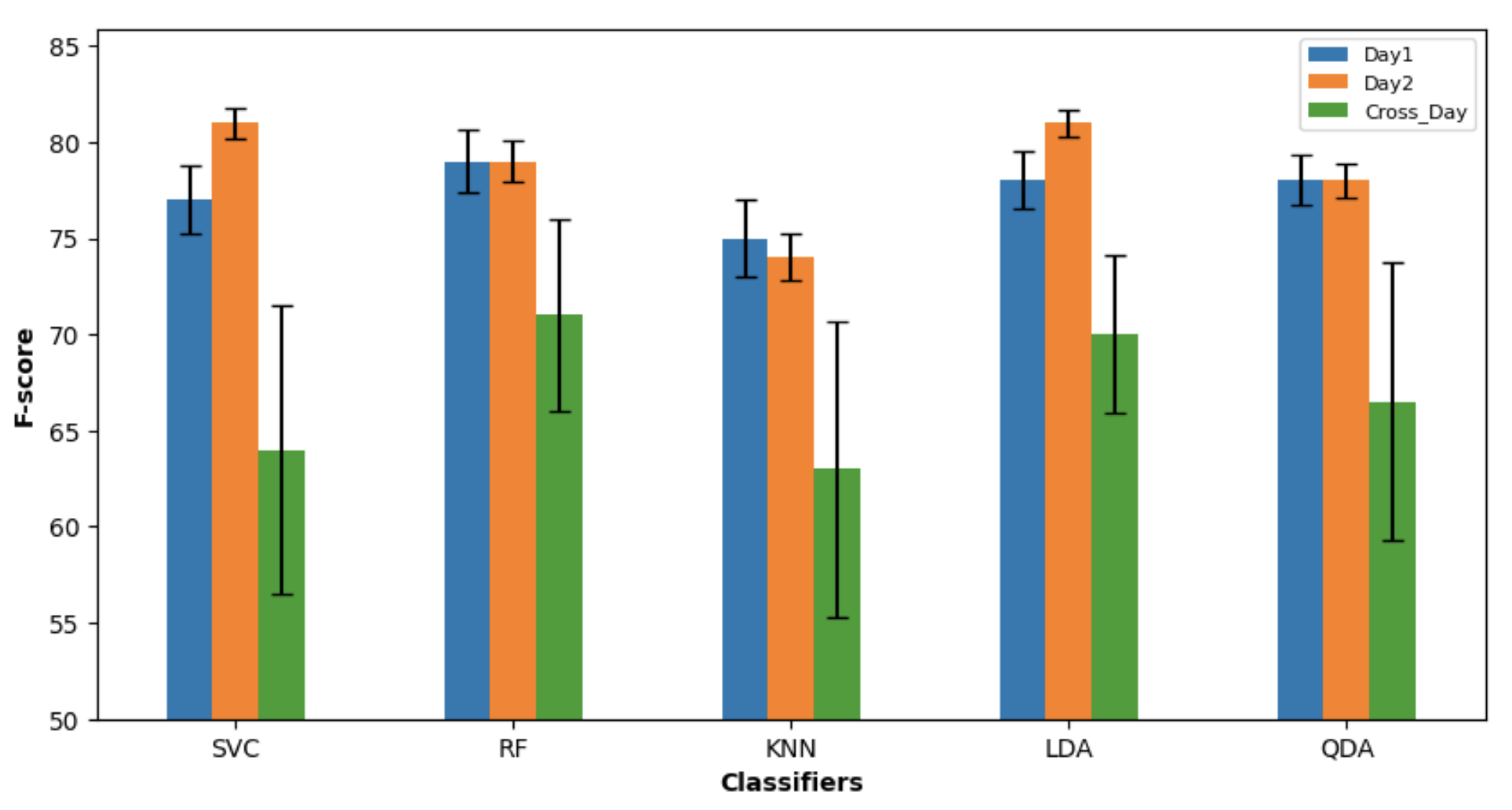
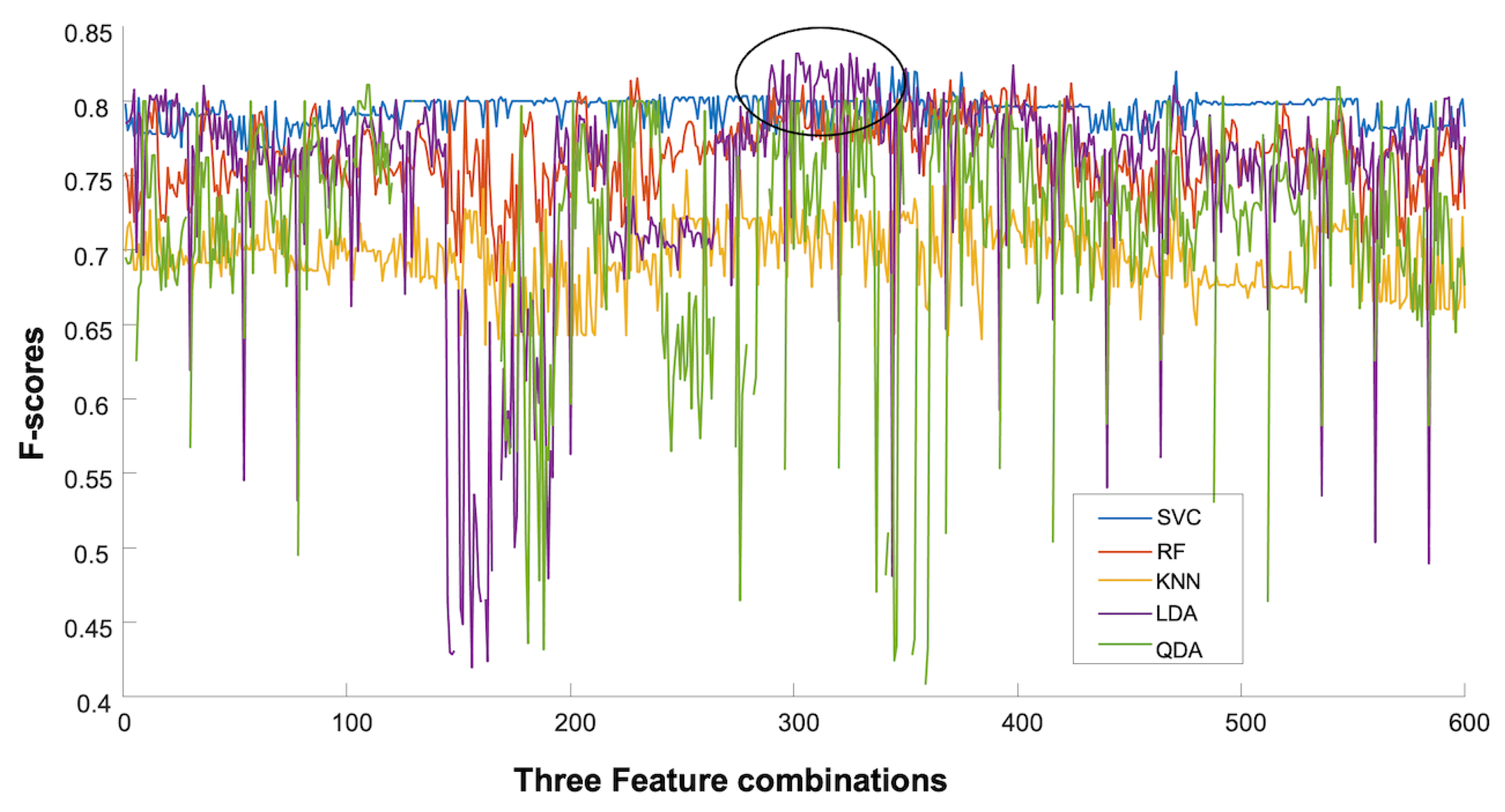
3.1. Classification
3.1.1. Day 1
3.1.2. Day 2
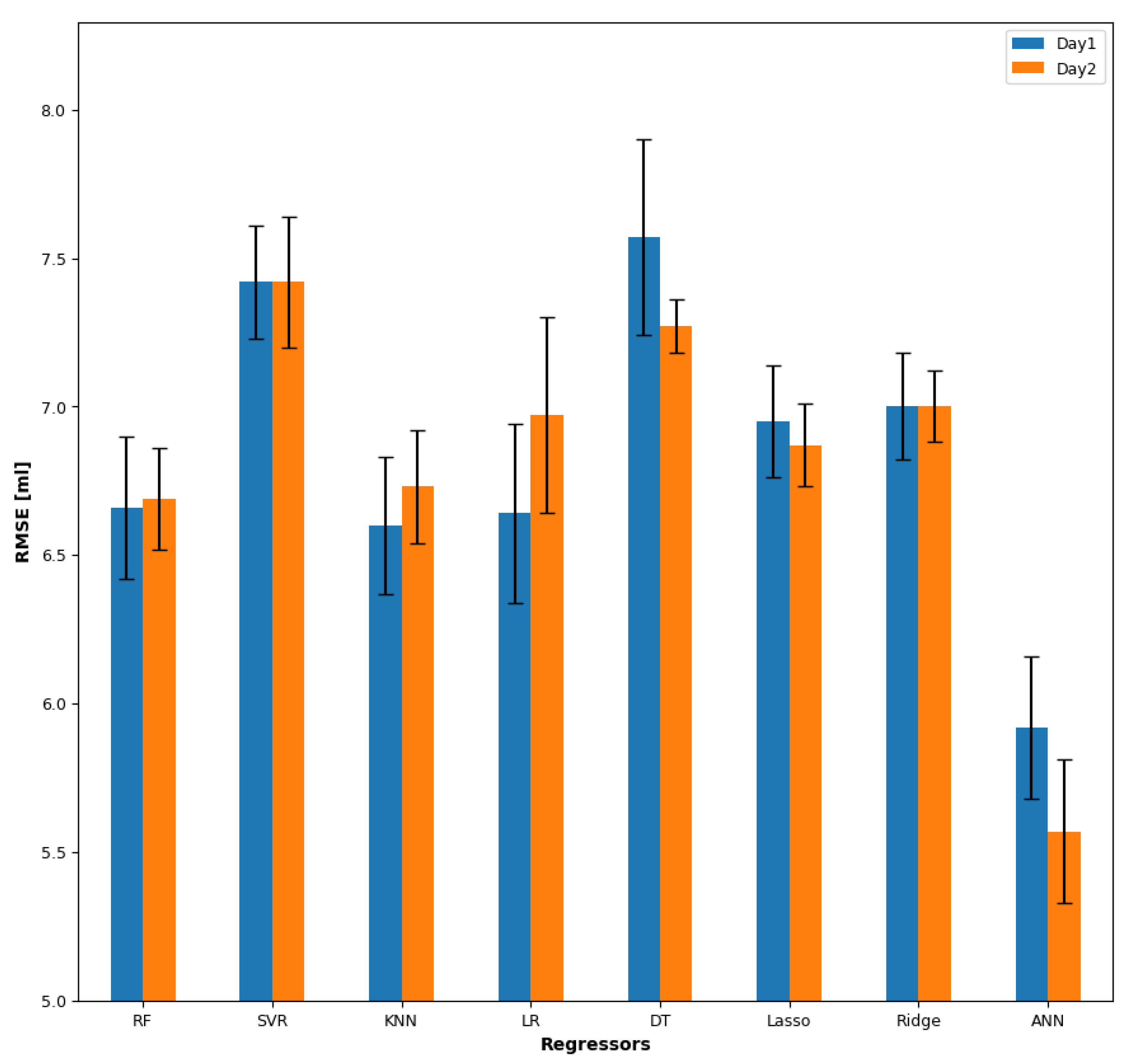
3.2. Estimation
3.2.1. Day 1
3.2.2. Day 2
3.2.3. Cross-Day
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Sharkawy, A.M.; Sahota, O.; Maughan, R.J.; Lobo, D.N. The pathophysiology of fluid and electrolyte balance in the older adult surgical patient. Clin. Nutr. 2014, 33, 6–13. [Google Scholar] [PubMed]
- Cohen, R.; Fernie, G.; Fekr, A.R. Fluid intake monitoring systems for the elderly: A review of the literature. Nutrients 2021, 13, 2092. [Google Scholar]
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lavizzo-Mourey, R.J. Dehydration in the Elderly: A Short Review. J. Natl. Med. Assoc. 1987, 79, 1033–1038. [Google Scholar] [PubMed]
- Alcorn, E. Improving fluid balance charts through staff education on a general medical ward: A quality improvement project. Future Healthc. J. 2022, 9, 114. [Google Scholar] [CrossRef]
- Malvuccio, C.; Kamavuako, E.N. Detection of Swallowing Events and Fluid Intake Volume Estimation from Surface Electromyography Signals. In Proceedings of the 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Langkawi Island, Malaysia, 1–3 March 2021; pp. 245–250. [Google Scholar]
- Asogan, H.; Raoof, A. Education and training as key drivers for improving the quality of fluid balance charts: Findings from a quality improvement project. BMJ Open Qual. 2021, 10, e001137. [Google Scholar]
- Huhn, S.; Axt, M.; Gunga, H.C.; Maggioni, M.A.; Munga, S.; Obor, D.; Sie, A.; Boudo, V.; Bunker, A.; Sauerborn, R.; et al. The impact of wearable technologies in health research: Scoping review. JMIR mHealth uHealth 2022, 10, e34384. [Google Scholar]
- Eskandari, S. Bite Detection and Differentiation Using Templates of Wrist Motion. Master’s Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- Liu, K.-C.; Hsieh, C.-Y.; Huang, H.-Y.; Chiu, L.-T.; Hsu, S.J.-P.; Chan, C.-T. Drinking Event Detection and Episode Identification Using 3D-Printed Smart Cup. IEEE Sens. J. 2020, 20, 13743–13751. [Google Scholar] [CrossRef]
- Gomes, D.; Sousa, I. Realtime Drink Trigger Detection in Free-living Conditions Using Inertial Sensors. Sensors 2019, 19, 2145. [Google Scholar] [CrossRef]
- Flutura, S.; Seiderer, A.; Aslan, I.; Dang, C.T.; Schwarz, R.; Schiller, D.; André, E. DrinkWatch: A Mobile Wellbeing Application Based on Interactive and Cooperative Machine Learning. In Proceedings of the 2018 International Conference on Digital Health, Lyon, France, 23–26 April 2018; ACM: New York, NY, USA, 2018. [Google Scholar]
- Ren, Y.; Tan, S.; Zhang, L.; Wang, Z.; Yang, J. Liquid Level Sensing Using Commodity WiFiina Smart Home Environment. Proc. Interact. Mobile Wearable Ubiquitous Technol. Arch. 2020, 4, 1–30. [Google Scholar] [CrossRef]
- Fan, M.; Truong, K.N. SoQr: Sonically Quantifying the Content Level inside Containers. In Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing—UbiComp’15, Osaka, Japan, 7–11 September 2015; ACM Press: New York, NY, USA, 2015; pp. 3–14. [Google Scholar]
- Amft, O.; Bannach, D.; Pirkl, G.; Kreil, M.; Lukowicz, P. Towards wearable sensing-based assessment of fluid intake. In Proceedings of the 2010 8th IEEE International Conference on Pervasive Computing and Communications Workshops (PERCOM Workshops), Mannheim, Germany, 29 March–2 April 2010; pp. 298–303. [Google Scholar]
- Wellnitz, A.; Wolff, J.P.; Haubelt, C.; Kirste, T. Fluid intake recognition using inertial sensors. In Proceedings of the 6th international Workshop on Sensor-based Activity Recognition and Interaction, Rostock, Germany, 16–17 September 2019; pp. 1–7. [Google Scholar]
- Zhou, B.; Cheng, J.; Sundholm, M.; Reiss, A.; Huang, W.; Amft, O.; Lukowicz, P. Smart table surface: A novel approach to pervasive dining monitoring. In Proceedings of the 2015 IEEE International Conference on Pervasive Computing and Communications (PerCom), St. Louis, MO, USA, 23–27 March 2015; pp. 155–162. [Google Scholar]
- Chang, K.H.; Liu, S.Y.; Chu, H.H.; Hsu, J.Y.J.; Chen, C.; Lin, T.Y.; Chen, C.Y.; Huang, P. The diet-aware dining table: Observing dietary behaviors over a tabletop surface. In Proceedings of the Pervasive Computing: 4th International Conference, PERVASIVE 2006, Dublin, Ireland, 7–10 May 2006; Springer: Berlin/Heidelberg, Germany, 2006; pp. 366–382. [Google Scholar]
- Huang, H.Y.; Hsieh, C.Y.; Liu, K.C.; Hsu, S.J.P.; Chan, C.T. Fluid intake monitoring system using a wearable inertial sensor for fluid intake management. Sensors 2020, 20, 6682. [Google Scholar] [CrossRef] [PubMed]
- Anderez, D.O.; Lotfi, A.; Langensiepen, C. A hierarchical approach in food and drink intake recognition using wearable inertial sensors. In Proceedings of the 11th PErvasive Technologies Related to Assistive Environments Conference, Corfu, Greece, 26–29 June 2018; pp. 552–557. [Google Scholar]
- Malvuccio, C.; Kamavuako, E.N. The Effect of EMG Features on the Classification of Swallowing Events and the Estimation of Fluid Intake Volume. Sensors 2022, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.A.; Kamavuako, E.N. Estimation of Fluid Intake Volume from Surface Electromyography Signals: A Comparative Study of Seven Regression Techniques. In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023), Lisbon, Portugal, 16–18 February 2023; Volume 4, pp. 118–124. [Google Scholar]
- Amft, O.; Troster, G. Methods for detection and classification of normal swallowing from muscle activation and sound. In Proceedings of the 2006 Pervasive Health Conference and Workshops, Innsbruck, Austria, 21 May 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1–10. [Google Scholar]
- Nicholls, B.; Ang, C.S.; Kanjo, E.; Siriaraya, P.; Bafti, S.M.; Yeo, W.H.; Tsanas, A. An EMG-based Eating Behaviour Monitoring system with haptic feedback to promote mindful eating. Comput. Biol. Med. 2022, 149, 106068. [Google Scholar] [CrossRef] [PubMed]
- Vaiman, M.; Segal, S.; Eviatar, E. Surface electromyographic studies of swallowing in normal children, age 4–12 years. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 65–73. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.; Jansen, A. Recording of swallowing events using electromyography as a non-invasive measurement of salivation. Appetite 1999, 33, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Vinyard, C.J.; Fiszman, S. Using electromyography as a research tool in food science. Curr. Opin. Food Sci. 2016, 9, 50–55. [Google Scholar]
- Huang, Q.; Wang, W.; Zhang, Q. Your glasses know your diet: Dietary monitoring using electromyography sensors. IEEE Internet Things J. 2017, 4, 705–712. [Google Scholar] [CrossRef]
- BioCloud 3D Anatomy. Available online: https://biocloud3d.com/index.php (accessed on 10 July 2023).
- Tkach, D.; Huang, H.; Kuiken, T.A. Study of stability of time-domain features for electromyographic pattern recognition. J. Neuroeng. Rehabil. 2010, 7, 1–13. [Google Scholar] [CrossRef]
- Zardoshti-Kermani, M.; Wheeler, B.C.; Badie, K.; Hashemi, R.M. EMG feature evaluation for movement control of upper extremity prostheses. IEEE Trans. Rehabil. Eng. 1995, 3, 324–333. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, H.H.; Moon, C.S.; Mun, C.W. Comparison of k-nearest neighbor, quadratic discriminant and linear discriminant analysis in classification of electromyogram signals based on the wrist-motion directions. Curr. Appl. Phys. 2011, 11, 740–745. [Google Scholar]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39, 7420–7431. [Google Scholar]
- Welcome to TSFEL Documentation! Welcome to TSFEL Documentation!—TSFEL 0.1.4 Documentation. Available online: https://tsfel.readthedocs.io/en/latest/ (accessed on 30 June 2023).
- Barona-Lopez, L.I.; Valdivieso-Caraguay, A.L.; Benalcazar, M.E.; Aguas, X.; Zea, J.A. Feature evaluation of EMG signals for hand gesture recognition based on mutual information, fuzzy entropy and RES index. In Proceedings of the Advances and Applications in Computer Science, Electronics and Industrial Engineering: Proceedings of CSEI 2020, Xinxiang, China, 14–16 June 2020; Springer: Singapore, 2021; pp. 101–119. [Google Scholar]
- Altın, C.; Er, O. Comparison of different time and frequency domain feature extraction methods on elbow gesture’s EMG. Eur. J. Interdiscip. Stud. 2016, 2, 35–44. [Google Scholar] [CrossRef]
- Nazarpour, K.; Al-Timemy, A.H.; Bugmann, G.; Jackson, A. A note on the probability distribution function of the surface electromyogram signal. Brain Res. Bull. 2013, 90, 88–91. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, E.L.; Schut, M.H.; Westerink, J.H.; van Herk, J.; Tuinenbreijer, K. Computing emotion awareness through facial electromyography. In Proceedings of the International Conference on Computer Vision in Human–Computer Interaction, Graz, Austria, 13 May 2006; pp. 52–63. [Google Scholar]
- Oskoei, M.A.; Hu, H. Support vector machine-based classification scheme for myoelectric control applied to upper limb. IEEE Trans. Biomed. Eng. 2008, 55, 1956–1965. [Google Scholar] [CrossRef]
- Kendell, C.; Lemaire, E.D.; Losier, Y.; Chan, A.; Hudgins, B. A novel approach to surface electromyography: An exploratory study of electrode-pair selection based on signal characteristics. J. Neuroeng. Rehabil. 2012, 9, 1–18. [Google Scholar] [CrossRef]
- Hudgins, B.; Parker, P.; Scott, R.N. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993, 40, 82–94. [Google Scholar] [CrossRef]
- Khushaba, R.N.; Al-Ani, A.; Al-Jumaily, A. Orthogonal fuzzy neighborhood discriminant analysis for multifunction myoelectric hand control. IEEE Trans. Biomed. Eng. 2010, 57, 1410–1419. [Google Scholar] [CrossRef]
- Yavuz, E.; Eyupoglu, C. A cepstrum analysis-based classification method for hand movement surface EMG signals. Med. Biol. Eng. Comput. 2019, 57, 2179–2201. [Google Scholar]
| Feature Full Name | Abbreviation | References |
|---|---|---|
| Autoregressive Coefficients | AC | [30,31] |
| Avergare Amplitude Change | AAC | [32,33] |
| Calc Centroid | CC | [34] |
| Different Absolute Standard Deviation Value | DASDV | [32,33] |
| Entropy | MYop | [35] |
| Empirical Cumulative Distribution | ECDF | [34] |
| Frequency Ratio | FR | [33] |
| Linear Prediction Cepstral Coefficients | LPCC | [34] |
| Log Detector | LOG | [30,36] |
| Kurtosis | Kurt | [37,38] |
| Mean Power | MNP | [33,39] |
| Mean Frequency | MNF | [39,40] |
| Median Frequency | MDF | [33,39] |
| Mean Absolute Value | MAV | [41,42] |
| Myopulse Percentage Rate | MYOP | [33] |
| Mel Frequency Cepstral Coefficients | MFCC | [43] |
| Peak Frequency | PKF | [33] |
| Power Spectrum Density Bandwidth | PW | [36] |
| Skewness | Skew | [38,42] |
| Spectral Centroid | SC | [34] |
| Spectral Entropy | SE | [34] |
| Spectrogram Frequency | SF | [34] |
| Variance | VAR | [30,31] |
| Wavelength | WL | [30,41] |
| Willison Amplitude Change | WAMP | [30,31] |
| Zero Crossing Rate | ZC | [30,41] |
| Subjects | 1F | 2F | 3F | 4F |
|---|---|---|---|---|
| S1 | (LogD) SVC, QDA | (Kurtosis, PW) RF | (LogD, WL, FR) (MAV, LogD, PW) RF, QDA | (PW, CC, Kurt, AAC) RF |
| S2 | (MNF) (MDF) (AC) LDA | (MNP, MNF) (FR, MNP) RF, SVC, KNN, LDA | (Fr, MP, MF) (MP, MF, MDF) (Fr, MP, MF) (FR, MNP, SF) RF, SVC, KNN, LDA, QDA | (FR, MNP, MNF, MDF) (MNP, MNF, MDF, PF) (FR, MNP, MNF, MDF) RF, SVC, KNN, LDA |
| S3 | (MAV) (LogD) (MAV) (WL) (AAC) SVC, QDA, LDA | (SC, Skew) (AC, DASD) RF, LDA | (AC, Entropy, SC) LDA | (MDF, PF, AC, MAV) LDA |
| S4 | ZC LDA, QDA | (MNP, ZC) QDA | (MNP, MNF, ZC) QDA | (AAC, DASDV, ZC, MNP) QDA |
| S5 | SC, CC LDA | (MNF, PW) (MFCC, MNP) RF, QDA | (MNP, MNF, ZC) LDA | (Entropy, ECDF, SC, LogD) LDA |
| S6 | SF, SVC | (myop, LPCC) LDA | (MDF, PF, myop) SVC | (FR, MNP, MNF, ZC) QDA |
| S7 | Kurt SVC | (AC, SC) LDA | (AC, myop, ECDF) LDA | (PW, CC, Kurtosis, AAC) RF |
| S8 | AC QDA | (myop, Kurt) SVC | (kur, skew, WL) RF | (LogD, WL, AAC, PW) RF |
| S9 | myop SVC | (MNP, WL) QDA | (MNP, MDF, PW) RF | (MNP, MNF, MDF, MFCC) RF (DASDV, ZC, WAMP, PW) KNN |
| S10 | WAMP RF, LogD, LDA | (SF, MDF) RF | (MF, MDF, PW) LDA | (WAMP, myop, VAR, Kurt) LDA (WAMP, myop, VAR, PW) QDA (DASDV, ZC, WAMP, PW) KNN |
| S11 | AC QDA | (MNP, myop) LDA | (myop, VAR, MNP) LDA | (PF, AC, myop, MDF) LDA |
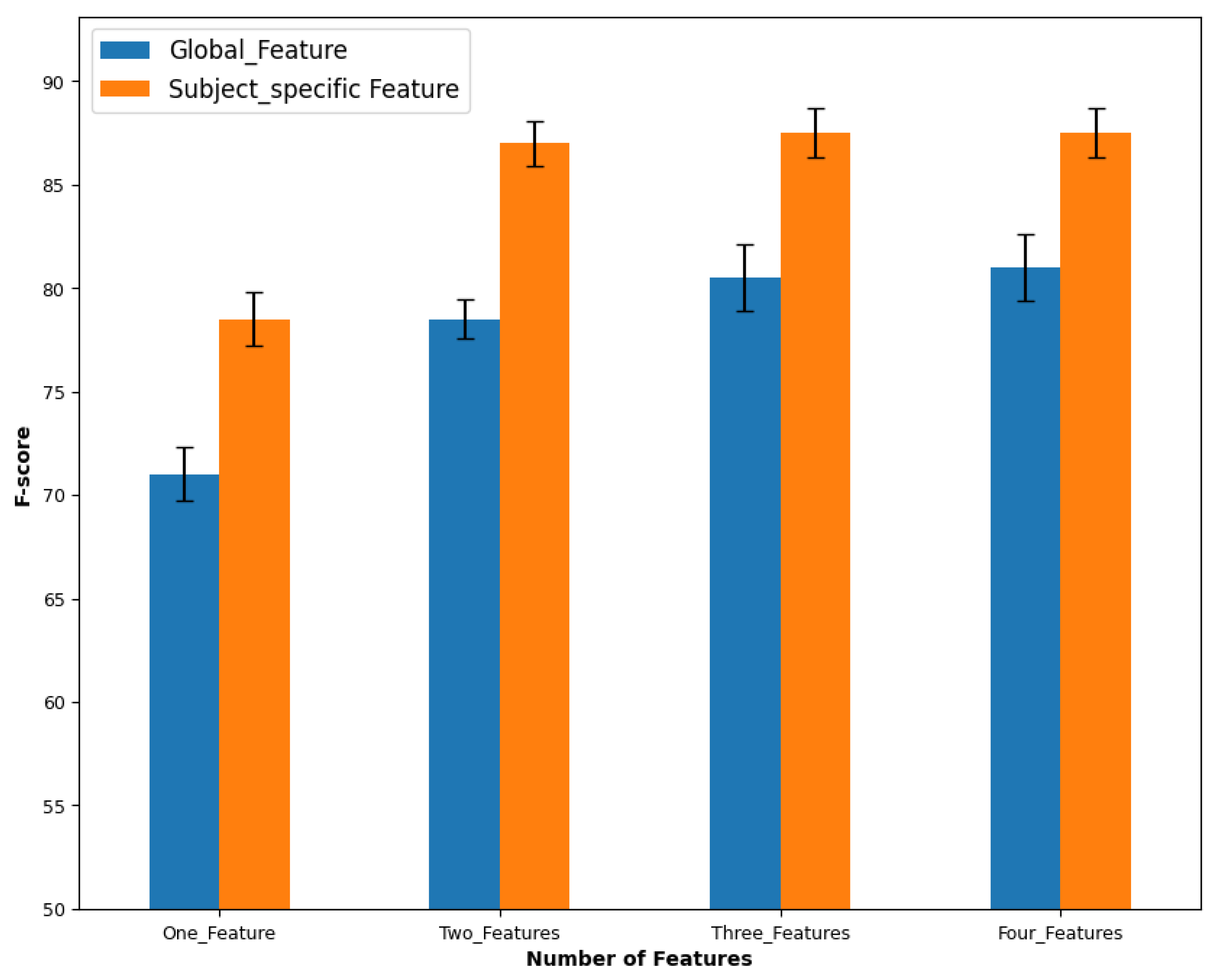
| – | Number of Features | Best Global Features | F-Score | Best Calssifiers |
|---|---|---|---|---|
| Day 1 | 1F | AAC | RF | |
| 2F | LPCC, MAV | LDA | ||
| 3F | LPCC, MAV, LogD | LDA | ||
| 4F | LPCC, MAV, LogD, WL | LDA | ||
| Day 2 | 1F | FR | SVC | |
| 2F | LogD, WAMP | SVC | ||
| 3F | LPCC, MAV, AAC | LDA | ||
| 4F | SC, SE, SF, ECDF | SVC |
| Subjects | RMSE | 1F |
|---|---|---|
| S1 | 4.67 | FR |
| S2 | 3.07 | MFCC |
| S3 | 5.04 | Kurt |
| S4 | 5 | MFCC |
| S5 | 4.58 | Kurt |
| S6 | 2.88 | Skew |
| S7 | 3.87 | FR |
| S8 | 5.07 | MDF |
| S9 | 5.59 | ZC |
| S10 | 6.05 | Kurt |
| S11 | 4.94 | SF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, I.; Niazi, I.K.; Haavik, H.; Kamavuako, E.N. A Cross-Day Analysis of EMG Features, Classifiers, and Regressors for Swallowing Events Detection and Fluid Intake Volume Estimation. Sensors 2023, 23, 8789. https://doi.org/10.3390/s23218789
Ismail I, Niazi IK, Haavik H, Kamavuako EN. A Cross-Day Analysis of EMG Features, Classifiers, and Regressors for Swallowing Events Detection and Fluid Intake Volume Estimation. Sensors. 2023; 23(21):8789. https://doi.org/10.3390/s23218789
Chicago/Turabian StyleIsmail, Iman, Imran Khan Niazi, Heidi Haavik, and Ernest N. Kamavuako. 2023. "A Cross-Day Analysis of EMG Features, Classifiers, and Regressors for Swallowing Events Detection and Fluid Intake Volume Estimation" Sensors 23, no. 21: 8789. https://doi.org/10.3390/s23218789
APA StyleIsmail, I., Niazi, I. K., Haavik, H., & Kamavuako, E. N. (2023). A Cross-Day Analysis of EMG Features, Classifiers, and Regressors for Swallowing Events Detection and Fluid Intake Volume Estimation. Sensors, 23(21), 8789. https://doi.org/10.3390/s23218789








