Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies
Abstract
:1. Introduction
2. Materials and Methods
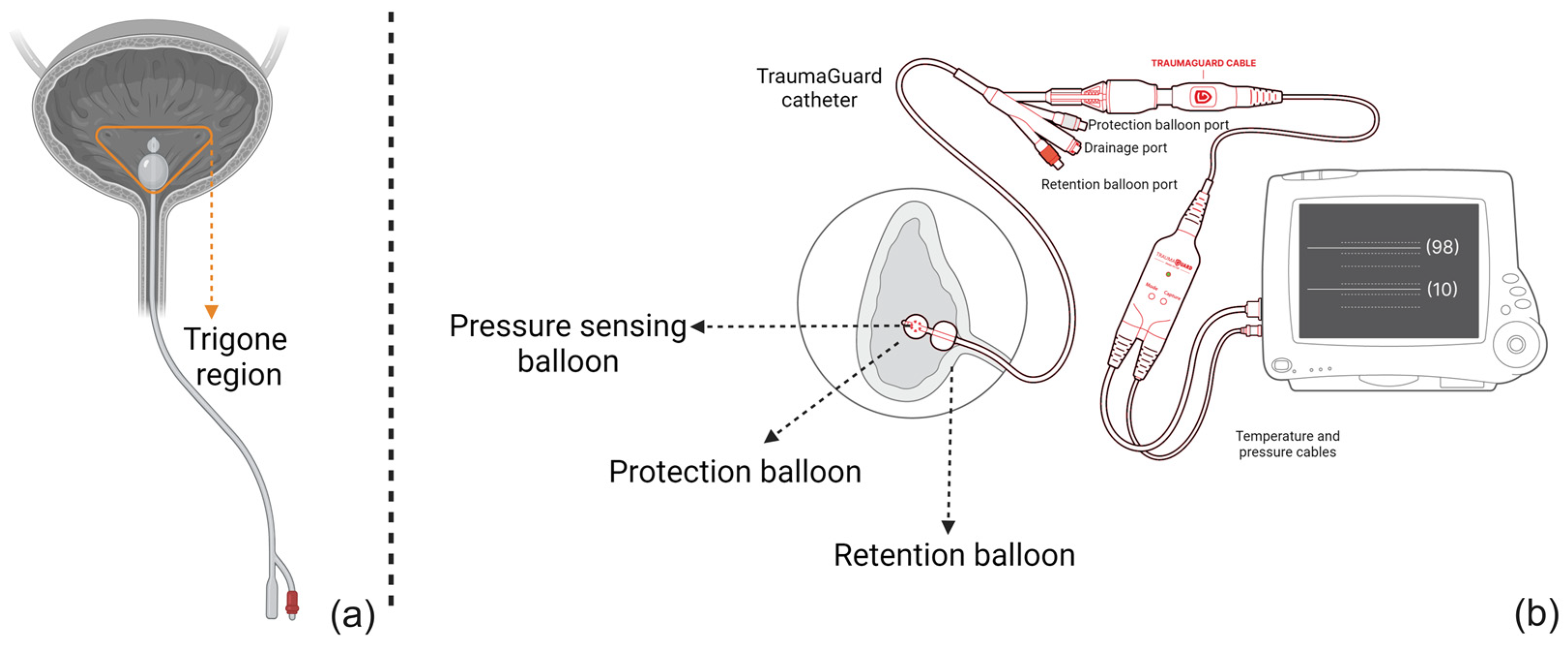
2.1. TraumaGuard System
2.2. Pig Study
2.3. Cadaver Study
2.4. Ethical Considerations
2.5. Statistical Analysis
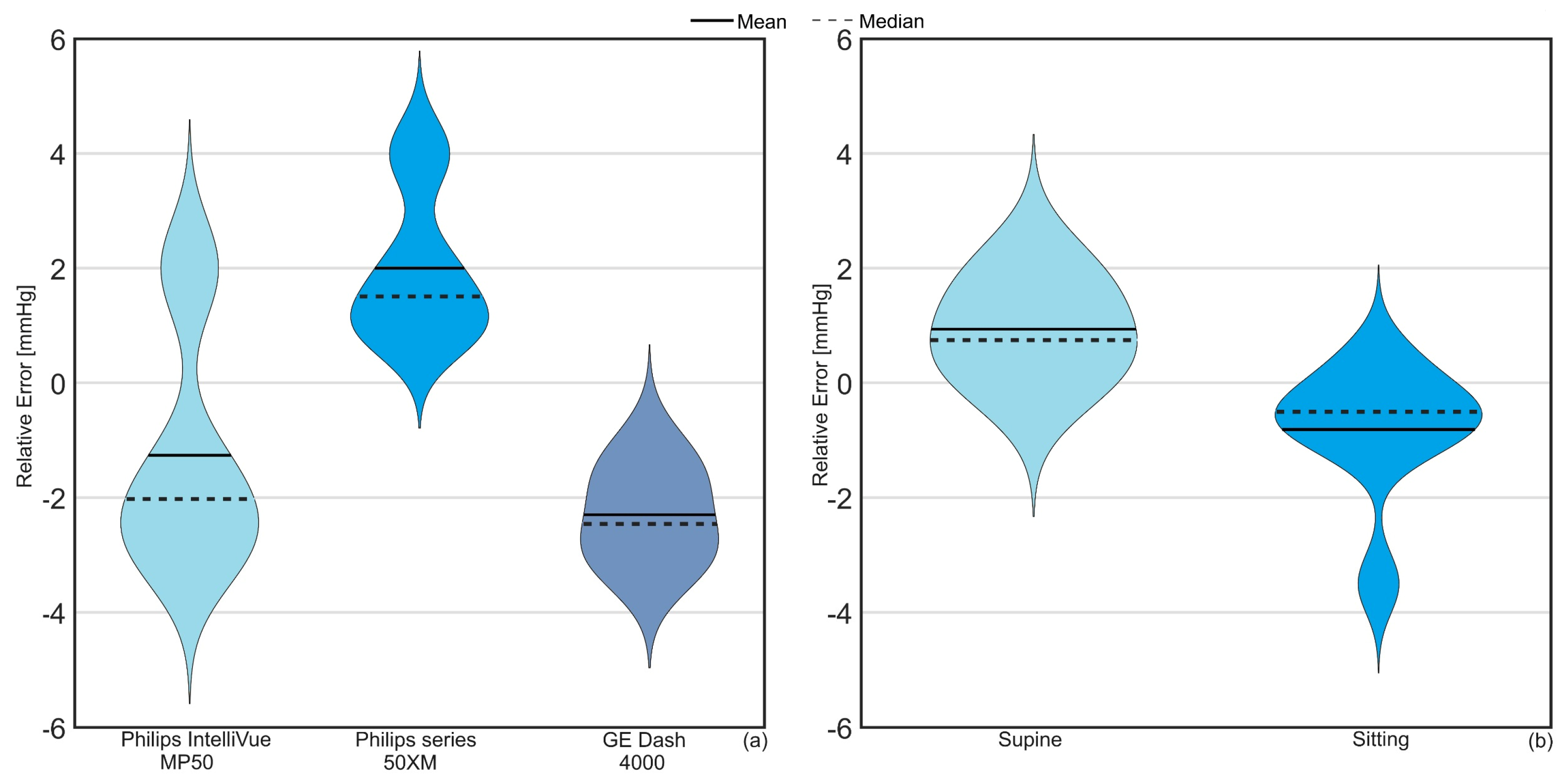
3. Results
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, B.M.; Howard, C. A 6th Vital Sign--Potential Use of Nasogastric Tube for Intra-abdominal Pressure Monitoring Method to Detect Feeding Intolerance in Very Low Birth-Weight Preterm Infants (<1500 g). Adv. Neonatal Care 2015, 15, 176–181. [Google Scholar] [PubMed]
- Smit, M.; van Meurs, M.; Zijlstra, J.G. Intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients: A narrative review of past, present, and future steps. Scand. J. Surg. 2022, 111, 14574969211030128. [Google Scholar] [CrossRef]
- Van Hee, R. Historical highlights in concept and treatment of abdominal compartment syndrome. Acta Clin. Belg. 2007, 62 (Suppl. S1), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Strang, S.G.; van der Hoven, B.; Monkhorst, K.; Ali, S.; van Lieshout, E.M.M.; van Waes, O.J.F.; Verhofstad, M.H.J. Relation between intra-abdominal pressure and early intestinal ischemia in rats. Trauma. Surg. Acute Care Open 2020, 5, e000595. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saleh, N.; Aronson, D.; Khamaisi, M.; Khoury, E.E.; Awad, H.; Kabala, A.; Ramadan, R.; Karram, T.; Kakiashvili, E.; Bishara, B.; et al. Increased Intra-abdominal Pressure Induces Acute Kidney Injury in an Experimental Model of Congestive Heart Failure. J. Card. Fail. 2019, 25, 468–478. [Google Scholar] [CrossRef]
- Fiedler, M.O.; Simeliunas, E.; Deutsch, B.L.; Diktanaite, D.; Harms, A.; Brune, M.; Dietrich, M.; Uhle, F.; Weigand, M.A.; Kalenka, A. Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model. J. Clin. Med. 2021, 10, 306. [Google Scholar] [CrossRef]
- Khanna, A.K.; Minear, S.; Kurz, A.; Moll, V.; Stanton, K.; Essakalli, L.; Prabhakar, A. Intra-abdominal hypertension in cardiac surgery patients: A multicenter observational sub-study of the Accuryn registry. J. Clin. Monit. Comput. 2023, 37, 189–199. [Google Scholar] [CrossRef]
- Pereira, R.; Buglevski, M.; Perdigoto, R.; Marcelino, P.; Saliba, F.; Blot, S.; Starkopf, J. Intra-abdominal hypertension and abdominal compartment syndrome in the critically ill liver cirrhotic patient-prevalence and clinical outcomes. A multicentric retrospective cohort study in intensive care. PLoS ONE 2021, 16, e0251498. [Google Scholar] [CrossRef]
- Xu, X.; He, Z.; Shan, Y.; Mao, Q.; Feng, J.; Gao, G. Intra-Abdominal Pressure Measurements in Neurocritical Patients. J. Vis. Exp. 2021, 171, e62557. [Google Scholar] [CrossRef]
- Jacobs, R.; Wise, R.D.; Myatchin, I.; Vanhonacker, D.; Minini, A.; Mekeirele, M.; Kirkpatrick, A.W.; Pereira, B.M.; Sugrue, M.; De Keulenaer, B.; et al. Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review. Life 2022, 12, 1390. [Google Scholar] [CrossRef]
- Ott, D.E. Abdominal Compliance and Laparoscopy: A Review. J. Soc. Laparoendosc. Surg. 2019, 23, e2018. [Google Scholar] [CrossRef]
- Coleman, T.J.; Thomsen, J.C.; Maass, S.D.; Hsu, Y.; Nygaard, I.E.; Hitchcock, R.W. Development of a wireless intra-vaginal transducer for monitoring intra-abdominal pressure in women. Biomed. Microdevices 2012, 14, 347–355. [Google Scholar] [CrossRef]
- Iacubovici, L.; Karol, D.; Baar, Y.; Beri, A.; Herzberg, H.; Zarour, S.; Goren, O.; Cohen, B. Assessment of Intra-Abdominal Pressure with a Novel Continuous Bladder Pressure Monitor-A Clinical Validation Study. Life 2023, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Sugrue, M.; McKee, J.L.; Pereira, B.M.; Roberts, D.J.; De Waele, J.J.; Leppaniemi, A.; Ejike, J.C.; Reintam Blaser, A.; D’Amours, S.; et al. Update from the Abdominal Compartment Society (WSACS) on intra-abdominal hypertension and abdominal compartment syndrome: Past, present, and future beyond Banff 2017. Anaesthesiol. Intensive Ther. 2017, 49, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Damaser, M.S.; Niblett, P.; Rosier, P.; Toozs-Hobson, P.; Hosker, G.; Kightley, R.; Gammie, A. Air filled, including "air-charged," catheters in urodynamic studies: Does the evidence justify their use? Neurourol. Urodyn. 2017, 36, 1234–1242. [Google Scholar] [CrossRef]
- See, K.C.; Tayebi, S.; Sum, C.L.; Phua, J.; Stiens, J.; Wise, R.; Mukhopadhyay, A.; Malbrain, M. Feasibility analysis of a novel non-invasive ultrasonographic method for the measurement of intra-abdominal pressure in the intensive care unit. J. Clin. Monit. Comput. 2023, 37, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Dai, Y.; Zhao, D.; Sun, Z.; Chen, F.; Zhu, Y.; Liang, H.; Cao, H.; Zhang, L. Deep Domain Adaptation for Predicting Intra-Abdominal Pressure with Multichannel Attention Fusion Radar Chip. Adv. Intell. Syst. 2022, 4, 2100209. [Google Scholar] [CrossRef]
- Tayebi, S.; Pourkazemi, A.; Malbrain, M.; Stiens, J. Non-Invasive Intra-Abdominal Pressure Measurement by Means of Transient Radar Method: In Vitro Validation of a Novel Radar-Based Sensor. Sensors 2021, 21, 5999. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Xu, Z.; Sivaperuman Kalairaj, M.; Ponraj, G.; Huang, H.; Ng, C.-F.; Wu, Q.H.; Ren, H. Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring. Biosensors 2021, 11, 156. [Google Scholar] [CrossRef]
- David, M.; Raviv, A.; Peretz, A.; Berkovich, U.; Pracca, F. Towards a continuous non-invasive assessment of intra-abdominal pressure based on bioimpedance and microwave reflectometry: A pilot run on a porcine model. Biomed. Signal Process. Control. 2018, 44, 96–100. [Google Scholar] [CrossRef]
- Farmer, A.D.; Scott, S.M.; Hobson, A.R. Gastrointestinal motility revisited: The wireless motility capsule. United Eur. Gastroenterol. J. 2013, 1, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kušar, M.; Djokić, M.; Djordjević, S.; Hribernik, M.; Krašna, S.; Trotovšek, B. Preliminary study of reliability of transcutaneous sensors in measuring intraabdominal pressure. Sci. Rep. 2022, 12, 8268. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Cheatham, M.L.; Malbrain, M.L.; Kirkpatrick, A.W.; Sugrue, M.; Balogh, Z.; Ivatury, R.; De Keulenaer, B.; Kimball, E.J. Recommendations for research from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Acta Clin. Belg. 2009, 64, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Device Approvals, Denials, and Clearances. Available online: https://www.fda.gov/medical-devices/510k-clearances/october-2021-510k-clearances (accessed on 10 September 2023).
- Tayebi, S.; Wise, R.; Zarghami, A.; Malbrain, L.; Khanna, A.K.; Dabrowski, W.; Stiens, J.; Malbrain, M.L.N.G. In Vitro Validation of a Novel Continuous Intra-Abdominal Pressure Measurement System (TraumaGuard). J. Clin. Med. 2023, 12, 6260. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
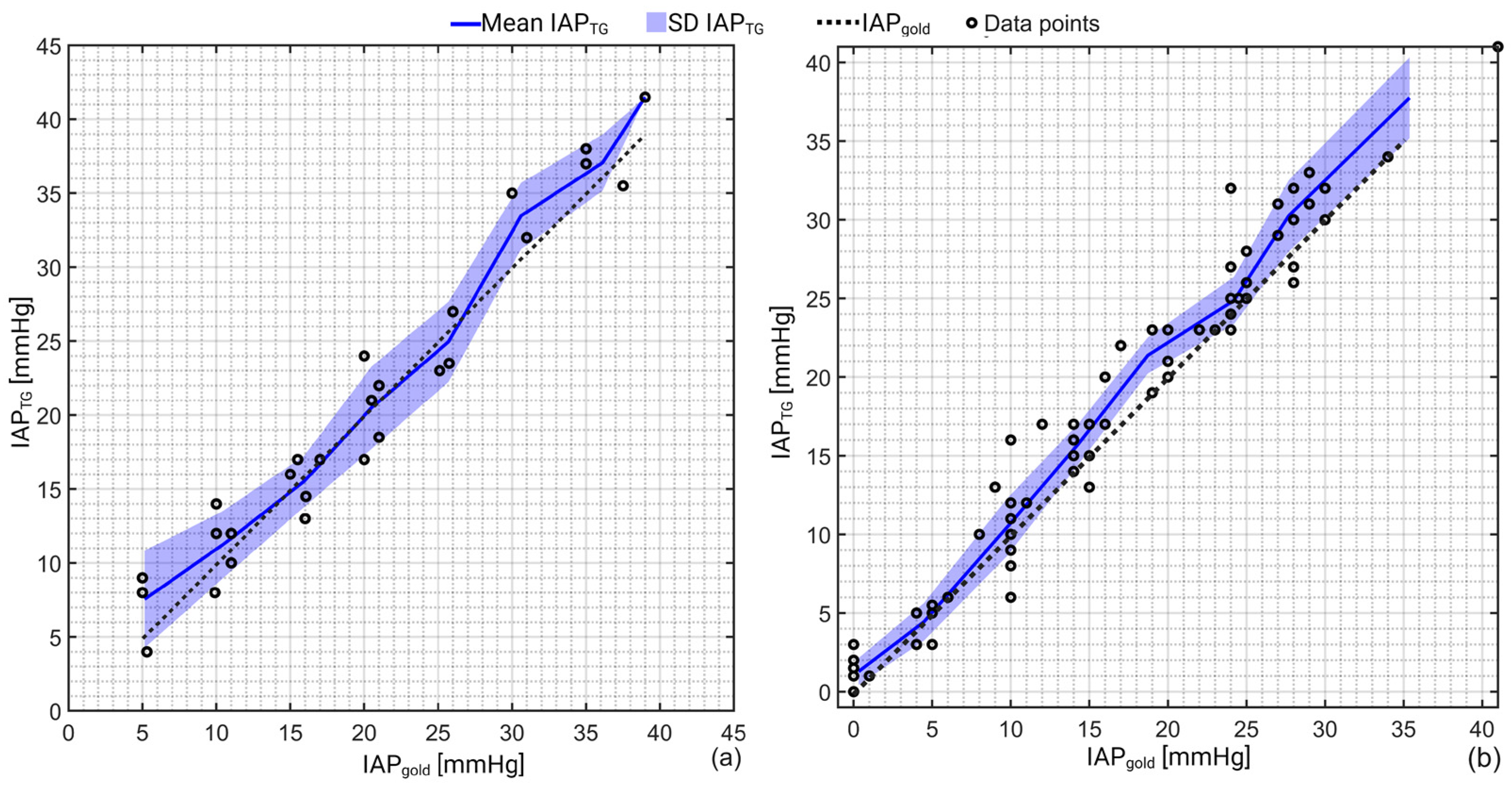
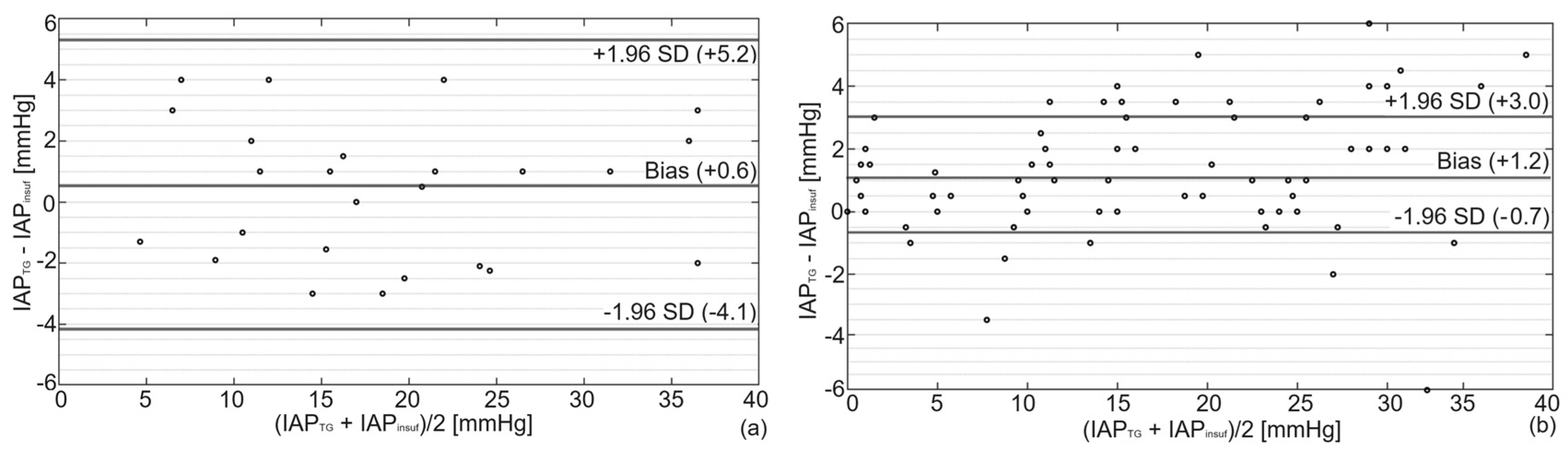
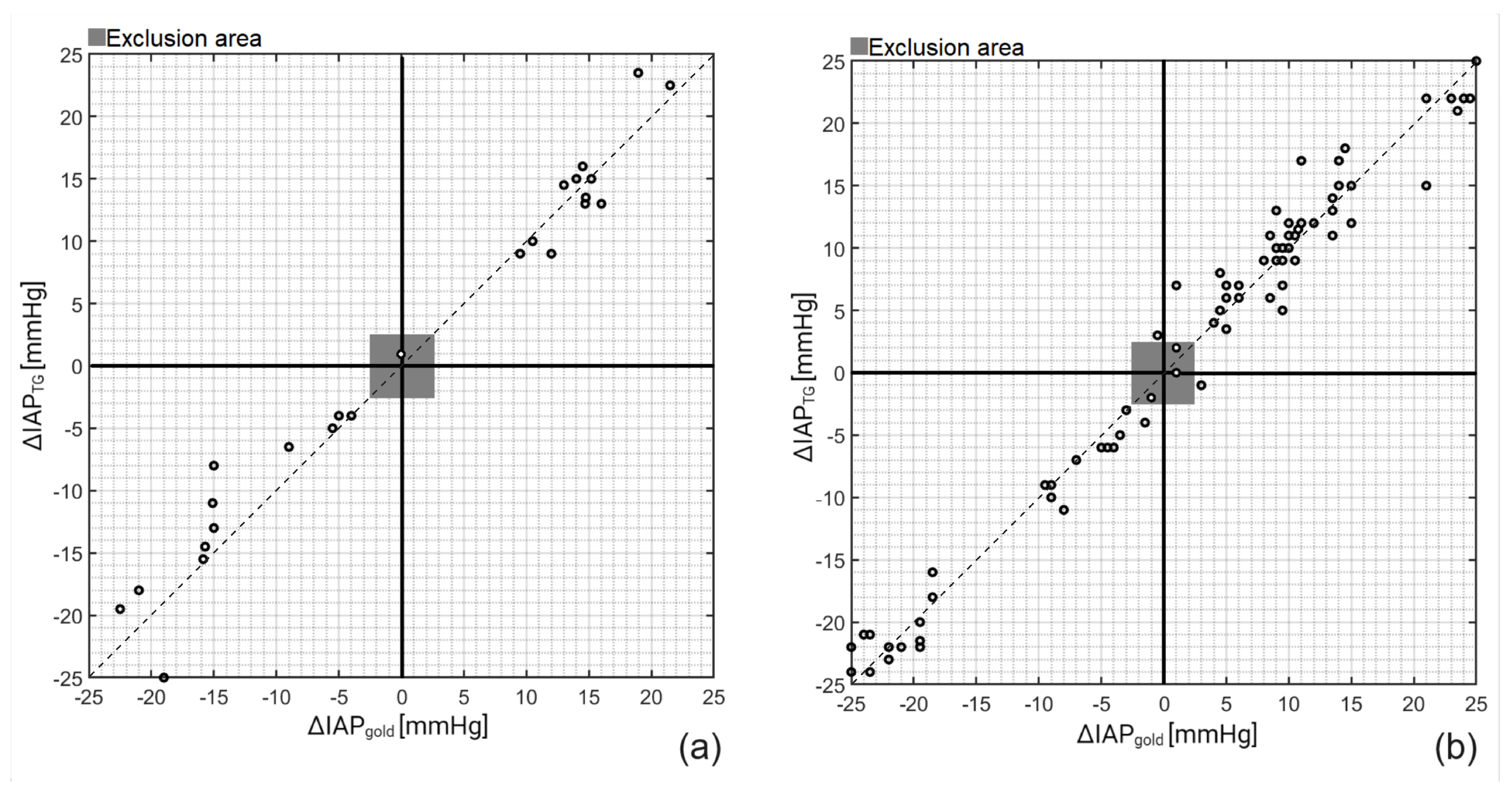

| Mean [mmHg] | Bias [mmHg] | Precision [mmHg] | LLA [mmHg] | ULA [mmHg] | PE [%] | CV [-] | |
|---|---|---|---|---|---|---|---|
| Porcine | 20.4 | 0.6 | 2.4 | −4.1 | +5.3 | 24 | 0.5 |
| Cadaver | 15.6 | 1.2 | 1.9 | −0.7 | +3.1 | 24 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebi, S.; McKinney, T.; McKinney, C.; Delvadia, D.; Levine, M.-A.; Spofford, E.S., Jr.; Malbrain, L.; Stiens, J.; Dabrowski, W.; Malbrain, M.L.N.G. Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies. Sensors 2023, 23, 8806. https://doi.org/10.3390/s23218806
Tayebi S, McKinney T, McKinney C, Delvadia D, Levine M-A, Spofford ES Jr., Malbrain L, Stiens J, Dabrowski W, Malbrain MLNG. Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies. Sensors. 2023; 23(21):8806. https://doi.org/10.3390/s23218806
Chicago/Turabian StyleTayebi, Salar, Tim McKinney, Cynthia McKinney, Dipak Delvadia, Marc-Alan Levine, Edward S. Spofford, Jr., Luca Malbrain, Johan Stiens, Wojciech Dabrowski, and Manu L. N. G. Malbrain. 2023. "Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies" Sensors 23, no. 21: 8806. https://doi.org/10.3390/s23218806
APA StyleTayebi, S., McKinney, T., McKinney, C., Delvadia, D., Levine, M.-A., Spofford, E. S., Jr., Malbrain, L., Stiens, J., Dabrowski, W., & Malbrain, M. L. N. G. (2023). Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies. Sensors, 23(21), 8806. https://doi.org/10.3390/s23218806









