Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 1: Laboratory Validation
Abstract
1. Introduction
- Implementing sensor networks in real-world structures is still a difficult task. The aforementioned examples have been individually validated by laboratory testing. Nevertheless, as monitoring a structure requires many control points to analyse different zones, it is necessary to generate sensor networks that work in a coordinated manner rather than isolated elements;
- These systems must be autonomous. This involves having to implement central units that manage the data collected by the sensors distributed all over the structure and are capable of processing it to offer clear data directly and automatically. As other authors have pointed out, this includes data transfer, processing, plotting, and even websites to store data and present results [1,68]. This requirement also implies making hardware and system communication investments;
- The stakeholders who intervene in developing and building civil infrastructure still need to be made aware of the importance of embedded monitoring systems. More often than not, there are still no concerns at all about structure maintenance, and standards do not significantly highlight the importance of structure management;
- The method to set up systems in real work and its integration with the other tasks to be performed while building works. Embedded monitoring systems must be set up simply and quickly with no maintenance requirements to considerably lower their cost. For this purpose, the durability and robustness of the employed components (durability longer than the structure’s foreseeable service life) must also be taken into account. Some authors point out that the main constraints of these systems are still linked to sensors’ durability and stability over time [69];
- The economic aspect. It is fundamental to be competitive compared with traditional evaluation and management systems, such as visual inspection or on-site corrosion measurements.
2. Materials and Methods
2.1. Test Specimens
2.2. Materials
2.3. Exposure Conditions
2.4. Testing Procedure
- First, the corrosion potentials () of sensors were measured by a high-impedance voltmeter (multimeter Keithley 2000) using a calomel reference electrode (SCE of Radiometer Analytical XR110) following Standard ASTM C876 [79]. The reference electrode was partially immersed in the exposure solution, depending on each group. The value was recorded 3 min after measurements commenced to ensure that the recorded signal was stable enough;
- Second, the corrosion rate () of each sensor was determined by the linear polarisation resistance (LPR) technique. In this method, Stern and Geary’s expression is used to determine by estimating polarisation resistance () according to Equation (1):where is the surface of the working electrode (1571 mm2) and parameter can adopt values within a range from 13 to 52 mV [80]. In this case, the average value (26 mV) was used, with 2 being the maximum error factor of the prediction [81,82]. Firstly, the corrosion potential () of the working electrode was recorded when the variation reached a value that equaled or was less than 0.03 mV/s. Furthermore, a linear voltammetric scan (LVS) was applied from − 20 mV to + 20 mV at a scanning speed of 0.2 mV/s to obtain [83,84]. This measurement was taken with an Autolab PGSTAT 100 Potentiostat, and the Nova 1.11 software was used for signal processing. The measurement cell configuration was a 3-electrode one; each sensor was the working electrode. A stainless-steel piece partially immersed in the exposure solution was employed as the counter electrode. The reference electrode was an SCE (Radiometer Analytical XR110), which was also partially immersed in solution.
- Later, the corrosion rate of each sensor was determined by the Potential Step Volmametry (PSV) method (). This technique, which is used by the INESSCOM system, was introduced in previous works and has been previously validated [71,72,73,74,76]. This measurement was also taken with an Autolab PGSTAT 100 Potentiostat, and the Nova 1.11 software was used for signal processing. The measurement cell configuration was also a 3-electrode one;
- In addition, the sensors’ double-layer capacitance () was determined from the voltammogram () obtained after applying CSV, ± 50 mV × 2 cycles at a sweep speed of 1 mV/s. This procedure, previously used by other authors in the corrosion field [21,22,85], consists of determining the intensity increase corresponding to the voltammeter width in () and replacing it in Equation (2) together with the applied sweep speed (), which allows to calculate .
- Finally, the corrosion rate was also determined by the Tafel Extrapolation (TE) method () as a reference technique [86]. To do this, the polarisation curves ( vs. ) were obtained by applying a linear potential sweep at a sweep speed of 0.2 mV/s [86]. Initially, the sweep was applied in a positive direction from to + 140 mV. Subsequently, a 24 h period was used to ensure that returned to the initially recorded values (with a difference of ±5 mV), and then the scan was applied in a negative direction from to − 140 mV [87,88]. Later, was determined by extrapolating the straight sections (from ± 59 mV) of the anodic and cathodic curves to according to [89];
- To complement and compare the information obtained from the sensors by the electrochemical methods described above, a visual inspection of the test specimens was also carried out to visually detect any appreciable corrosion symptoms. To be able to inspect the sensor state, three test specimens from all three groups (A, B, C) were broken once the study was completed.
3. Results
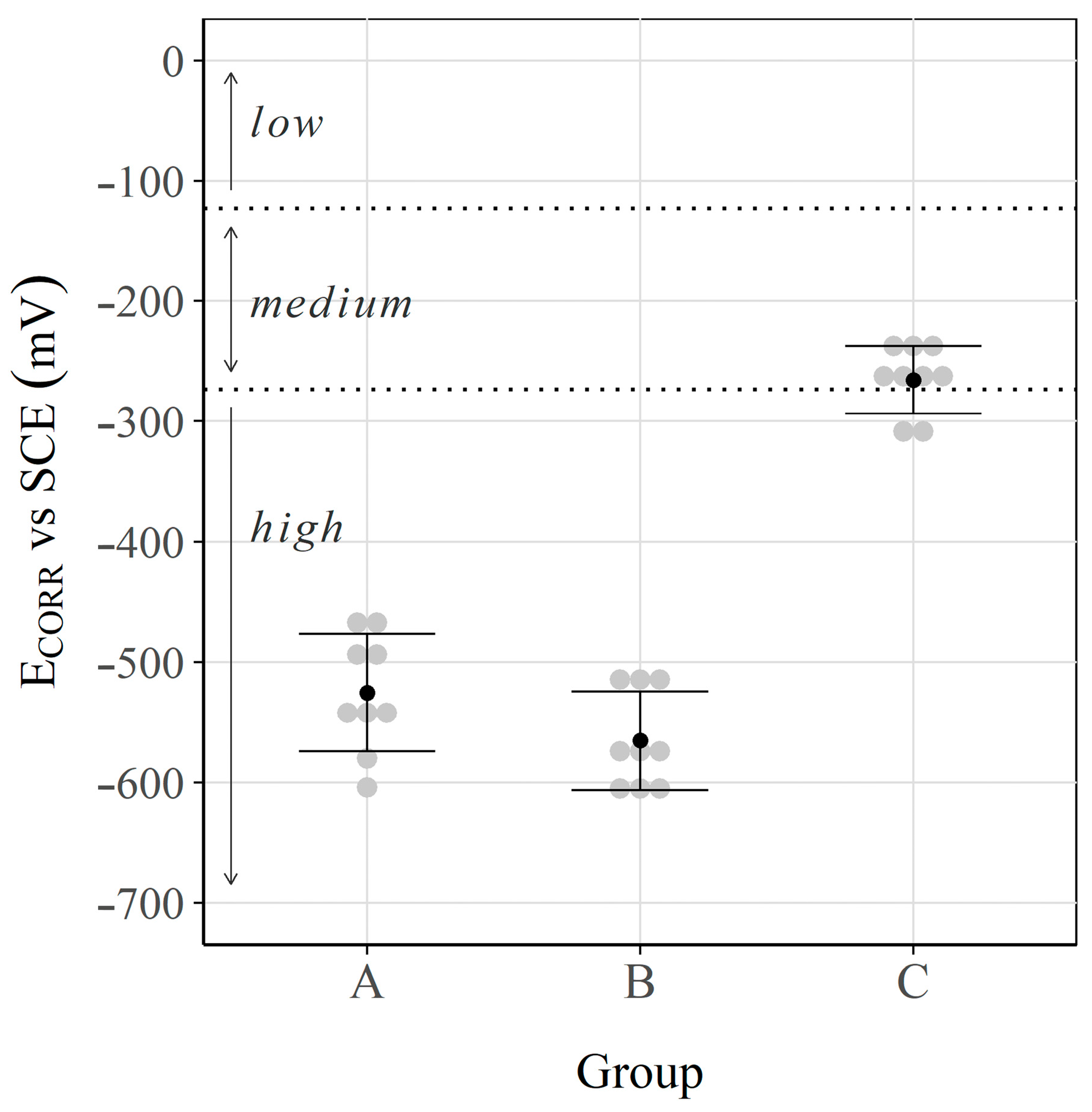
3.1. Corrosion Potential and Corrosion Rate
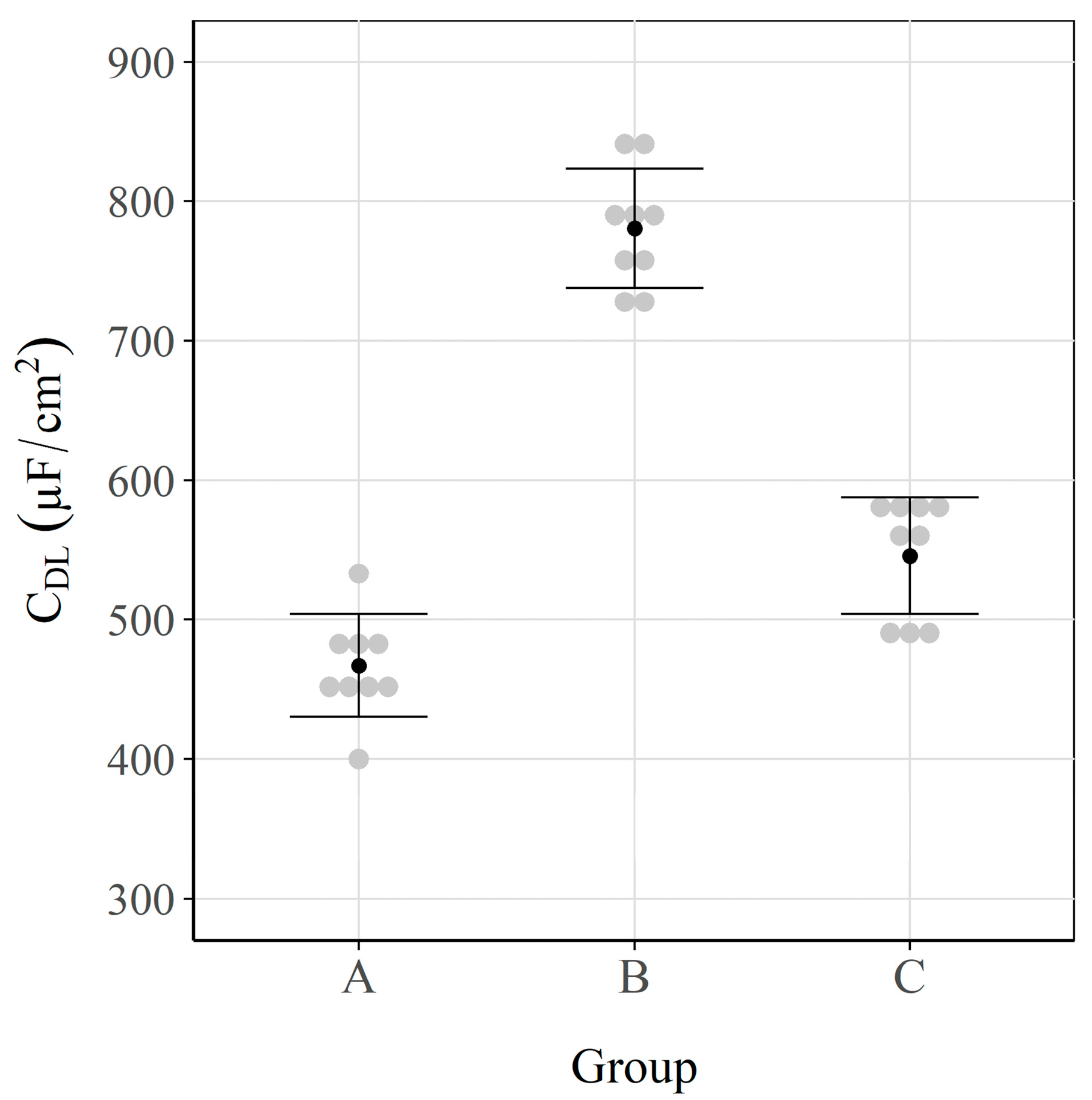
3.2. Double-Layer Capacitance
3.3. Visual Inspection
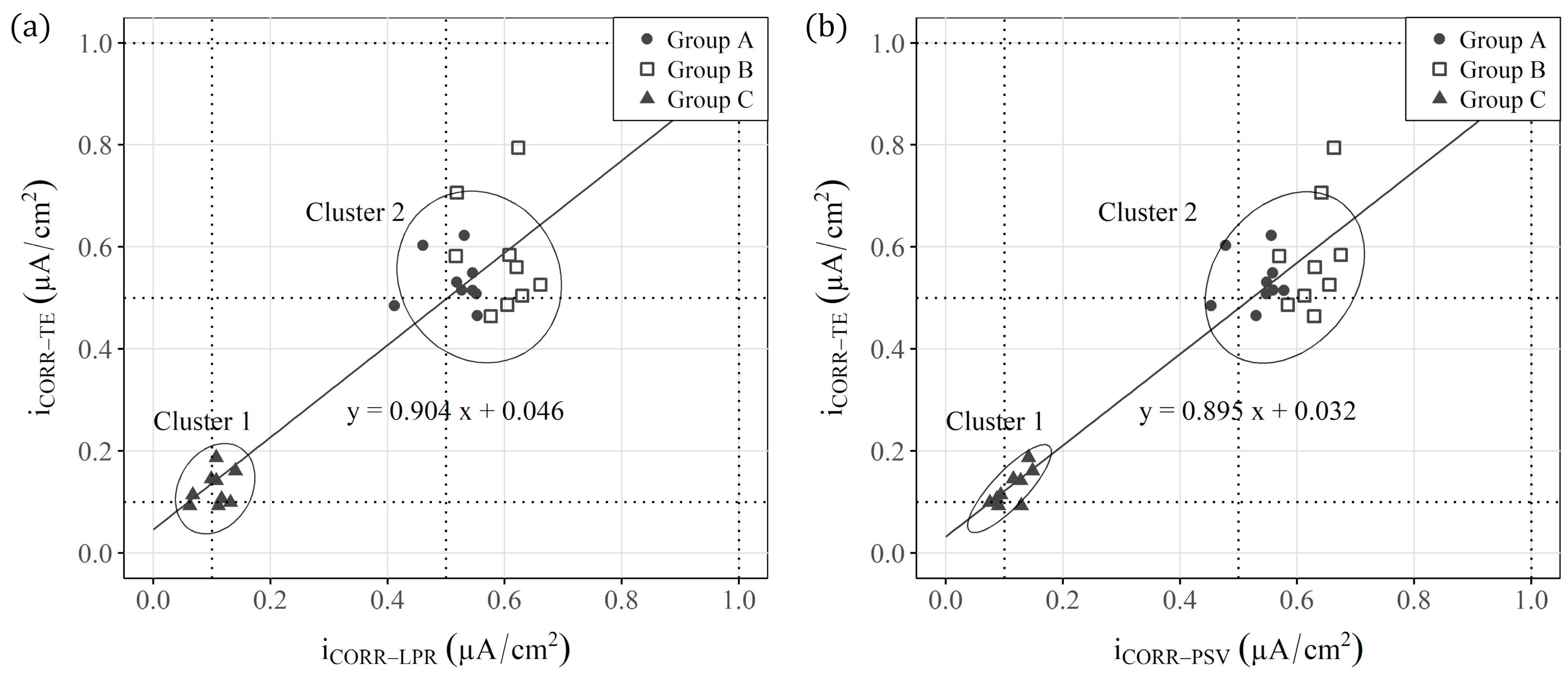
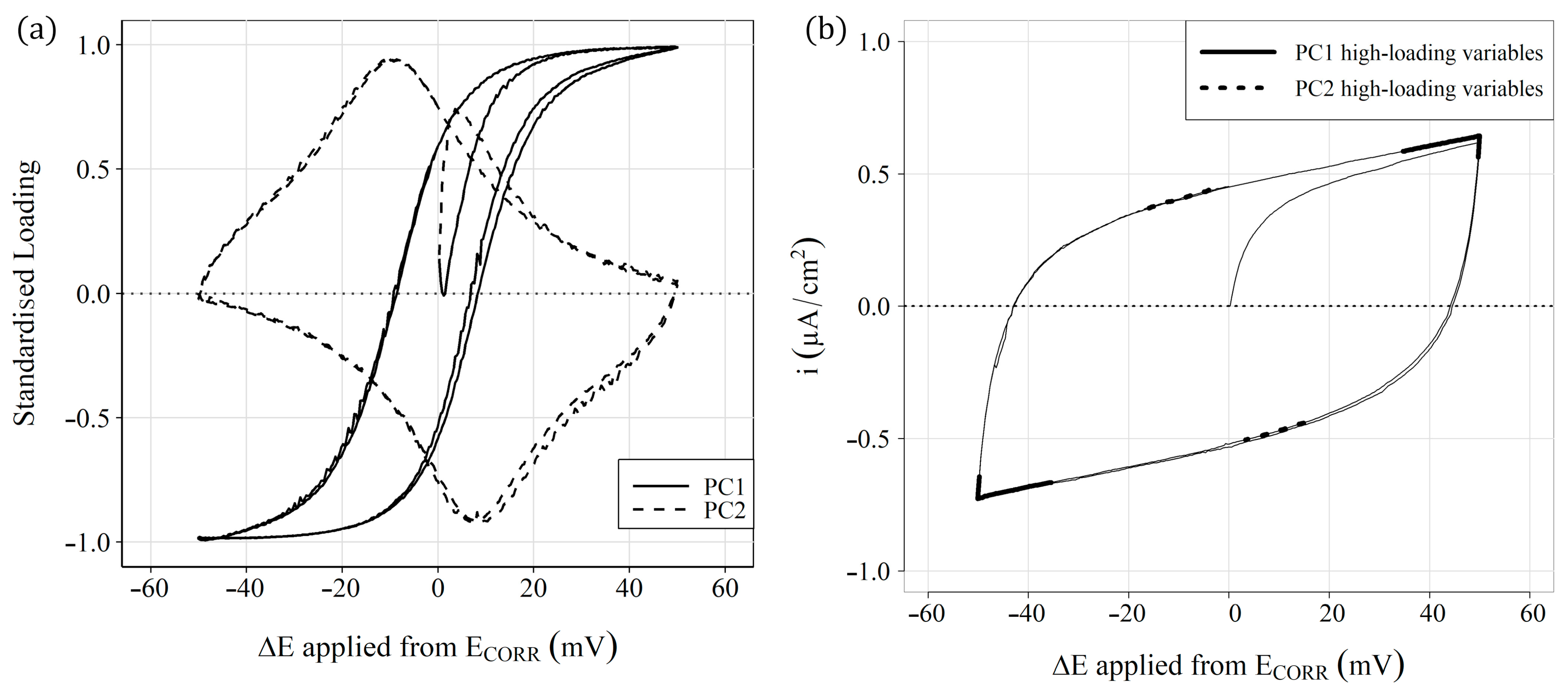
3.4. Statistical Analyses
4. Conclusions
- Obtaining the , , and allows for monitoring the kinetic activity of the embedded sensors in RCSs. Nevertheless, analysing these parameters independently can lead to mistaken interpretations;
- Conversely, the analysis performed by multivariate tools (PCA) of sensors’ ( and ) responses allows a classification that distinguishes the different study scenarios;
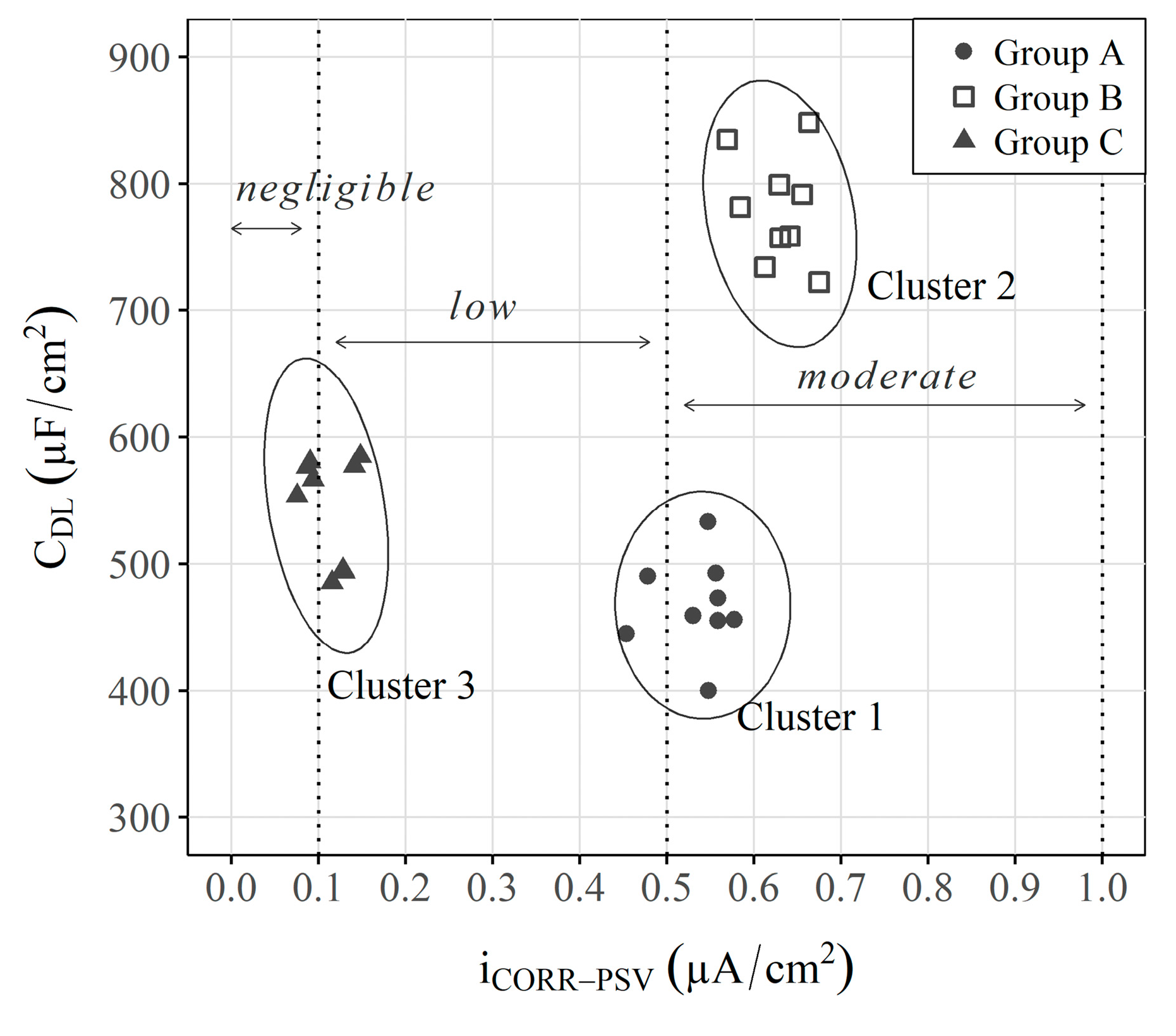
- To facilitate PCA understanding, this work also proposes using comparative graphs of both parameters ( and ) to distinguish the three study scenarios, but with a much clearer representation in which each axis corresponds to a given parameter, unlike the PCA;
- The obtained results demonstrate that implementing a new measurement protocol in INESSCOM to, in this case, analyse , would be extremely useful for simply and quickly determining the precursor corrosion agent, even when the recorded corrosion kinetics are similar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakiyama, F.I.H.; Lehmann, F.; Garrecht, H. Structural health monitoring of concrete structures using fibre-optic-based sensors: A review. Mag. Concr. Res. 2021, 73, 174–194. [Google Scholar] [CrossRef]
- Sun, M.; Staszewski, W.J.; Swamy, R.N. Swamy, Smart sensing technologies for structural health monitoring of civil engineering structures. Adv. Civ. Eng. 2010, 2010, 724962. [Google Scholar] [CrossRef]
- Angst, U.M.; Hooton, R.D.; Marchand, J.; Page, C.L.; Flatt, R.J.; Elsener, B.; Gehlen, C.; Gulikers, J. Present and future durability challenges for reinforced concrete structures. Mater. Corros. 2012, 63, 1047–1051. [Google Scholar] [CrossRef]
- Wangler, T.; Roussel, N.; Bos, F.P.; Salet, T.A.; Flatt, R.J. Digital Concrete: A Review. Cem. Concr. Res. 2019, 123, 105780. [Google Scholar] [CrossRef]
- Brandt, A.M. Fibre reinforced cement-based (FRC) composites after over 40 years of development in building and civil engineering. Compos. Struct. 2008, 86, 3–9. [Google Scholar] [CrossRef]
- Shafei, B.; Kazemian, M.; Dopko, M.; Najimi, M. State-of-the-art review of capabilities and limitations of polymer and glass fibers used for fiber-reinforced concrete. Materials 2021, 14, 409. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Andrade, C.; Li, Z. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- El-Joukhadar, N.; Pantazopoulou, S. Effectiveness of UHPFRC cover in delaying bar corrosion. Constr. Build. Mater. 2020, 269, 121288. [Google Scholar] [CrossRef]
- El-Sayed, A.K.; Hussain, R.R.; Shuraim, A.B. Effect of stirrup corrosion on the shear strength of reinforced concrete short beams. J. Civ. Eng. Manag. 2016, 22, 491–499. [Google Scholar] [CrossRef]
- Azam, R.; El-Sayed, A.K.; Soudki, K. Behaviour of reinforced concrete beams without stirrups subjected to steel reinforcement corrosion. J. Civ. Eng. Manag. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Wang, J.; Ng, P.-L.; Wang, W.; DU, J.; Song, J. Modelling chloride diffusion in concrete with influence of concrete stress state. J. Civ. Eng. Manag. 2017, 23, 955–965. [Google Scholar] [CrossRef][Green Version]
- Moradi-Marani, F.; Shekarchi, M.; Dousti, A.; Mobasher, B. Investigation of Corrosion Damage and Repair System in a Concrete Jetty Structure. J. Perform. Constr. Facil. 2010, 24, 294–301. [Google Scholar] [CrossRef]
- Yuan, Y.; Ji, Y.; Shah, S.P. Comparison of Two Accelerated Corrosion Techniques for Concrete Structures. ACI Struct. J. 2007, 104, 344–347. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Claisse, P. A Review of Corrosion and Protection of Steel in Concrete. Arab. J. Sci. Eng. 2018, 43, 5035–5055. [Google Scholar] [CrossRef]
- Bertolini, L.; Bertolini, L.; Milano, P.; Chimica, D.; Chimica, I. Steel corrosion and service life of reinforced concrete structures. Struct. Infrastruct. Eng. 2008, 4, 123–137. [Google Scholar] [CrossRef]
- Beushausen, H.; Torrent, R.; Alexander, M.G. Performance-based approaches for concrete durability: State of the art and future research needs. Cem. Concr. Res. 2019, 119, 11–20. [Google Scholar] [CrossRef]
- González, J.; Cobo, A.; González, M.; Feliu, S. On-site determination of corrosion rate in reinforced concrete structures by use of galvanostatic pulses. Corros. Sci. 2001, 43, 611–625. [Google Scholar] [CrossRef]
- Brenna, A.; Bolzoni, F.; Beretta, S.; Ormellese, M. Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings. Constr. Build. Mater. 2013, 48, 734–744. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Chemical and Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar] [CrossRef]
- González, J.A.; Feliú, S.; Rodríguez, P.; Ramírez, E.; Alonso, C.; Andrade, C. Some questions on the corrosion of steel in concrete—Part I: When, how and how much steel corrodes. Mater. Struct. Constr. 1996, 29, 40–46. [Google Scholar] [CrossRef]
- González, J.A.; Feliú, S.; Rodríquez, P.; López, W.; Ramírez, E.; Alonso, C.; Andrade, C. Some questions on the corrosion of steel in concrete. Part II: Corrosion mechanism and monitoring, service life prediction and protection methods. Mater. Struct. Constr. 1996, 29, 97–104. [Google Scholar] [CrossRef]
- Ministerio de Transportes Movilidad y Agenda Urbana. Gobierno de España, Código Estructural, 2022. Available online: https://www.mitma.gob.es/organos-colegiados/comision-permanente-de-estructuras-de-acero/cpa/codigo-estructural (accessed on 2 February 2023).
- Ministerio de Fomento. Gobierno de España, EHE-08. Instrucción de Hormigón Estructural, 2008. Available online: https://www.mitma.gob.es/organos-colegiados/mas-organos-colegiados/comision-permanente-del-hormigon/cph/instrucciones/ehe-08-version-en-castellano (accessed on 2 February 2023).
- Concu, G.; De Nicolo, B.; Pani, L. Non-destructive testing as a tool in reinforced concrete buildings refurbishments. Struct. Surv. 2011, 29, 147–161. [Google Scholar] [CrossRef]
- Zhu, Z.; German, S.; Brilakis, I. Detection of large-scale concrete columns for automated bridge inspection. Autom. Constr. 2010, 19, 1047–1055. [Google Scholar] [CrossRef]
- Moore, M.; Phares, B.; Graybeal, B.; Rolander, D.; Washer, G. Reliability of Visual Inspection for Highway Bridges Volume I: Final Report, Fed. Highw. Adm. II (2001) 486. Available online: http://www.tfhrc.gov/hnr20/nde/01020.htm (accessed on 2 February 2023).
- Koch, C.; Georgieva, K.; Kasireddy, V.; Akinci, B.; Fieguth, P. A review on computer vision based defect detection and condition assessment of concrete and asphalt civil infrastructure. Adv. Eng. Inform. 2015, 29, 196–210. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Gastaldi, M.; Lollini, F.; Redaelli, E. Corrosion assessment and restoration strategies of reinforced concrete buildings of the cultural heritage. Mater. Corros. 2011, 62, 146–154. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Jamali, A.; Adey, B. Concrete cover cracking owing to reinforcement corrosion—Theoretical considerations and practical experience. Mater. Corros. 2012, 63, 1069–1077. [Google Scholar] [CrossRef]
- Hornbostel, K.; Larsen, C.K.; Geiker, M.R. Relationship between concrete resistivity and corrosion rate—A literature review. Cem. Concr. Compos. 2013, 39, 60–72. [Google Scholar] [CrossRef]
- Polder, R.B. Test methods for on site measurement of resistivity of concrete—A RILEM TC-154 technical recommendation. Constr. Build. Mater. 2001, 15, 125–131. [Google Scholar] [CrossRef]
- du Plooy, R.; Lopes, S.P.; Villain, G.; Dérobert, X. Development of a multi-ring resistivity cell and multi-electrode resistivity probe for investigation of cover concrete condition. NDT E Int. 2013, 54, 27–36. [Google Scholar] [CrossRef]
- Leelalerkiet, V.; Kyung, J.-W.; Ohtsu, M.; Yokota, M. Analysis of half-cell potential measurement for corrosion of reinforced concrete. Constr. Build. Mater. 2004, 18, 155–162. [Google Scholar] [CrossRef]
- Mietz, J.; Isecke, B. Monitoring of concrete structures with respect to rebar corrosion. Constr. Build. Mater. 1996, 10, 367–373. [Google Scholar] [CrossRef]
- Song, H.-W.; Saraswathy, V. Corrosion Monitoring of Reinforced Concrete Structures—A Review. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar] [CrossRef]
- Torrent, R.J. A two-chamber vacuum cell for measuring the coefficient of permeability to air of the concrete cover on site. Mater. Struct. Constr. 1992, 25, 358–365. [Google Scholar] [CrossRef]
- Tarussov, A.; Vandry, M.; De La Haza, A. Condition assessment of concrete structures using a new analysis method: Ground-penetrating radar computer-assisted visual interpretation. Constr. Build. Mater. 2013, 38, 1246–1254. [Google Scholar] [CrossRef]
- Hugenschmidt, J.; Loser, R. Detection of chlorides and moisture in concrete structures with ground penetrating radar. Mater. Struct. Constr. 2008, 41, 785–792. [Google Scholar] [CrossRef]
- Millard, S.G.; Gowers, K.R.; Gill, R.P. Practical field measurement of reinforcement corrosion in concrete using linear polarisation methods. Eur. J. Non-Destr. Test. 1992, 2, 17–25. [Google Scholar]
- Law, D.; Millard, S.; Bungey, J. Linear polarisation resistance measurements using a potentiostatically controlled guard ring. NDT E Int. 2000, 33, 15–21. [Google Scholar] [CrossRef]
- Feliu, S.; González, J.A.; Escudero, M.L.; Andrade, M.C. Possibilities of the guard ring for electrical signal confinement in the polarization measurements of reinforcements. Corrosion 1990, 46, 1015–1020. [Google Scholar] [CrossRef]
- Feliu, S.; González, J.A.; Andrade, C.S.F., Jr. Confinement of the Electrical Signal for in Situ Measurement of Polarization Resistance in Reinforced Concrete. Mater. J. 1990, 87, 457–460. Available online: https://www.unhcr.org/publications/manuals/4d9352319/unhcr-protection-training-manual-european-border-entry-officials-2-legal.html?query=excom1989 (accessed on 2 February 2023).
- Feliu, S.; Gonzalez, J.A.; Andrade, C. Errors in the On-site Measurements of Rebar Corrosion Rates Arising from Signal Un Confinement. Symp. Pap. 1994, 151, 183–196. [Google Scholar]
- Ramón, J.E.; Castillo, Á.; Martínez, I. On-site corrosion monitoring experience in concrete structures: Potential improvements on the current-controlled polarization resistance method. Mater. Constr. 2021, 71, e265. [Google Scholar] [CrossRef]
- Nygaard, P.V.; Geiker, M.R.; Elsener, B. Corrosion rate of steel in concrete: Evaluation of confinement techniques for on-site corrosion rate measurements. Mater. Struct. Constr. 2009, 42, 1059–1076. [Google Scholar] [CrossRef]
- Feliu, S.; Andrade, C.; González, J.A.; Alonso, C. A new method forin-situ measurement of electrical resistivity of reinforced concrete. Mater. Struct. Constr. 1996, 29, 362–365. [Google Scholar] [CrossRef]
- Song, G. Theoretical analysis of the measurement of polarisation resistance in reinforced concrete. Cem. Concr. Compos. 2000, 22, 407–415. [Google Scholar] [CrossRef]
- Wojtas, H. Determination of corrosion rate of reinforcement with a modulated guard ring electrode; analysis of errors due to lateral current distribution. Corros. Sci. 2004, 46, 1621–1632. [Google Scholar] [CrossRef]
- Liu, T.; Weyers, R. Modeling the Dynamic Corrosion Process in Chloride. Cem. Concr. Res. 1998, 28, 365–379. [Google Scholar] [CrossRef]
- Connection-Less Electrical Pulse Response Analysis (CEPRA). Test Method for Corrosion Rate Measurement; Giatech: Ottawa, ON, Canada, 2015.
- Fan, L.; Bao, Y. Review of fiber optic sensors for corrosion monitoring in reinforced concrete. Cem. Concr. Compos. 2021, 120, 104029. [Google Scholar] [CrossRef]
- Leung, C.K.; Wan, K.T.; Chen, L. A novel optical fiber sensor for steel corrosion in concrete structures. Sensors 2008, 8, 1960–1976. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, X.; Lei, Y. Monitoring corrosion of reinforcement in concrete structures via fiber Bragg grating sensors. Front. Mech. Eng. China 2009, 4, 316–319. [Google Scholar] [CrossRef]
- Martínez, I.; Andrade, C. Examples of reinforcement corrosion monitoring by embedded sensors in concrete structures. Cem. Concr. Compos. 2009, 31, 545–554. [Google Scholar] [CrossRef]
- Pereira, E.; Figueira, R.; Salta, M.M.; Fonseca, I. Embedded sensors for corrosion monitoring of existing reinforced concrete structures. Mater. Sci. Forum 2008, 587–588, 677–681. [Google Scholar] [CrossRef]
- Xu, C.; Li, Z.; Jin, W. A new corrosion sensor to determine the start and development of embedded rebar corrosion process at coastal concrete. Sensors 2013, 13, 13258–13275. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, J.P.; Davies, K.; Hladky, K. The use of permanent corrosion monitoring in new and existing reinforced concrete structures. Cem. Concr. Compos. 2002, 24, 27–34. [Google Scholar] [CrossRef]
- Karthick, S.; Muralidharan, S.; Saraswathy, V.; Thangavel, K. Long-term relative performance of embedded sensor and surface mounted electrode for corrosion monitoring of steel in concrete structures. Sens. Actuators B Chem. 2014, 192, 303–309. [Google Scholar] [CrossRef]
- Duffó, G.S.; Farina, S.B. Development of an embeddable sensor to monitor the corrosion process of new and existing reinforced concrete structures. Constr. Build. Mater. 2009, 23, 2746–2751. [Google Scholar] [CrossRef]
- Yu, H.; Caseres, L. An embedded multi-parameter corrosion sensor for reinforced concrete structures. Mater. Corros. 2012, 63, 1011–1016. [Google Scholar] [CrossRef]
- Legat, A. Monitoring of steel corrosion in concrete by electrode arrays and electrical resistance probes. Electrochim. Acta 2007, 52, 7590–7598. [Google Scholar] [CrossRef]
- Gandía-Romero, J.M.; Bataller, R.; Monzón, P.; Campos, I.; García-Breijo, E.; Valcuende, M.; Soto, J. Characterization of embeddable potentiometric thick-film sensors for monitoring chloride penetration in concrete. Sens. Actuators B Chem. 2016, 222, 407–418. [Google Scholar] [CrossRef]
- Gandía-Romero, J.; Campos, I.; Valcuende, M.; García-Breijo, E.; Marcos; Payá, J.; Soto, J. Potentiometric thick-film sensors for measuring the pH of concrete. Cem. Concr. Compos. 2016, 68, 66–76. [Google Scholar] [CrossRef]
- Pereira, E.V.; Figueira, R.B.; Salta, M.M.L.; Da Fonseca, I.T.E. A galvanic sensor for monitoring the corrosion condition of the concrete reinforcing steel: Relationship between the galvanic and the corrosion currents. Sensors 2009, 9, 8391–8398. [Google Scholar] [CrossRef]
- Park, Z.-T.; Choi, Y.-S.; Kim, J.-G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structure: Part 2. Laboratory electrochemical testing of sensors in concrete. Cem. Concr. Res. 2005, 35, 1814–1819. [Google Scholar] [CrossRef]
- Taffese, W.Z.; Nigussie, E. Autonomous corrosion assessment of reinforced concrete structures: Feasibility study. Sensors 2020, 20, 6825. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, F.J.; Betti, M.; Bartoli, G.; Pallarés, L. Structural health monitoring (SHM) and Nondestructive testing (NDT) of slender masonry structures: A practical review. Constr. Build. Mater. 2021, 297, 123768. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical sensors for monitoring the corrosion conditions of reinforced concrete structures: A review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef]
- Ramón, J.E.; Gandía-Romero, J.M.; Bataller, R.; López, J.A.; Valcuende, M.; Soto, J. Real-time corrosion monitoring of an ultra-high performance fibre-reinforced concrete offshore raft by using an autonomous sensor system. Struct. Control Health Monit. 2022, 29, e3102. [Google Scholar] [CrossRef]
- Ramon Zamora, J.E. Sistema de Sensores Embebidos para Monitorizar la Corrosión en Estructuras de Hormigón Armado. Fundamentos, Metodología y Aplicaciones. Doctoral Dissertation, Universitat Politècnica de València, Valencia, Spain, 2018. [Google Scholar]
- Ramón, J.E.; Gandía-Romero, J.M.; Valcuende, M.; Bataller, R. Integrated sensor network for monitoring steel corrosion in concrete structures. Vitr.-Int. J. Arch. Technol. Sustain. 2016, 1, 65–79. [Google Scholar] [CrossRef]
- Ramón, J.; Martínez-Ibernón, A.; Gandía-Romero, J.; Fraile, R.; Bataller, R.; Alcañiz, M.; García-Breijo, E.; Soto, J. Characterization of electrochemical systems using potential step voltammetry. Part I: Modeling by means of equivalent circuits. Electrochim. Acta 2019, 323, 134702. [Google Scholar] [CrossRef]
- Ramón, J.; Gandía-Romero, J.; Bataller, R.; Alcañiz, M.; Valcuende, M.; Soto, J. Potential step voltammetry: An approach to corrosion rate measurement of reinforcements in concrete. Cem. Concr. Compos. 2020, 110, 103590. [Google Scholar] [CrossRef]
- Lliso-Ferrando, J.R. Monitorizacion de la Durabilidad de Estructuras Existentes de Hormigon Armado Mediante la Inserción de una red de Sensors. Ph.D. Thesis, Universitat Politècnica de València, València, Spain, 2022. [Google Scholar]
- Martínez-Ibernón, A.; Ramón, J.; Gandía-Romero, J.; Gasch, I.; Valcuende, M.; Alcañiz, M.; Soto, J. Characterization of electrochemical systems using potential step voltammetry. Part II: Modeling of reversible systems. Electrochim. Acta 2019, 328, 135111. [Google Scholar] [CrossRef]
- Ramón, J.E.; Martínez, I.; Gandía-Romero, J.M.; Soto, J. Improved Tafel-Based Potentiostatic Approach for Corrosion Rate Monitoring of Reinforcing Steel. J. Nondestruct. Eval. 2022, 41, 1–25. [Google Scholar] [CrossRef]
- Ramón, J.E.; Martínez, I.; Gandía-Romero, J.M.; Soto, J. An embedded-sensor approach for concrete resistivity measurement in on-site corrosion monitoring: Cell constants determination. Sensors 2021, 21, 2481. [Google Scholar] [CrossRef] [PubMed]
- ASTM C876-15; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in. Stand. Test Method Corros. Potentials Uncoated Reinf. Steel Concr. USA. 2017; pp. 1–8. Available online: https://cdn.standards.iteh.ai/samples/94013/0af25737abb44bf1802863d273a7ae6b/ASTM-C876-15.pdf (accessed on 2 February 2023).
- González, J.A.; Miranda, J.M. Consideraciones sobre los posibles mecanismos de corrosión de las estructuras de hormigón armado y sobre los factores que controlan su cinética. Rev. Metal. 2004, 40, 89–100. [Google Scholar] [CrossRef][Green Version]
- González, J.; Molina, A.; Escudero, M.; Andrade, C. Errors in the electrochemical evaluation of very small corrosion rates—II. Other electrochemical techniques applied to corrosion of steel in concrete. Corros. Sci. 1985, 25, 519–530. [Google Scholar] [CrossRef]
- González, J.; Molina, A.; Escudero, M.; Andrade, C. Errors in the electrochemical evaluation of very small corrosion rates—I. Polarization resistance method applied to corrosion of steel in concrete. Corros. Sci. 1985, 25, 917–930. [Google Scholar] [CrossRef]
- AENOR. UNE 112072:2011; Determinación de la Velocidad de Corrosión de Armaduras en Laboratorio Mediante Medida de la Resistencia a la Polarización. Spain. 2011. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0048528 (accessed on 2 February 2023).
- American Society of Testing Materials (ASTM). Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; American Society of Testing Materials (ASTM): West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Rodríguez, P.; Ramírez, E.; González, J.A. Methods for studying corrosion in reinforced concrete. Mag. Concr. Res. 1994, 46, 81–90. [Google Scholar] [CrossRef]
- Wagner, C.; Traud, W. On the Interpretation of Corrosion Processes through the Superposition of Electrochemical Partial Processes and on the Potential of Mixed Electrodes. Corrosion 2006, 62, 843–855. [Google Scholar] [CrossRef]
- Chang, Z.-T.; Cherry, B.; Marosszeky, M. Polarisation behaviour of steel bar samples in concrete in seawater. Part 2: A polarisation model for corrosion evaluation of steel in concrete. Corros. Sci. 2008, 50, 3078–3086. [Google Scholar] [CrossRef]
- Chang, Z.-T.; Cherry, B.; Marosszeky, M. Polarisation behaviour of steel bar samples in concrete in seawater. Part 1: Experimental measurement of polarisation curves of steel in concrete. Corros. Sci. 2008, 50, 357–364. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Luciano, G.; Traverso, P.; Letardi, P. Applications of chemometric tools in corrosion studies. Corros. Sci. 2010, 52, 2750–2757. [Google Scholar] [CrossRef]
- Luciano, G.; Leardi, R.; Letardi, P. Principal component analysis of colour measurements of patinas and coating systems for outdoor bronze monuments. J. Cult. Herit. 2009, 10, 331–337. [Google Scholar] [CrossRef]
- Xu, Q.; Ding, C.; Liu, J.; Luo, B. PCA-guided search for K-means. Pattern Recognit. Lett. 2015, 54, 50–55. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Gulikers, J.; Polder, R.; Cigna, R.; Vennesland, Ø.; Salta, M.; Raharinaivo, A. Elsener, Recommendations of RILEM TC-154-EMC: “Electrochemical techniques for measuring metallic corrosion” Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. Constr. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Lliso-Ferrando, J.; Gasch, I.; Martínez-Ibernón, A.; Valcuende, M. Effect of macrocell currents on rebar corrosion in reinforced concrete structures exposed to a marine environment. Ocean Eng. 2022, 257, 111680. [Google Scholar] [CrossRef]
- Sibatov, R.T.; Uchaikin, V.V. Fractional kinetics of charge carriers in supercapacitors. In Volume 8 Applications in Engineering, Life and Social Sciences, Part B; De Gruyter: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Conway, B.E.; Pell, W.G. Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]






| Cement | Water | Sand (0/2) | Sand (2/4) | Gravel (4/6) | w/c Ratio |
|---|---|---|---|---|---|
| 250 | 220 | 735.5 | 735.5 | 638 | 0.8 |
| Exposure Condition | Sample | vs. SCE (mV) | (µF/cm2) | (µA/cm2) | (µA/cm2) | (µA/cm2) |
|---|---|---|---|---|---|---|
| Group A (Carbonation + Chlorides) | 1 | −604 | 473 | 0.527 | 0.559 | 0.515 |
| 2 | −470 | 459 | 0.553 | 0.530 | 0.465 | |
| 3 | −580 | 456 | 0.545 | 0.578 | 0.514 | |
| 4 | −465 | 490 | 0.461 | 0.478 | 0.603 | |
| 5 | −538 | 455 | 0.546 | 0.559 | 0.549 | |
| 6 | −482 | 400 | 0.519 | 0.548 | 0.531 | |
| 7 | −532 | 492 | 0.531 | 0.556 | 0.622 | |
| 8 | −552 | 445 | 0.412 | 0.454 | 0.485 | |
| 9 | −505 | 533 | 0.552 | 0.548 | 0.508 | |
| Mean | −525 | 467 | 0.52 | 0.53 | 0.53 | |
| CoV 1 | 9.0% | 7.9% | 9.6% | 7.7% | 9.4% | |
| Group B (Chlorides) | 1 | −514 | 835 | 0.517 | 0.569 | 0.582 |
| 2 | −515 | 848 | 0.623 | 0.663 | 0.795 | |
| 3 | −514 | 759 | 0.518 | 0.641 | 0.706 | |
| 4 | −595 | 781 | 0.605 | 0.584 | 0.487 | |
| 5 | −607 | 757 | 0.620 | 0.630 | 0.560 | |
| 6 | −564 | 722 | 0.608 | 0.674 | 0.584 | |
| 7 | −579 | 734 | 0.630 | 0.612 | 0.505 | |
| 8 | −585 | 791 | 0.661 | 0.655 | 0.526 | |
| 9 | −615 | 799 | 0.576 | 0.629 | 0.464 | |
| Mean | −565 | 781 | 0.59 | 0.629 | 0.58 | |
| CoV 1 | 7.3% | 5.5% | 8.5% | 5.6% | 18.9% | |
| Group C (Saturated Ca(OH)2 solution) | 1 | −230 | 554 | 0.132 | 0.075 | 0.099 |
| 2 | −240 | 576 | 0.116 | 0.087 | 0.106 | |
| 3 | −251 | 494 | 0.111 | 0.123 | 0.093 | |
| 4 | −308 | 567 | 0.067 | 0.0937 | 0.114 | |
| 5 | −245 | 580 | 0.062 | 0.090 | 0.093 | |
| 6 | −309 | 495 | 0.107 | 0.128 | 0.142 | |
| 7 | −272 | 485 | 0.099 | 0.115 | 0.146 | |
| 8 | −274 | 577 | 0.107 | 0.141 | 0.187 | |
| 9 | −258 | 585 | 0.140 | 0.148 | 0.161 | |
| Mean | −265 | 546 | 0.11 | 0.112 | 0.13 | |
| CoV 1 | 10.6% | 7.7% | 27.3% | 23.5% | 23.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramón-Zamora, J.E.; Lliso-Ferrando, J.R.; Martínez-Ibernón, A.; Gandía-Romero, J.M. Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 1: Laboratory Validation. Sensors 2023, 23, 8869. https://doi.org/10.3390/s23218869
Ramón-Zamora JE, Lliso-Ferrando JR, Martínez-Ibernón A, Gandía-Romero JM. Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 1: Laboratory Validation. Sensors. 2023; 23(21):8869. https://doi.org/10.3390/s23218869
Chicago/Turabian StyleRamón-Zamora, José Enrique, Josep Ramon Lliso-Ferrando, Ana Martínez-Ibernón, and José Manuel Gandía-Romero. 2023. "Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 1: Laboratory Validation" Sensors 23, no. 21: 8869. https://doi.org/10.3390/s23218869
APA StyleRamón-Zamora, J. E., Lliso-Ferrando, J. R., Martínez-Ibernón, A., & Gandía-Romero, J. M. (2023). Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 1: Laboratory Validation. Sensors, 23(21), 8869. https://doi.org/10.3390/s23218869







