Divalent Aptamer-Functionalized Nanochannels for Facile Detection of Cancer Cell-Derived Exosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture and Exosomes Purification
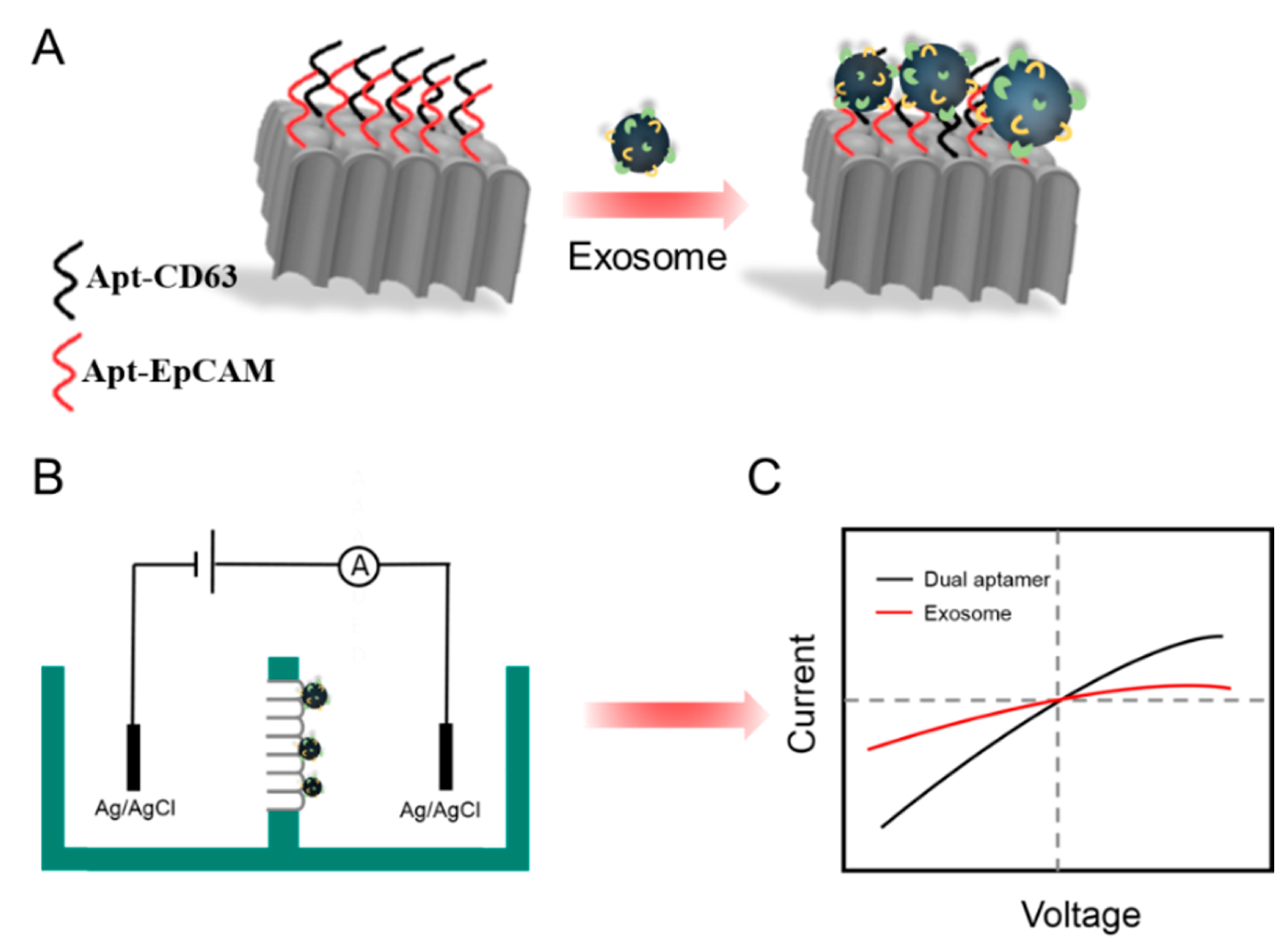
2.3. Functionalization of PAA Film and Detection of Exosomes
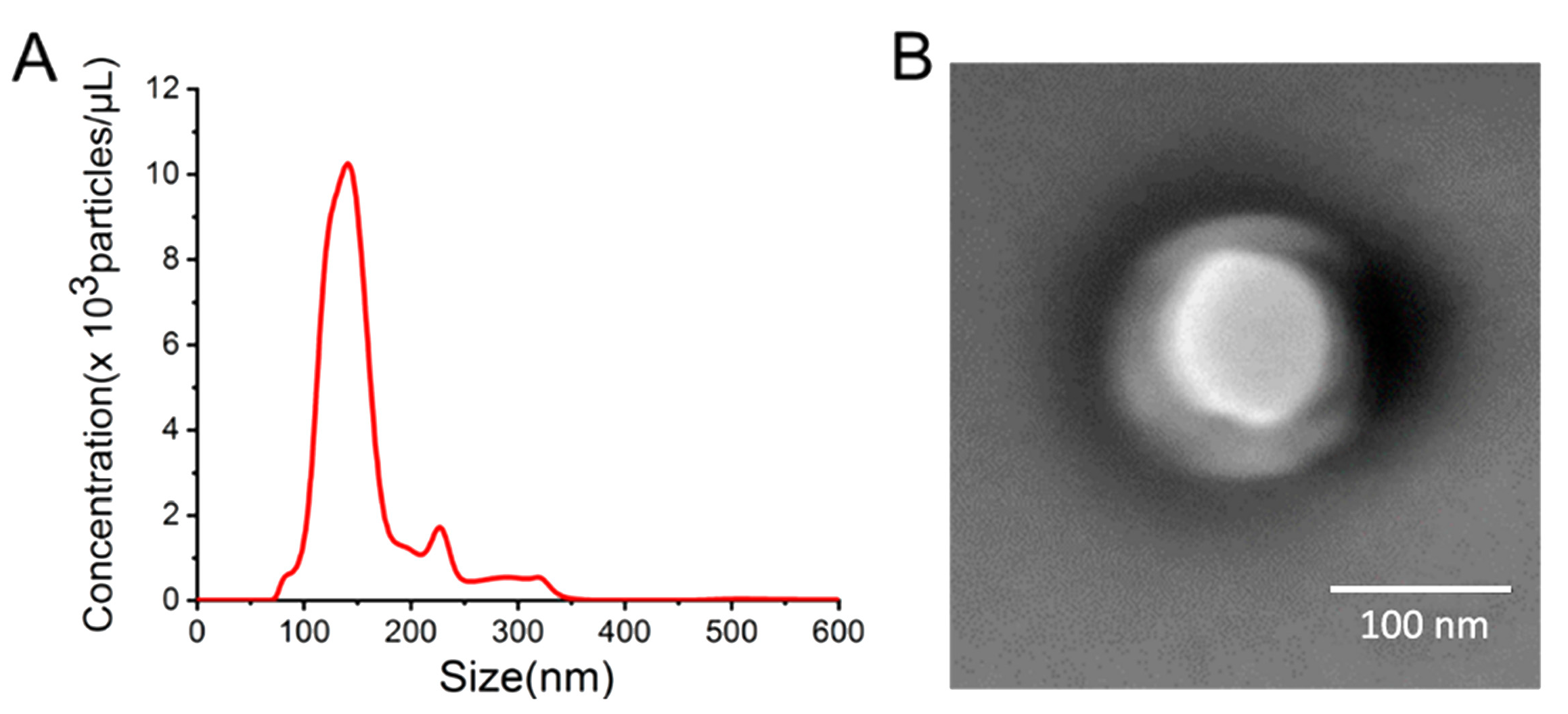
2.4. Characterization of Exosomes and PAA Films
2.5. Electrochemical Measurement
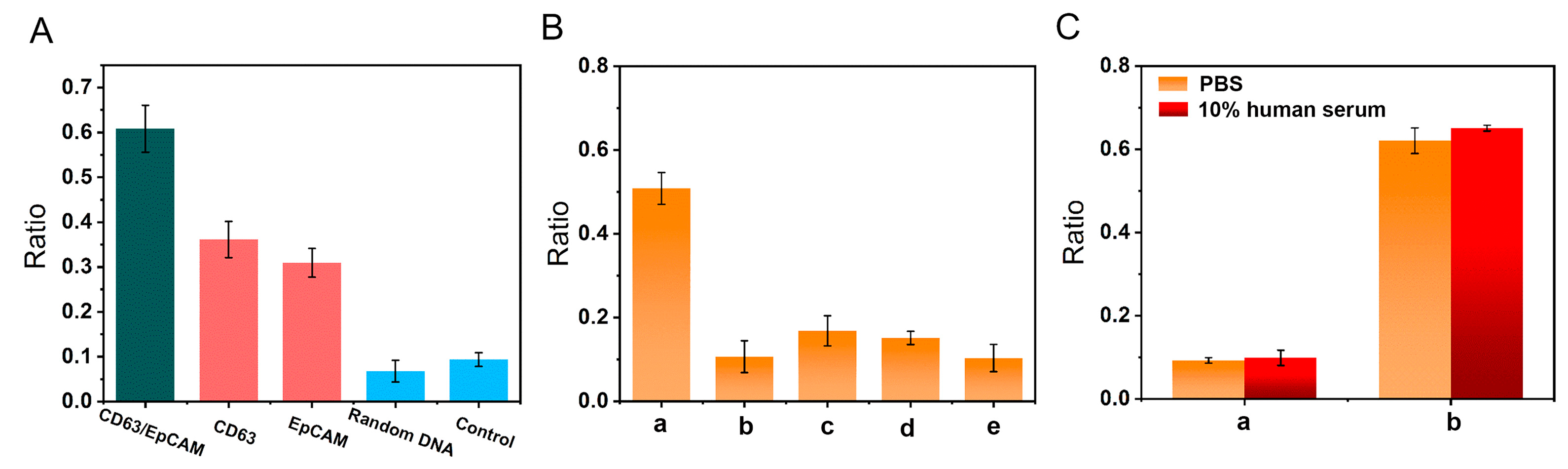
3. Results and Discussion
3.1. Characterization of Exosomes
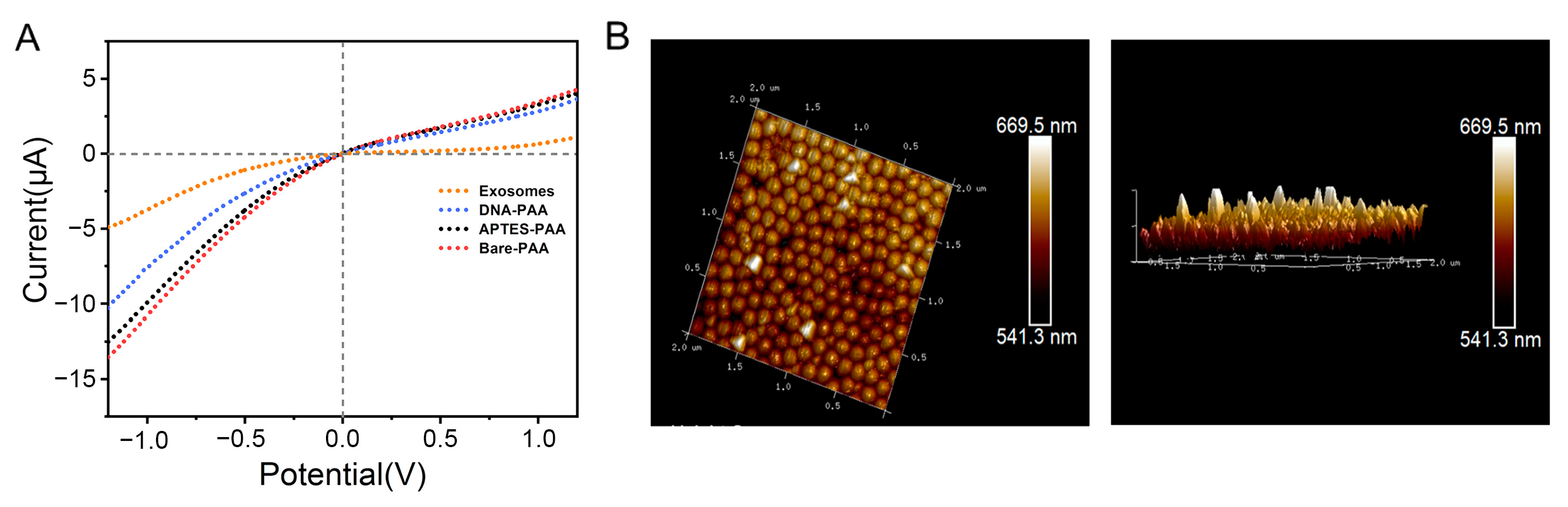
3.2. Characterization and Functionalization of the PAA Film
3.3. Feasibility Investigation
3.4. Optimization of Experimental Conditions
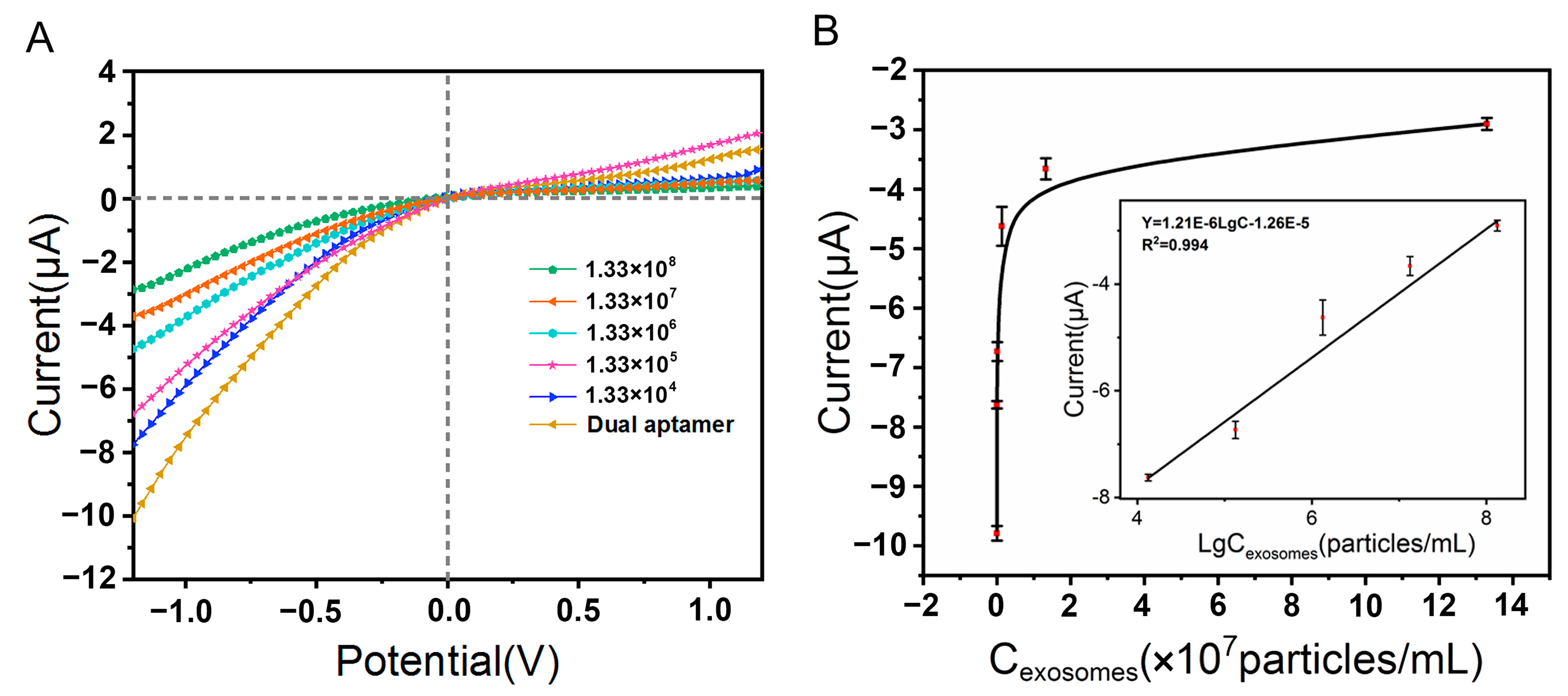
3.5. Investigation of Detection Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.-Y.; Li, D.-P.; Hou, S.-P.; Zhu, X. The cancer exosomes: Clinical implications, applications and challenges. Int. J. Cancer 2020, 146, 2946–2959. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Slack, F.J. Malicious exosomes. Science 2014, 346, 1459–1460. [Google Scholar] [CrossRef]
- Xia, R.-P.; Chai, H.; Jiao, J.; Miao, P. Assembly of DNA triangular pyramid frustum for ultrasensitive quantification of exosomal miRNA. Biosens. Bioelectron. 2023, 231, 115297. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.-L.; Yang, J.-G.; Wang, B.-K.; Sun, H.-H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rew. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.-Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C.; et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef]
- Su, J.; Chen, S.-H.; Dou, Y.-Z.; Zhao, Z.-H.; Jia, X.-L.; Ding, X.-T.; Song, S.-P. Smartphone-based electrochemical biosensors for directly detecting serum-derived exosomes and monitoring their secretion. Anal. Chem. 2022, 94, 3235–3244. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.-Y.; Yang, X.-H.; Liu, X.-F.; Nie, W.-Y.; Zheng, Y.; Cheng, Q.; Wang, K.-M. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef]
- Shao, H.-L.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Niu, R.-Y.; Chen, X.-H.; Sun, Z.-X.; Wang, L.; Wang, Z.-N.; Zhang, C.; Ding, D.; Yang, J.-C.; Wang, Y.-Z.; Luo, Y. A smart TESTER for reliable discrimination of cancer-derived small extracellular vesicles. Anal. Chem. 2023, 1276, 341636. [Google Scholar] [CrossRef]
- Lu, C.; Xiao, W.; Su, Y.-Q.; Zhang, X.-C.; Chen, Y.-X.; Lu, K.-J.; Teng, P.-J.; Liang, J.-J.; Yang, H.-W.; Song, Q.-F.; et al. Rapid Evaluation of Lung Adenocarcinoma Progression by Detecting Plasma Extracellular Vesicles with Lateral Flow Immunoassays. ACS Sens. 2023, 8, 1950–1959. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Huang, Z.-P.; Li, T.-D.; Deng, A.-M.; Kong, J.-L. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 2018, 184, 219–226. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Tian, Y.; Jiang, L. Fundamental studies and practical applications of bio-inspired smart solid-state nanopores and nanochannels. Nano Today 2016, 11, 61–81. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sato, K.; Kinbara, K. Calcium-induced reversible assembly of phosphorylated amphiphile within lipid bilayer membranes. Chem. Comm. 2021, 57, 4106–4109. [Google Scholar] [CrossRef]
- Ma, W.-R.; Liu, L.-L.; Zhang, X.; Liu, X.-F.; Xu, Y.; Li, S.-B.; Zeng, M.-L. Heterostructured nanochannels with modulated ionic current rectification for ultrasensitive detection of Hg2+. J. Mater. Chem. C 2022, 10, 16388–16396. [Google Scholar] [CrossRef]
- Shi, L.; Mu, C.-L.; Gao, T.; Chai, W.-X.; Sheng, A.-Z.; Chen, T.-S.; Yang, J.; Zhu, X.-L.; Li, G.-X. Rhodopsin-like ionic gate fabricated with graphene oxide and isomeric DNA switch for efficient photocontrol of ion transport. J. Am. Chem. Soc. 2019, 141, 8239–8243. [Google Scholar] [CrossRef]
- Lv, R.; Wang, X.; Mao, Z.-Q.; Bai, Y.-R.; Hao, J.-X.; Zhang, F. Engineering Sandwiched Nanochannel Aptasensor for Efficiently Screening Cancer Cells. Chem.-Eur. J. 2023, 29, 202203380. [Google Scholar] [CrossRef]
- Shi, L.; Jia, F.-J.; Wang, L.; Jalalah, M.; Al-Assiri, M.S.; Gao, T.; Harraz, F.A.; Li, G.-X. Fabrication of an artificial ionic gate inspired by mercury-resistant bacteria for simple and sensitive detection of mercury ion. Sens. Actuators B-Chem. 2021, 326, 128976. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Ma, X.-M.; Fang, X.-N.; Xiang, L.-L.; Yi, Y.-X.; Li, J.-L.; Luo, Z.-F.; Li, G.-X. Aptamer-functionalized nanochannels for one-step detection of SARS-CoV-2 in samples from COVID-19 patients. Anal. Chem. 2021, 93, 16646–16654. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, X.-P.; Younis, M.R.; Li, Z.-Q.; Xia, X.-H.; Wang, C. Ultrasensitive capture, detection, and release of circulating tumor cells using a nanochannel–ion channel hybrid coupled with electrochemical detection technique. Anal. Chem. 2017, 89, 10957–10964. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, J.; Wang, L.; Duan, X.-G.; Wang, Y.-S.; Xiang, Y.; Li, G.-X. Sensitive detection of chloramphenicol based on Ag-DNAzyme-mediated signal amplification modulated by DNA/metal ion interaction. Biosens. Bioelectron. 2019, 127, 45–49. [Google Scholar] [CrossRef]
- Yang, L.F.; Ling, M.; Kacherovsky, N.; Pun, S.H. Aptamers 101: Aptamer discovery and in vitro applications in biosensors and separations. Chem. Sci. 2023, 14, 4961–4978. [Google Scholar] [CrossRef]
- Qu, H.; Zheng, M.-Y.; Ma, Q.-H.; Wang, L.; Mao, Y.; Eisenstein, M.; Tom Soh, H.; Zheng, L. Allosteric regulation of aptamer affinity through mechano-chemical coupling. Angew. Chem. Int. Ed. 2023, 62, e202214045. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.-J.; Niu, X.-M.; Qian, L.-Q.; Zhang, Y.; Fan, L.-Y.; Cao, C.-X.; et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta 2017, 982, 84–95. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, J.-X.; Tian, F.; Cai, L.-L.; Zhang, W.; Feng, Q.; Chang, J.-Q.; Wan, F.-N.; Yang, Y.-J.; Dai, B.; et al. Low-cost thermophoretic profiling of extracellular-vesicle surface proteins for the early detection and classification of cancers. Nat. Biomed. Eng. 2019, 3, 183–193. [Google Scholar] [CrossRef]
- Xiong, T.-Y.; Zhang, K.-L.; Jiang, Y.-N.; Yu, P.; Mao, L.-Q. Ion current rectification: From nanoscale to microscale. Sci. China Chem. 2019, 62, 1346–1359. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhou, F.; Jia, F.; Yang, N. Divalent Aptamer-Functionalized Nanochannels for Facile Detection of Cancer Cell-Derived Exosomes. Sensors 2023, 23, 9139. https://doi.org/10.3390/s23229139
Huang Y, Zhou F, Jia F, Yang N. Divalent Aptamer-Functionalized Nanochannels for Facile Detection of Cancer Cell-Derived Exosomes. Sensors. 2023; 23(22):9139. https://doi.org/10.3390/s23229139
Chicago/Turabian StyleHuang, Yue, Fangfang Zhou, Fengjie Jia, and Nana Yang. 2023. "Divalent Aptamer-Functionalized Nanochannels for Facile Detection of Cancer Cell-Derived Exosomes" Sensors 23, no. 22: 9139. https://doi.org/10.3390/s23229139



