Uncovering Subtle Gait Deterioration in People with Early-Stage Multiple Sclerosis Using Inertial Sensors: A 2-Year Multicenter Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcome and Outcome Measures
2.3.1. Disability
2.3.2. Walking Endurance
2.3.3. Fatigue
2.3.4. Instrumented Walking Parameters
- Cadence (stride/min): computed as 60/Tstride, where Tstride is the stride duration (i.e., the time interval between two consecutive heel-strikes of the same foot, estimated following Salarian et al. [23]).
- Stride regularity (unitless): quantified using the method proposed by Moe-Nilssen & Helbostad [24]. In particular, the normalized autocorrelation function was computed from the trunk acceleration modulus. The first and second peak values of this function, corresponding, respectively, to a time lag equal to step and stride duration, were used to quantify the regularity of consecutive steps and strides (see Angelini et al. [25]). Increasing values, from 0 to 1, indicate higher step and stride regularity. In this study, stride regularity was preferred to step regularity since the latter has been often used as a measure of gait symmetry (see [26,27]).
- Gait instability (unitless): quantified by the short term Lyapunov exponent (sLyE) computed from the lower back antero-posterior (AP) and medio-lateral (ML) accelerations, as detailed by Caronni et al. [13]. Given that sLyE is affected by data length [28,29,30], each 10-stride steady state walking bout was re-sampled to 1000 frames (10 strides × 100 frames) to maintain equal data length across walking bouts and participants. Hence, sLyE was computed on each time-normalized walking bout and then averaged over the whole test, as proposed by Sloot et al. [31]. Larger values of sLyE mean decreased local dynamic stability, that is decreased ability of the balance control system to deal with small perturbations typically occurring during locomotion, such as internal control errors or external disturbances [28,32].
- Gait symmetry (%): quantified by the improved Harmonic Ratio (iHR) calculated from trunk AP and ML accelerations [15,18]. In summary, we used a fast discrete Fourier transform to decompose acceleration signals into harmonics. Hence, iHR was computed as the percentage ratio between the sum of the powers of the first 10 in-phase harmonics to the sum of the powers of the first 20 (in-phase and out-of-phase) harmonics. A range from 0 (no symmetry) to 100% (perfect symmetry) was used to describe gait symmetry (see Pasciuto et al. [33]).
2.3.5. Perceived Walking Ability
2.4. Statistical Analysis
3. Results
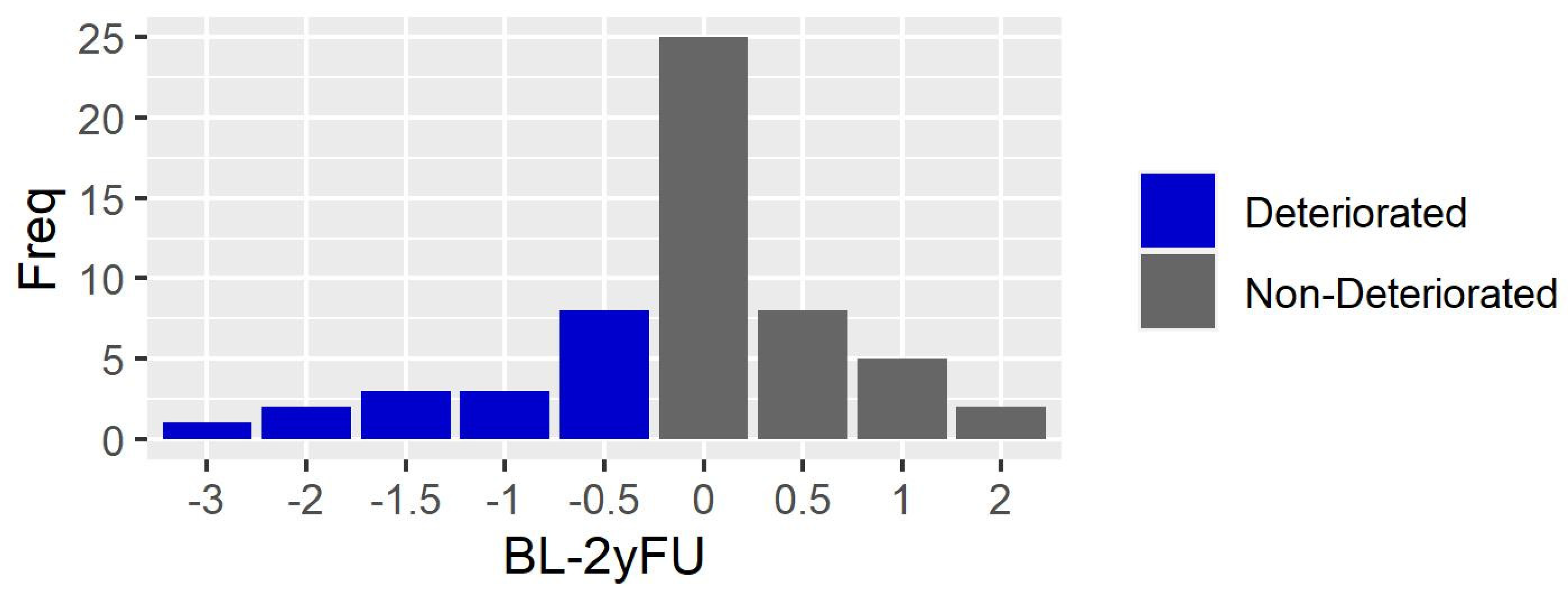
3.1. Clinical Assessment
3.2. Instrumented Walking Assessment
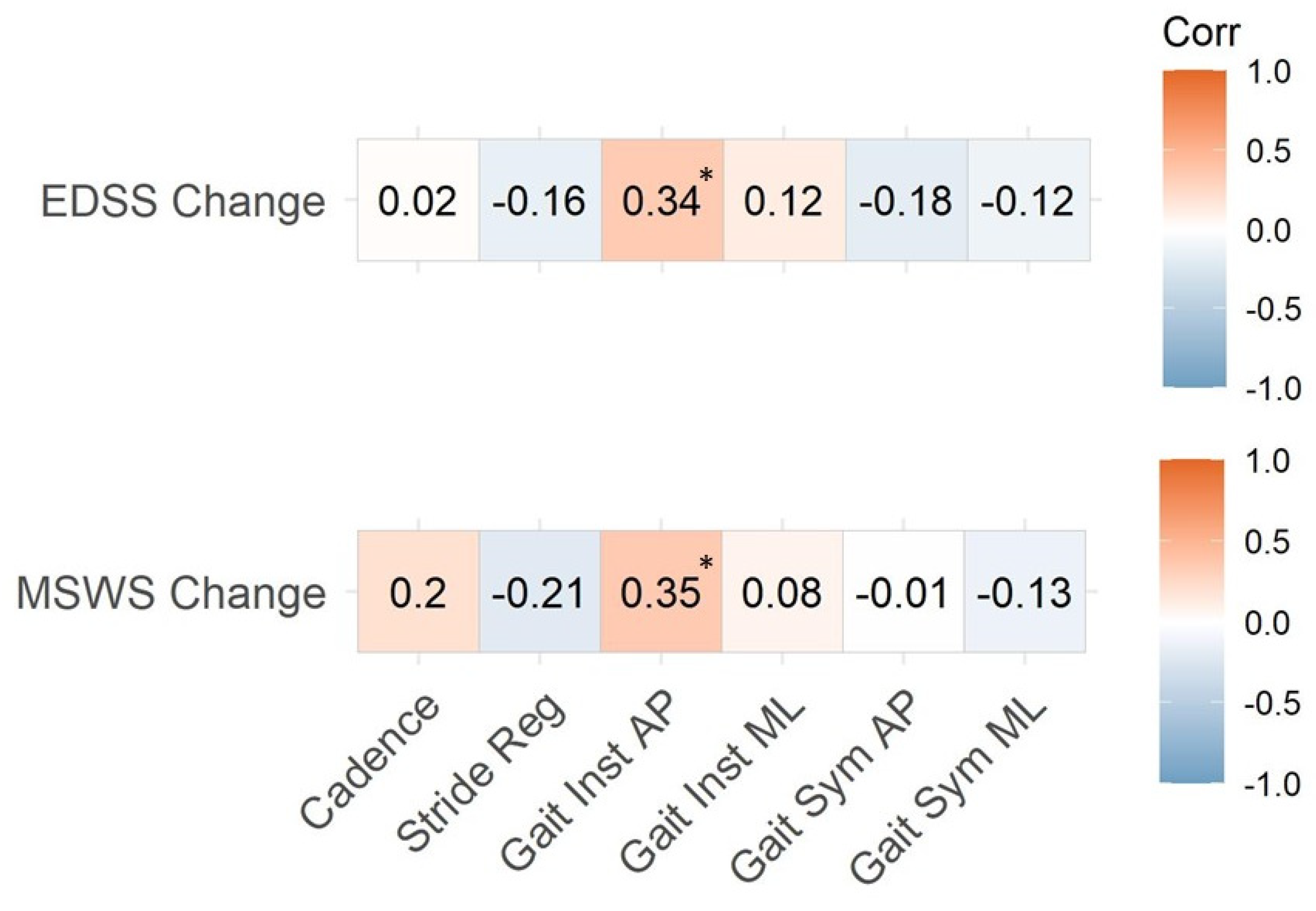
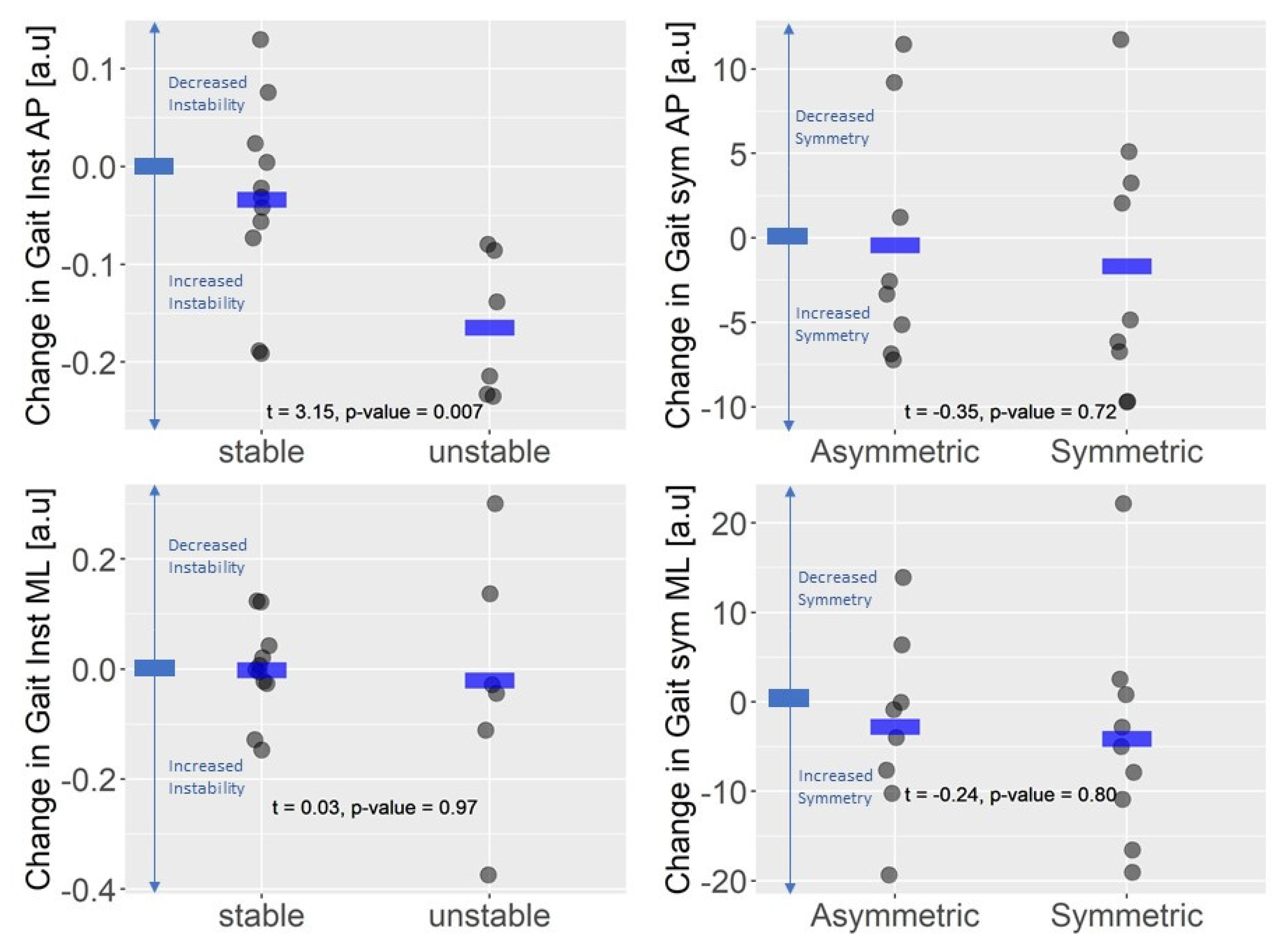
3.3. Instrumented Gait Variables and EDSS
3.4. Instrumented Gait Variables and Perceived Assessment of Gait
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comi, G.; Radaelli, M.; Sørensen, S. Multiple Sclerosis 2 Evolving Concepts in the Treatment of Relapsing Multiple Sclerosis. Lancet 2017, 389, 1347–1356. [Google Scholar] [CrossRef]
- Zörner, B.; Hostettler, P.; Meyer, C.; Killeen, T.; Gut, P.; Linnebank, M.; Weller, M.; Straumann, D.; Filli, L. Prognosis of Walking Function in Multiple Sclerosis Supported by Gait Pattern Analysis. Mult. Scler. Relat. Disord. 2022, 63, 103802. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A. Gait Variability across the Disability Spectrum in People with Multiple Sclerosis. J. Neurol. Sci. 2016, 361, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling Walking Dysfunction in Multiple Sclerosis: Characterisation, Classification and Progression over Time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef]
- Comber, L.; Galvin, R.; Coote, S. Gait Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Peebles, A.T.; Bruetsch, A.P.; Lynch, S.G.; Huisinga, J.M. Dynamic Balance in Persons with Multiple Sclerosis Who Have a Falls History Is Altered Compared to Non-Fallers and to Healthy Controls. J. Biomech. 2017, 63, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Bruijn, S.M.; Galea, M.P. Gait Stability at Early Stages of Multiple Sclerosis Using Different Data Sources. Gait Posture 2020, 77, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait Abnormalities in Minimally Impaired Multiple Sclerosis Patients. Mult. Scler. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Flachenecker, F.; Gaßner, H.; Hannik, J.; Lee, D.-H.; Flachenecker, P.; Winkler, J.; Eskofier, B.; Linker, R.A.; Klucken, J. Objective Sensor-Based Gait Measures Reflect Motor Impairment in Multiple Sclerosis Patients: Reliability and Clinical Validation of a Wearable Sensor Device. Mult. Scler. Relat. Disord. 2020, 39, 101903. [Google Scholar] [CrossRef]
- Dreyer-Alster, S.; Menascu, S.; Dolev, M.; Givon, U.; Magalashvili, D.; Achiron, A.; Kalron, A. Longitudinal Relationships between Disability and Gait Characteristics in People with MS. Sci. Rep. 2022, 12, 3653. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.P.; Cofré Lizama, L.E.; Butzkueven, H.; Kilpatrick, T.J. Gait and Balance Deterioration over a 12-Month Period in Multiple Sclerosis Patients with EDSS Scores ≤ 3. NeuroRehabilitation 2017, 40, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Caronni, A.; Gervasoni, E.; Ferrarin, M.; Anastasi, D.; Brichetto, G.; Confalonieri, P.; Giovanni, R.D.I.; Prosperini, L.; Tacchino, A.; Solaro, C.; et al. Local Dynamic Stability of Gait in People with Early Multiple Sclerosis and No-to-Mild Neurological Impairment. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, D.; Feys, P.; Baert, I.; Riemenschneider, M.; Stenager, E.; Dalgas, U. Performed and Perceived Walking Ability in Relation to the Expanded Disability Status Scale in Persons with Multiple Sclerosis. J. Neurol. Sci. 2017, 382, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Rovaris, M.; Solaro, C.; Ferrarin, M.; et al. Instrumentally Assessed Gait Quality Is More Relevant than Gait Endurance and Velocity to Explain Patient-Reported Walking Ability in Early-Stage Multiple Sclerosis. Eur. J. Neurol. 2021, 28, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Vienne-Jumeau, A.; Quijoux, F.; Vidal, P.P.; Ricard, D. Wearable Inertial Sensors Provide Reliable Biomarkers of Disease Severity in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2020, 63, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Brichetto, G.; Carpinella, I.; Cavalla, P.; Confalonieri, P.; Groppo, E.; Prosperini, L.; et al. Prevalence and Patterns of Subclinical Motor and Cognitive Impairments in Non-Disabled Individuals with Early Multiple Sclerosis: A Multicenter Cross-Sectional Study. Ann. Phys. Rehabil. Med. 2022, 65, 101491. [Google Scholar] [CrossRef]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Solaro, C.; Rovaris, M.; Ferrarin, M.; et al. Walking With Horizontal Head Turns Is Impaired in Persons With Early-Stage Multiple Sclerosis Showing Normal Locomotion. Front. Neurol. 2022, 12, 821640. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic Criteria for Multiple Sclerosis: 2010 Revisions to the McDonald Criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Goldman, M.D.; Marrie, R.A.; Cohen, J.A. Evaluation of the Six-Minute Walk in Multiple Sclerosis Subjects and Healthy Controls. Mult. Scler. 2008, 14, 383–390. [Google Scholar] [CrossRef]
- Krupp, L.B.; Larocca, N.G.; Muir Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to Patients with Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Angelini, L.; Carpinella, I.; Cattaneo, D.; Ferrarin, M.; Gervasoni, E.; Sharrack, B.; Paling, D.; Nair, K.P.S.; Mazzà, C. Is a Wearable Sensor-Based Characterisation of Gait Robust Enough to Overcome Differences between Measurement Protocols? A Multi-Centric Pragmatic Study in Patients with Multiple Sclerosis. Sensors 2020, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Russmann, H.; Vingerhoets, F.J.G.; Dehollain, C.; Blanc, Y.; Burkhard, P.R.; Aminian, K. Gait Assessment in Parkinson’s Disease: Toward an Ambulatory System for Long-Term Monitoring. IEEE Trans. Biomed. Eng. 2004, 51, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Moe-Nilssen, R.; Helbostad, J.L. Estimation of Gait Cycle Characteristics by Trunk Accelerometry. J. Biomech. 2004, 37, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.; Hodgkinson, W.; Smith, C.; Dodd, J.M.; Sharrack, B.; Mazzà, C.; Paling, D. Wearable Sensors Can Reliably Quantify Gait Alterations Associated with Disability in People with Progressive Multiple Sclerosis in a Clinical Setting. J. Neurol. 2020, 267, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Raggi, M.; Rocchi, L.; Cutti, A.G.; Chiari, L. Gait Symmetry and Regularity in Transfemoral Amputees Assessed by Trunk Accelerations. J. Neuroeng. Rehabil. 2010, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.; Buckley, E.; Bonci, T.; Radford, A.; Sharrack, B.; Paling, D.; Nair, K.P.S.; Mazza, C. A Multifactorial Model of Multiple Sclerosis Gait and Its Changes Across Different Disability Levels. IEEE Trans. Biomed. Eng. 2021, 68, 3196–3204. [Google Scholar] [CrossRef]
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieen, J.H. Assessing the Stability of Human Locomotion: A Review of Current Measures. J. R. Soc. Interface 2013, 10, 20120999. [Google Scholar] [CrossRef]
- England, S.A.; Granata, K.P. The Influence of Gait Speed on Local Dynamic Stability of Walking. Gait Posture 2007, 25, 172–178. [Google Scholar] [CrossRef]
- Hussain, V.S.; Spano, M.L.; Lockhart, T.E. Effect of Data Length on Time Delay and Embedding Dimension for Calculating the Lyapunov Exponent in Walking. J. R. Soc. Interface 2020, 17, 20200311. [Google Scholar] [CrossRef]
- Sloot, L.H.; van Schooten, K.S.; Bruijn, S.M.; Kingma, H.; Pijnappels, M.; van Dieën, J.H. Sensitivity of Local Dynamic Stability of Over-Ground Walking to Balance Impairment Due to Galvanic Vestibular Stimulation. Ann. Biomed. Eng. 2011, 39, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Arpan, I.; Fino, P.C.; Fling, B.W.; Horak, F. Local Dynamic Stability during Long-Fatiguing Walks in People with Multiple Sclerosis. Gait Posture 2020, 76, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Pasciuto, I.; Bergamini, E.; Iosa, M.; Vannozzi, G.; Cappozzo, A. Overcoming the Limitations of the Harmonic Ratio for the Reliable Assessment of Gait Symmetry. J. Biomech. 2017, 53, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Galna, B.; Rochester, L.; Mazzà, C. Upper Body Accelerations as a Biomarker of Gait Impairment in the Early Stages of Parkinson’s Disease. Gait Posture 2019, 71, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Arcolin, I.; Corna, S.; Giardini, M.; Giordano, A.; Nardone, A.; Godi, M. Proposal of a New Conceptual Gait Model for Patients with Parkinson’s Disease Based on Factor Analysis. Biomed. Eng. Online 2019, 18, 70. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. Walking Speed: The Sixth Vital Sign. J. Geriatr. Phys. Ther. 2009, 32, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait Variability and Disability in Multiple Sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef]
- Tura, A.; Rocchi, L.; Raggi, M.; Cutti, A.G.; Chiari, L. Recommended Number of Strides for Automatic Assessment of Gait Symmetry and Regularity in Above-Knee Amputees by Means of Accelerometry and Autocorrelation Analysis. J. Neuroeng. Rehabil. 2012, 9, 11. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait Variability and Stability Measures: Minimum Number of Strides and within-Session Reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef]
- Brandstadter, R.; Ayeni, O.; Krieger, S.C.; Harel, N.Y.; Escalon, M.X.; Katz Sand, I.; Leavitt, V.M.; Fabian, M.T.; Buyukturkoglu, K.; Klineova, S.; et al. Detection of Subtle Gait Disturbance and Future Fall Risk in Early Multiple Sclerosis. Neurology 2020, 94, e1395–e1406. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Klöditz, A.; Trentzsch, K.; Ziemssen, T. Balance Testing in Multiple Sclerosis-Improving Neurological Assessment With Static Posturography? Front. Neurol. 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hsieh, K.L.; Sosnoff, J.J. Fall Risk Prediction in Multiple Sclerosis Using Postural Sway Measures: A Machine Learning Approach. Sci. Rep. 2019, 9, 16154. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, R.; Vaney, C.; Gattlen, B.; Meichtry, A.; Deriaz, O.; Lugon-Moulin, V.; Anchisi-Bellwald, A.-M.; Palaci, C.; Foinant, D.; Terrier, P. Local Dynamic Stability as a Responsive Index for the Evaluation of Rehabilitation Effect on Fall Risk in Patients with Multiple Sclerosis: A Longitudinal Study. BMC Res. Notes 2013, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and Limb Dominance in Able-Bodied Gait: A Review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Wagner, J.M.; Adusumilli, G.; Gottlieb, A.; Naismith, R.T. Gait Asymmetry, and Bilateral Coordination of Gait during a Six-Minute Walk Test in Persons with Multiple Sclerosis. Sci. Rep. 2020, 10, 12382. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Swinscow, T.D.V. Statistics at Square One; Thomas, D.V., Ed.; Wiley-Blackwell/BMJ Books: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hobart, J.C.; Riazi, A.; Lamping, D.L.; Fitzpatrick, R.; Thompson, A.J. Measuring the Impact of MS on Walking Ability: The 12-Item MS Walking Scale (MSWS-12). Neurology 2003, 60, 31–36. [Google Scholar] [CrossRef]
- Krieger, S.C.; Antoine, A.; Sumowski, J.F. EDSS 0 Is Not Normal: Multiple Sclerosis Disease Burden below the Clinical Threshold. Mult. Scler. 2022, 28, 2299–2303. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Hillel, I.; Mirelman, A.; Regev, K.; Hsieh, K.L.; Karni, A.; Devos, H.; Sosnoff, J.J.; Hausdorff, J.M. A Wearable Sensor Identifies Alterations in Community Ambulation in Multiple Sclerosis: Contributors to Real-World Gait Quality and Physical Activity. J. Neurol. 2020, 267, 1912–1921. [Google Scholar] [CrossRef]
- Williams, N.P.; Roland, P.S.; Yellin, W. Vestibular Evaluation in Patients with Early Multiple Sclerosis. Am. J. Otol. 1997, 18, 93–100. [Google Scholar]
- Tallantyre, E.C.; Bø, L.; Al-Rawashdeh, O.; Owens, T.; Polman, C.H.; Lowe, J.S.; Evangelou, N. Clinico-Pathological Evidence that Axonal Loss Underlies Disability in Progressive Multiple Sclerosis. Mult. Scler. 2010, 16, 406–411. [Google Scholar] [CrossRef]
- Gunn, H.; Markevics, S.; Haas, B.; Marsden, J.; Freeman, J. Systematic Review: The Effectiveness of Interventions to Reduce Falls and Improve Balance in Adults With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2015, 96, 1898–1912. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Dressendorfer, R.H. Exercise and Multiple Sclerosis. Sports Med. 2004, 34, 1077–1100. [Google Scholar] [CrossRef]
- White, L.J.; Castellano, V. Exercise and Brain Health—Implications for Multiple Sclerosis: Part 1—Neuronal Growth Factors. Sports Med. 2008, 38, 91–100. [Google Scholar] [CrossRef]
- Thrue, C.; Riemenschneider, M.; Hvid, L.G.; Stenager, E.; Dalgas, U. Time Matters: Early-Phase Multiple Sclerosis Is Accompanied by Considerable Impairments across Multiple Domains. Mult. Scler. 2021, 27, 1477–1485. [Google Scholar] [CrossRef]
- Diechmann, M.D.; Campbell, E.; Coulter, E.; Paul, L.; Dalgas, U.; Hvid, L.G. Effects of Exercise Training on Neurotrophic Factors and Subsequent Neuroprotection in Persons with Multiple Sclerosis-A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1499. [Google Scholar] [CrossRef]
- Dalgas, U.; Langeskov-Christensen, M.; Stenager, E.; Riemenschneider, M.; Hvid, L.G. Exercise as Medicine in Multiple Sclerosis—Time for a Paradigm Shift: Preventive, Symptomatic, and Disease-Modifying Aspects and Perspectives. Curr. Neurol. Neurosci. Rep. 2019, 19, 88. [Google Scholar] [CrossRef] [PubMed]
| Variable | Baseline (n = 56) | 2-Year Follow Up (n = 56) | 2-Year Follow Up Deteriorated Group (n = 17) | 2-Year Follow Up Non-Deteriorated Group (n = 39) |
|---|---|---|---|---|
| Age (years) | 38.2 ± 10.7 | 40.0 ± 11.0 | 44.6 ± 9.8 | 38.4 ± 10.8 |
| Female (n, %) | 35, 63% | 35, 63% | 10, 59% | 26, 67% |
| EDSS (points) | 1.5 ± 0.7 | 1.8 ± 1.0 | 2.7 ± 0.8 | 1.4 ± 0.7 |
| Years since diagnosis | 2.2 ± 1.8 | 4.2 ± 1.9 | 4.1 ± 1.5 | 4.2 ± 2.1 |
| 6MWT (m) | 564.0 ± 78.7 | 574.8 ± 87.6 | 554.5 ± 108.1 | 585.5 ± 76.9 |
| MSWS-12 (points) | 31.3 ± 14.1 | 31.4 ± 14.2 | 38.9 ± 17.0 | 28.8 ± 12.3 |
| FSS (points) | 3.1 ± 1.7 | 3.2 ± 1.8 | 3.7 ± 1.8 | 3.0 ± 1.8 |
| Variable | Baseline (n = 56) | 2-Year Follow Up (n = 56) | 2-Year Follow Up Deteriorated Group (n = 17) | 2-Year Follow Up Non-Deteriorated Group (n = 39) |
|---|---|---|---|---|
| Cadence (stride/min) | 63.4 ± 4.5 | 64.2 ± 5.6 | 63.8 ± 7.7 | 64.7 ± 4.8 |
| Stride Regularity (a.u.) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Gait Symmetry AP (a.u.) | 82.0 ± 6.3 | 84.2 ± 5.7 | 82.7 ± 7.0 | 84.8 ± 5.0 |
| Gait Symmetry ML (a.u.) | 80.2 ± 10.4 | 83.1 ± 8.9 | 80.5 ± 11.7 | 84.1 ± 7.2 |
| Gait Instability AP (a.u.) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Gait Instability ML (a.u.) | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Instrumented Variable | EDSS (n = 17) | MSWS-12 (n = 17) |
|---|---|---|
| Cadence | −0.35 | 0.17 |
| Stride regularity | −0.49 * | −0.24 |
| Gait instability AP | 0.36 | 0.73 * |
| Gait instability ML | 0.52 * | 0.20 |
| Gait symmetry AP | −0.45 | −0.09 |
| Gait symmetry ML | −0.55 * | −0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Solaro, C.; Rovaris, M.; Brichetto, G.; Confalonieri, P.; Tacchino, A.; Carpinella, I.; Cattaneo, D. Uncovering Subtle Gait Deterioration in People with Early-Stage Multiple Sclerosis Using Inertial Sensors: A 2-Year Multicenter Longitudinal Study. Sensors 2023, 23, 9249. https://doi.org/10.3390/s23229249
Gervasoni E, Anastasi D, Di Giovanni R, Solaro C, Rovaris M, Brichetto G, Confalonieri P, Tacchino A, Carpinella I, Cattaneo D. Uncovering Subtle Gait Deterioration in People with Early-Stage Multiple Sclerosis Using Inertial Sensors: A 2-Year Multicenter Longitudinal Study. Sensors. 2023; 23(22):9249. https://doi.org/10.3390/s23229249
Chicago/Turabian StyleGervasoni, Elisa, Denise Anastasi, Rachele Di Giovanni, Claudio Solaro, Marco Rovaris, Giampaolo Brichetto, Paolo Confalonieri, Andrea Tacchino, Ilaria Carpinella, and Davide Cattaneo. 2023. "Uncovering Subtle Gait Deterioration in People with Early-Stage Multiple Sclerosis Using Inertial Sensors: A 2-Year Multicenter Longitudinal Study" Sensors 23, no. 22: 9249. https://doi.org/10.3390/s23229249





