Preparation and Gas-Sensing Properties of Two-Dimensional Molybdenum Disulfide/One-Dimensional Copper Phthalocyanine Heterojunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MoS2 Nanosheets
2.3. Preparation of 1D-CuPc
2.4. Preparation of 2D-MoS2/1D-CuPc Heterojunction
2.5. Material Characterization
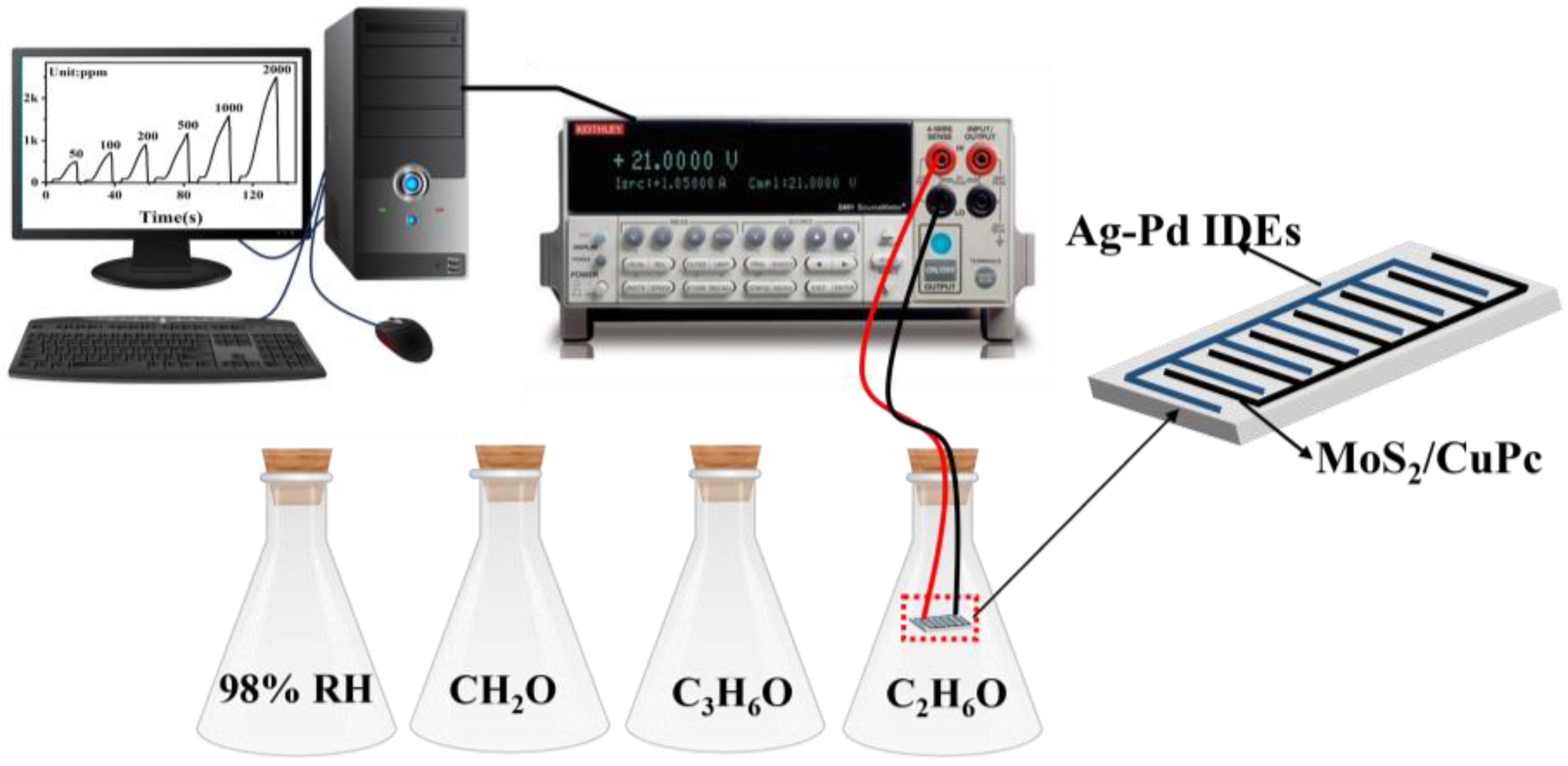
2.6. Gas-Sensing Performance Evaluation
3. Results and Discussion
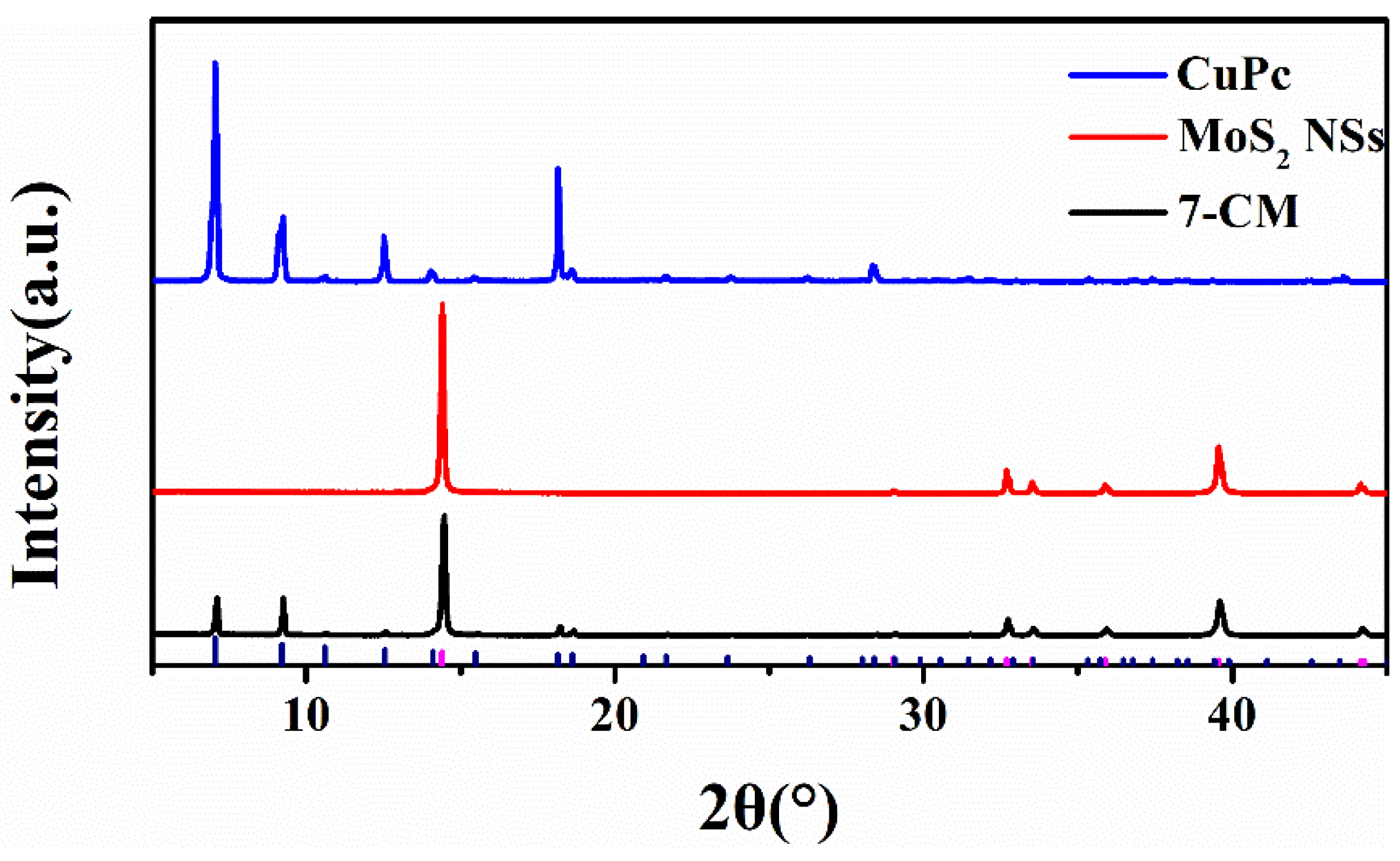
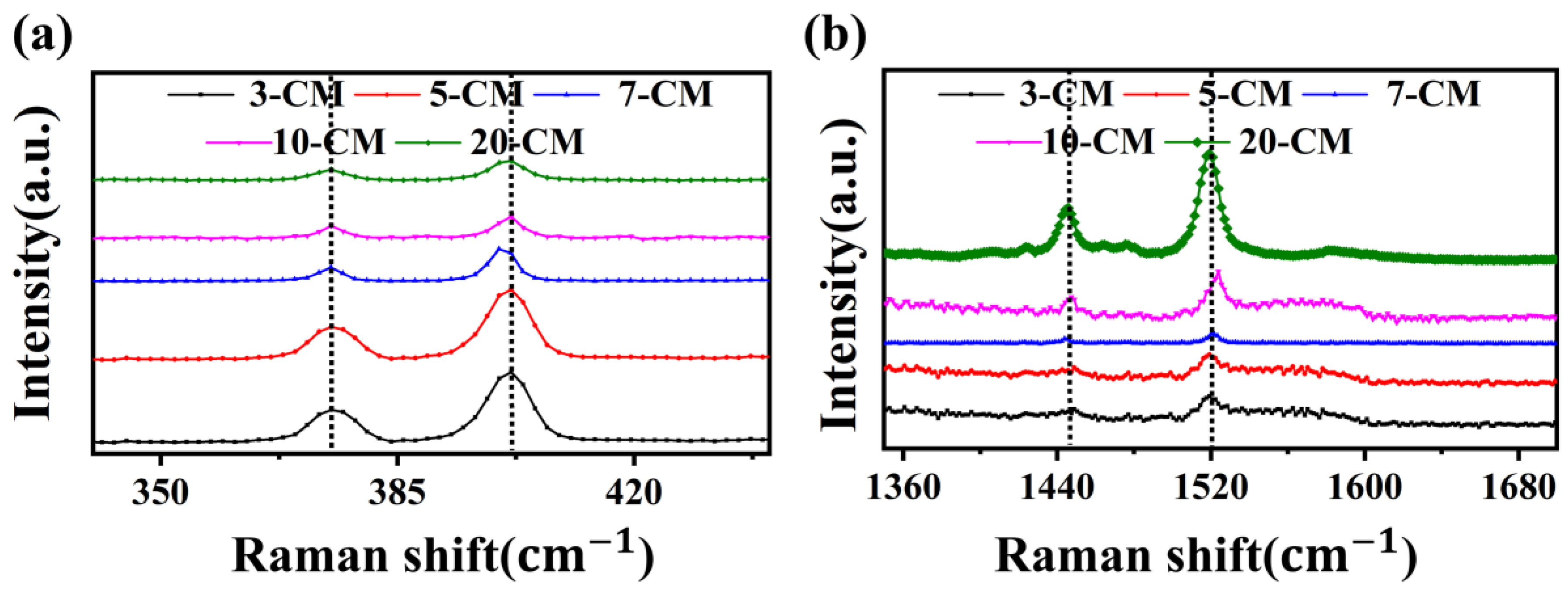
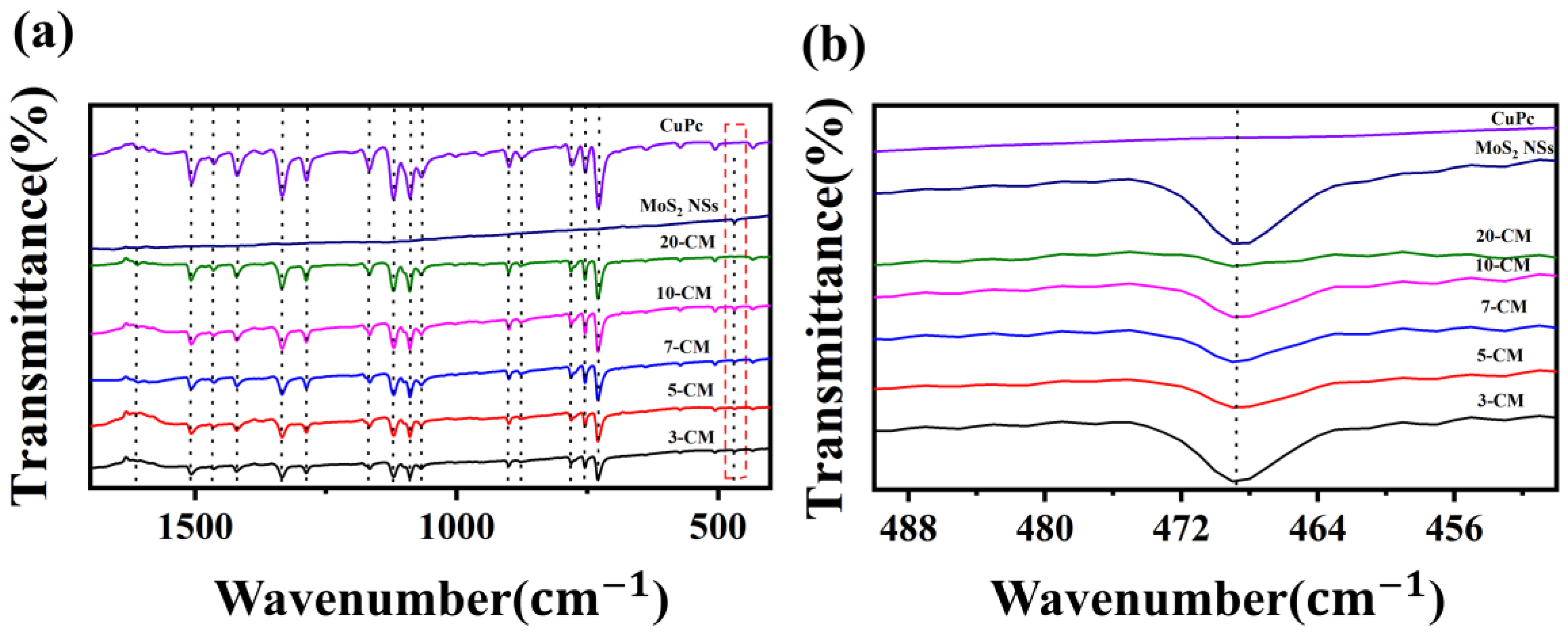
3.1. Material Characterization and Analysis
3.2. Gas-Sensing Studies
4. Analysis of Gas-Sensing Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haineng, B.; Hui, G.; Cheng, F.; Wang, J.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. Light-activated ultrasensitive NO2 gas sensor based on heterojunctions of CuO nanospheres/MoS2 nanosheets at room temperature. Sens. Actuators B Chem. 2022, 368, 132131. [Google Scholar]
- Yong, Y.; Wufei, G.; Xin, L.; Liu, Y.; Liang, Y.; Chen, B.; Yang, Y.; Luo, X.; Xu, K.; Yuan, C. Light-assisted room temperature gas sensing performance and mechanism of direct Z-scheme MoS2/SnO2 crystal faceted heterojunctions. J. Hazard. Mater. 2022, 436, 129246. [Google Scholar] [CrossRef]
- Robinson, M.T.; Tung, J.; Heydari Gharahcheshmeh, M.; Gleason, K.K. Humidity-Initiated Gas Sensors for Volatile Organic Compounds Sensing. Adv. Funct. Mater. 2021, 31, 2101310. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zheng, Z.; Yu, T.; Liu, C.; Qian, H.; Li, J.G. Seasonal and Diurnal Patterns of Outdoor Formaldehyde and Impacts on Indoor Environments and Health. Environ. Res. 2022, 205, 112550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ibrahim, A.A.; Nakate, U.T.; Albargi, H.; Alsaiari, M.A.; Ahmed, F.; Alharthi, F.A.; Alghamdi, A.A.; Al-Zaqri, N. Fabrication and Characterization of CuO Nanoplates Based Sensor Device for Ethanol Gas Sensing Application. Chem. Phys. Lett. 2021, 763, 138204. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Yang, C.; Meng, F.L. Strategies for Improving the Sensing Performance of Semiconductor Gas Sensors for High-Performance Formaldehyde Detection: A Review. Chemosensors 2021, 9, 179. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Zhang, Y.; Li, H.-Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room-Temperature Gas Sensors. Nano-Micro Lett. 2020, 12, 24–36. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.; Song, H.; Wang, J.; Song, Y.; Liang, Y.; Zhang, X. One-dimensional MoS2-decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloys Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S.; Oh, N.; Lee, H.; Lee, S.; Park, J.; Kim, H. Improved Sensitivity in Schottky Contacted Two-Dimensional MoS2 Gas Sensor. ACS Appl. Mater. Interfaces 2019, 11, 38902–38909. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhang, R. Progress in Preparation of Molybdenum Disulfide and its Application in Electrochemical Sensors. Hua Xue Shi Ji 2022, 44, 1063–1070. [Google Scholar]
- Meng, S.; Li, H.; Huang, C.; Zhang, C.; Jiang, C.; Li, W.; Xiao, X. MoS2 field-effect transistor-based biosensor for label-free detection. J. Light Scatt. 2023, 35, 135–141. [Google Scholar]
- Long, L.; Zhiyan, F.; Zhongzhou, D.; Tao, H.; Hu, C. Transition metal disulfide (MoTe2, MoSe2 and MoS2) were modified to improve NO2 gas sensitivity sensing. J. Ind. Eng. Chem. 2023, 118, 533–543. [Google Scholar]
- Ruijuan, D.; Wei, W. Adsorption of gas molecule on Rh, Ru doped monolayer MoS2 for gas sensing applications: A DFT study. Chem. Phys. Lett. 2022, 789, 139300. [Google Scholar]
- Wang, Z.; Zhang, T.; Zhao, C.; Han, T.; Fei, T.; Liu, S.; Lu, G. Rational synthesis of molybdenum disulfide nanoparticles decorated reduced graphene oxide hybrids and their application for high-performance NO2 sensing. Sens. Actuators B Chem. 2018, 260, 508–518. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Hai, L.; Zongyou, Y.; Qiyuan, H.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar]
- Li, L.; Wan, K.; Wang, Q.; Yang, A.; Yuan, H.; Wang, X.; Rong, M. Study on Sensitivity Characteristic of Decomposed Gas of MoS2 Sensor with Insulating Medium Feature. High Volt. Appar. 2021, 57, 36–43. [Google Scholar]
- Wenbo, L.; Rong, Q.; Shangjun, Z.; Hong, J.; Cheng, S.; Yueqin, Z. MoS2 with Different Morphologies: Preparation and Gas-sensing Property of NH3. J. Inorg. Mater. 2022, 37, 1135–1140. [Google Scholar]
- Shen, J.; Yang, Z.; Wang, Y.; Xu, L.-C.; Liu, R.; Liu, X. The gas sensing performance of borophene/MoS2 heterostructure. Appl. Surf. Sci. 2020, 504, 144412. [Google Scholar] [CrossRef]
- Xin, B.; Si-Jie, B.; Xiang-Feng, C.; Shi-Ming, L.; Chun-Shui, W.; Yu-Ying, B. Preparation and Gas⁃Sensing Properties of MoS2/Cd2SnO4 Composite Materials. Chin. J. Inorg. Chem. 2022, 38, 2173–2180. [Google Scholar]
- Lizhai, Z.; Chuangbei, M.; Jinniu, Z.; Huang, Y.; Xu, H.; Lu, H.; Xu, K.; Ma, F. Enhanced NO2-sensing performances of CeO2 nanoparticles on MoS2 at room temperature. Appl. Surf. Sci. 2022, 600, 154157. [Google Scholar]
- Hemalatha, P.; Jolly, B.; Amer, R.A.; Elhadrami, E.C.; Al-Thani, N.J. Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocompositesb-Based Sensor. Nanomaterials 2022, 12, 4423. [Google Scholar]
- Zhang, M.; Shao, C.; Guo, Z.; Zhang, Z.; Mu, J.; Cao, T.; Liu, Y. Hierarchical nanostructures of copper (II) phthalocyanine on electrospun TiO2 nanofibers: Controllable solvothermal-fabrication and enhanced visible photocatalytic properties. ACS Appl. Mater. Interfaces 2011, 3, 369–377. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Shaymurat, T. Effect of Different Solvents on Morphology and Gas-Sensitive Properties of Grinding-Assisted Liquid-Phase-Exfoliated MoS2 Nanosheets. Nanomaterials 2022, 12, 4485. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Talgar, S.; Peng, M. Preparation and humidity sensitive properties of cobaltphthalocyanine micro-nanowires. Electron. Compon. Mater. 2022, 41, 164–169. [Google Scholar]
- Huang, J.; Jiang, D.; Zhou, J.; Ye, J.; Sun, Y.; Li, X.; Geng, Y.; Wang, J.; Du, Y.; Qian, Z. Visible light-activated room temperature NH3 sensor base on CuPc-loaded ZnO nanorods. Sens. Actuators B Chem. 2021, 327, 128911. [Google Scholar] [CrossRef]
- Ikram, M.; Liu, L.; Liu, Y.; Ma, L.; Lv, H.; Ullah, M.; He, L.; Wu, H.; Wang, R.; Shi, K. Fabrication and characterization of a high-surface area MoS2@WS2 heterojunction for the ultra-sensitive NO2 detection at room temperature. J. Mater. Chem. A 2019, 7, 14602–14612. [Google Scholar] [CrossRef]
- Deng, D.R.; Wu, Q.H. Raman spectroscopy of copper phthalocyanine /graphene and 2, 3, 5, 6-tetrafluoro-tetracyano-quino-dimethane/graphene interfaces. Surf. Interface Anal. 2021, 53, 466–472. [Google Scholar] [CrossRef]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Design of hetero-nanostructures on MoS2 nanosheets to boost NO2 room-temperature sensing. ACS Appl. Mater. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.K.; Wang, J.; Sun, B.H.; Zhang, Y.; Wang, R.H.; Shi, K.Y. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xu, N.; Guan, R.; Yue, L.; Zhang, W. Highly sensitive ethanol gas sensor based on In2O3 spheres. Ionics 2021, 27, 3647–3653. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.J.; Choi, H.J.; Gwag, J.S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef]
- Zhang, D.; Mi, Q.; Wang, D.; Li, T. MXene/Co3O4 composite based formaldehyde sensor driven by ZnO/MXene nanowire arrays piezoelectric nanogenerator. Sens. Actuators B Chem. 2021, 339, 129923. [Google Scholar] [CrossRef]
- Lichtenstein, A.; Havivi, E.; Shacham, R.; Hahamy, E.; Leibovich, R.; Pevzner, A.; Krivitsky, V.; Davivi, G.; Presman, I.; Elnathan, R.; et al. Supersensitive fingerprinting of explosives by chemically modified nanosensors arrays. Nat. Commun. 2014, 5, 4195. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Cao, Y.; Guo, J.; Long, M.; Duan, H.; Jia, D. Chemiresistive sensor arrays based on noncovalently functionalized multi-walled carbon nanotubes for ozone detection. Sens. Actuators B Chem. 2019, 297, 126689. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Xie, Y.; Guo, B.; Hu, Z. Non-contact crack detection of high-speed blades based on principal component analysis and Euclidian angles using optical-fiber sensors. Sens. Actuators A Phys. 2013, 201, 66–72. [Google Scholar] [CrossRef]
- Qingyu, L.; Dengwang, L.; Wei, T. SnO2 quantum dots modified MoS2nanoflowers for enhanced ethanol sensing performance. Vacuum 2023, 213, 112168. [Google Scholar]
- Jianhua, Z.; Tingting, L.; Jingyu, G.; Hu, Y.; Zhang, D. Two-step hydrothermal fabrication of CeO2-loaded MoS2 nanoflowers for ethanol gas sensing application. Appl. Surf. Sci. 2021, 568, 150942. [Google Scholar]
- Song, Z.; Zhang, J.; Jiang, J. Morphological evolution, luminescence properties and a highsensitivity ethanol gas sensor based on 3D flower-like MoS2-ZnO micro/nanosphere arrays. Ceram. Int. 2020, 46, 6636–6640. [Google Scholar]
- Singh, S.; Raj, S.; Sharma, S. Ethanol sensing using MoS2/TiO2 composite prepared via hydrothermal method. Mater. Today Proc. 2021, 46, 6083–6086. [Google Scholar] [CrossRef]
- Priyanka, D.; Samaresh, D.; Saakshi, D. Wafer-Scale Synthesized MoS2/Porous Silicon Nanostructures for Efficient and Selective Ethanol Sensing at Room Temperature. ACS Appl. Mater. Interfaces 2017, 9, 21017–21024. [Google Scholar]
- Ali, A.; Wu, D.; Jianqiao, L.; Fu, C.; Wang, J.; Chu, X. The detection of ethanol vapors based on a p-type gas sensor fabricated from heterojunction MoS2-NiCo2O4. Mater. Chem. Phys. 2022, 282, 125964. [Google Scholar]
- Ma, H.; Yang, R.; Li, C.; Han, Y. Fabrication and Characteristics of MoS2 nanosheets on Photo-sensing and Gas-sensing. Mater. Rep. 2018, 32, 860–864. [Google Scholar]
- Liu, L.; Yang, W.; Zhang, H.; Yan, X.; Liu, Y. Ultra-High Response Detection of Alcohols Based on CdS/MoS2 Composite. Nanoscale Res. Lett. 2022, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takemura, S.; Watanabe, Y.; Ishii, A.; Tsuchida, I.; Akai, Y.; Sugiyama, T.; Hiramatsu, T.; Nanba, N.; Nishikawa, O.; et al. X-ray photoemission spectroscopy and Fourier transform infrared studies of electrochemical doping of copper phthalocyanine molecule in conducting polymer. J. Vac. Sci. Technol. A 2007, 25, 1147. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Zhang, M.; Qin, Z.; Cao, S.; Zhong, F.; Li, S.; Duan, H.M.; Zhang, J. Improved Gas-Sensitive Properties by a Heterojunction of Hollow Porous Carbon Microtubes Derived from Sycamore Fibers. ACS Sustain. Chem. Eng. 2021, 9, 14345–14352. [Google Scholar] [CrossRef]


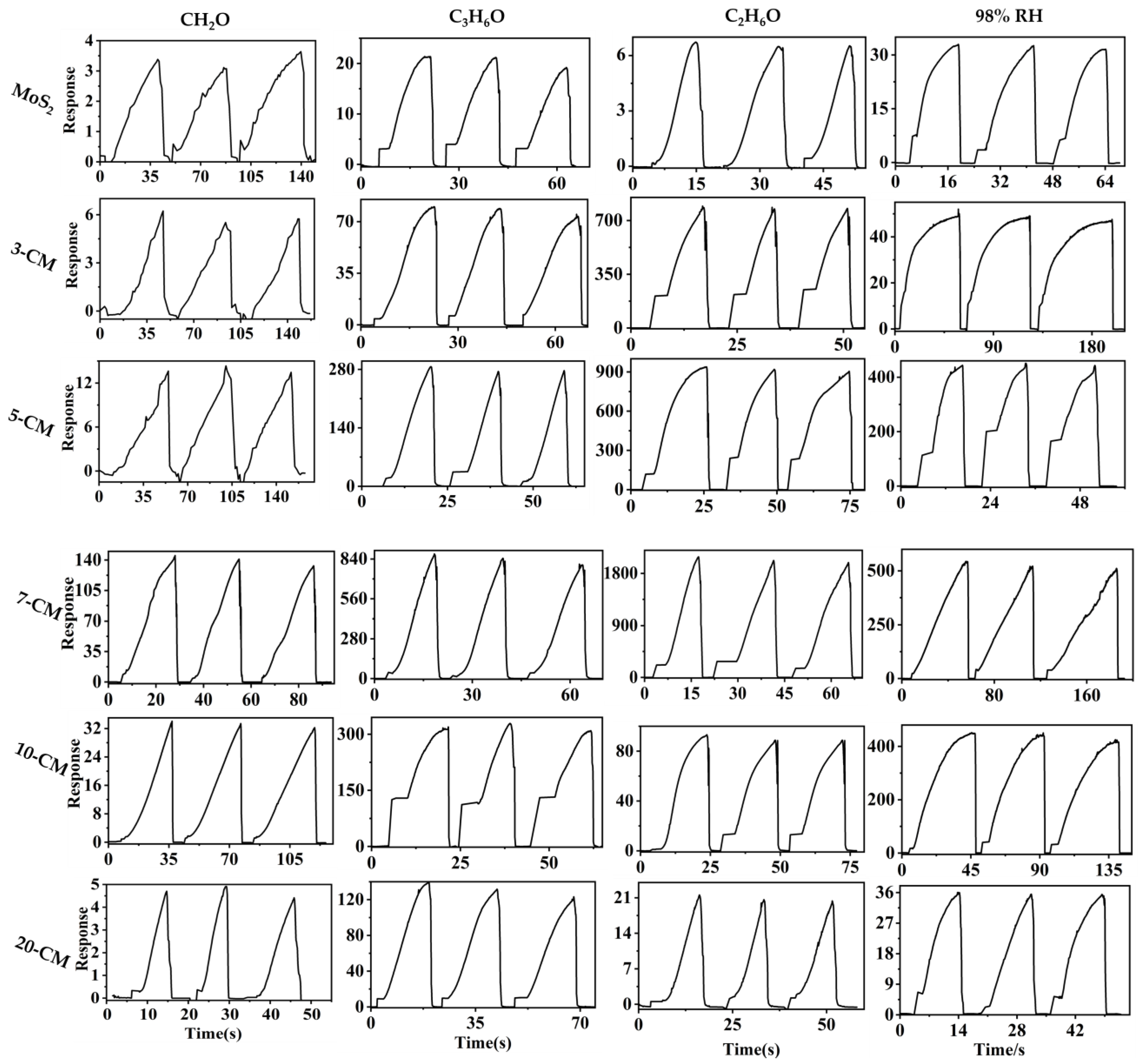

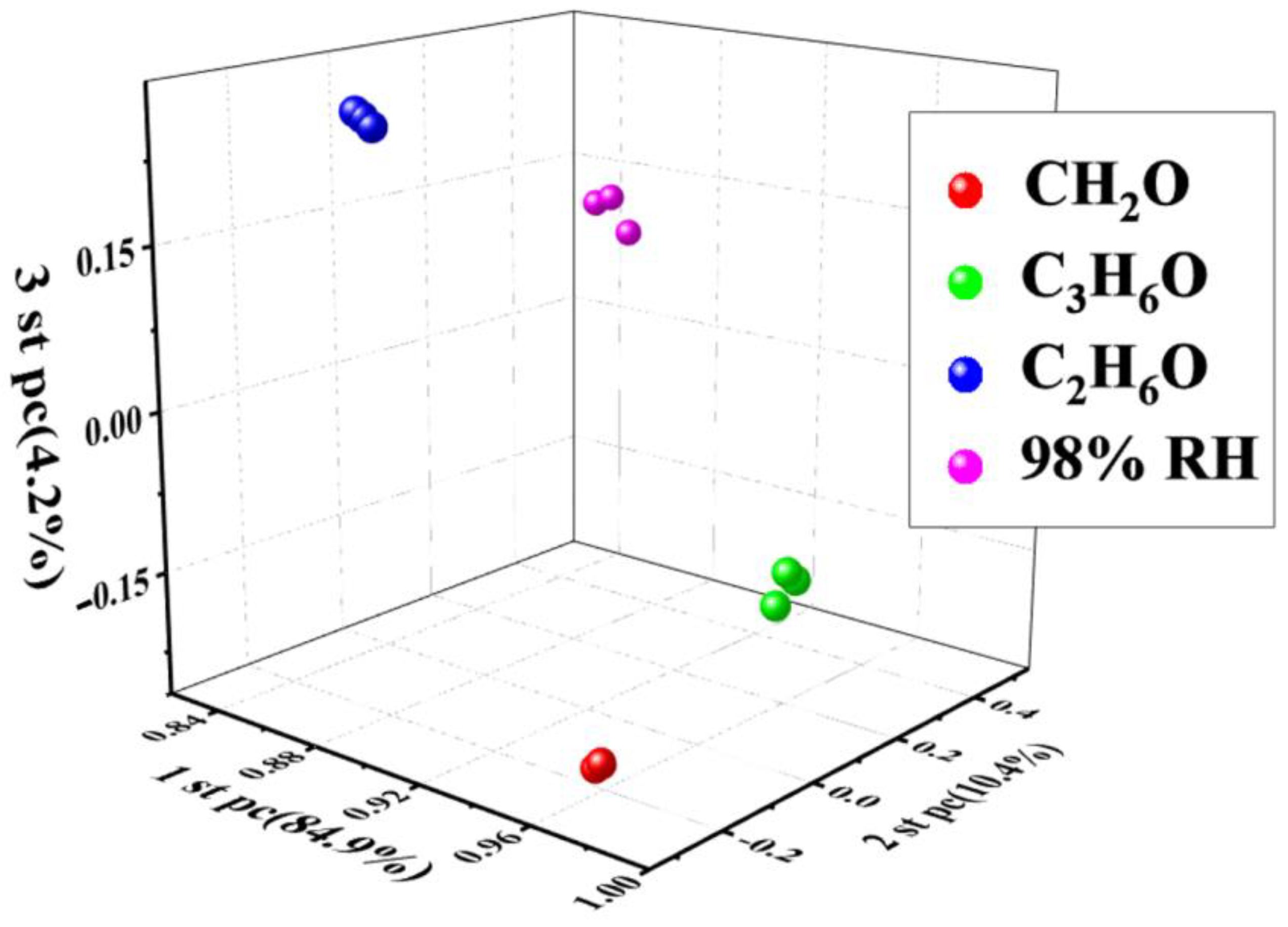

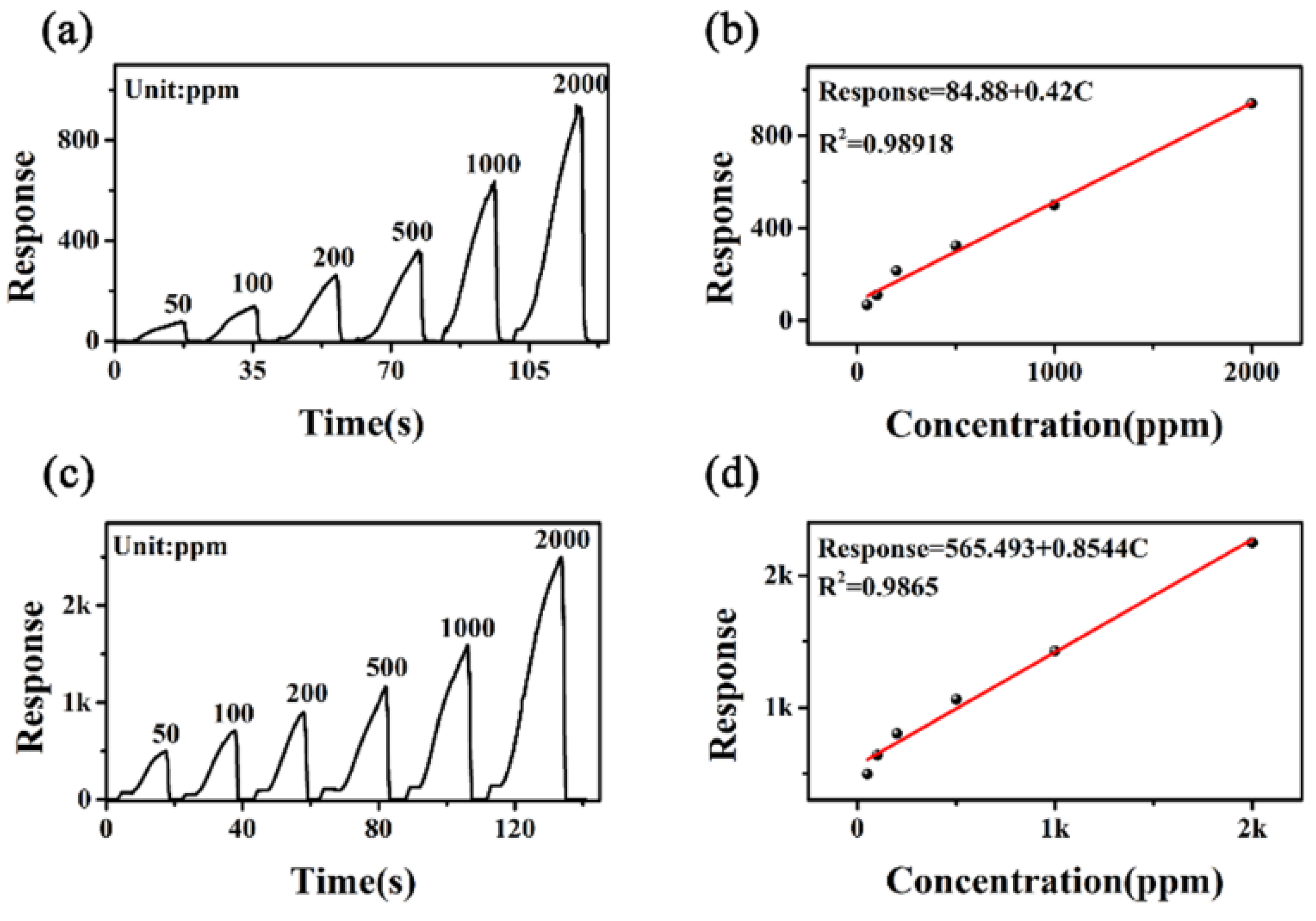


| Materials | Working Temperature | Concentration (ppm) | Response | Res/Rec Time (s) | Ref. |
|---|---|---|---|---|---|
| SnO2QD/MoS2 | 180 °C | 50 | 0.49 | 25/85 | [38] |
| MoS2/CeO2 | RT | 50 | 7.78 | 7/5 | [39] |
| 3D(MoS2)/ZnO | 220 °C | 500 | 12.08 | 30/10 | [40] |
| MoS2/TiO2 | 350 °C | 100 | 0.62 | 52/155 | [41] |
| MoS2/PSi | RT | 40 | 0.17 | --- | [42] |
| MoS2/NiCo2O4 | 170 °C | 100 | 9.00 | 15/6 | [43] |
| MoS2Nanosheets | RT | 114 | 1.14 | 2.4/29 | [44] |
| CdS/MoS2 | 210 °C | 100 | 0.95 | 20/9 | [45] |
| 2D-MoS2/1D-CuPc | RT | 100 | 710.42 | <10/<4 | This work |
| 50 | 497.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Xu, X.; Wang, H.; Shaymurat, T. Preparation and Gas-Sensing Properties of Two-Dimensional Molybdenum Disulfide/One-Dimensional Copper Phthalocyanine Heterojunction. Sensors 2023, 23, 9321. https://doi.org/10.3390/s23239321
Chen G, Xu X, Wang H, Shaymurat T. Preparation and Gas-Sensing Properties of Two-Dimensional Molybdenum Disulfide/One-Dimensional Copper Phthalocyanine Heterojunction. Sensors. 2023; 23(23):9321. https://doi.org/10.3390/s23239321
Chicago/Turabian StyleChen, Guoqing, Xiaojie Xu, Hao Wang, and Talgar Shaymurat. 2023. "Preparation and Gas-Sensing Properties of Two-Dimensional Molybdenum Disulfide/One-Dimensional Copper Phthalocyanine Heterojunction" Sensors 23, no. 23: 9321. https://doi.org/10.3390/s23239321
APA StyleChen, G., Xu, X., Wang, H., & Shaymurat, T. (2023). Preparation and Gas-Sensing Properties of Two-Dimensional Molybdenum Disulfide/One-Dimensional Copper Phthalocyanine Heterojunction. Sensors, 23(23), 9321. https://doi.org/10.3390/s23239321






