Surface Conditioning Effects on Submerged Optical Sensors: A Comparative Study of Fused Silica, Titanium Dioxide, Aluminum Oxide, and Parylene C
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Conditioning Film
2.4. Polystyrene Microbeads
2.5. Atomic Force Microscopy (AFM)
2.6. Water Contact Angle
2.7. Surface Charge
2.8. X-ray Photoelectron Spectroscopy (XPS)
2.9. Shear-Stress Flow Chamber
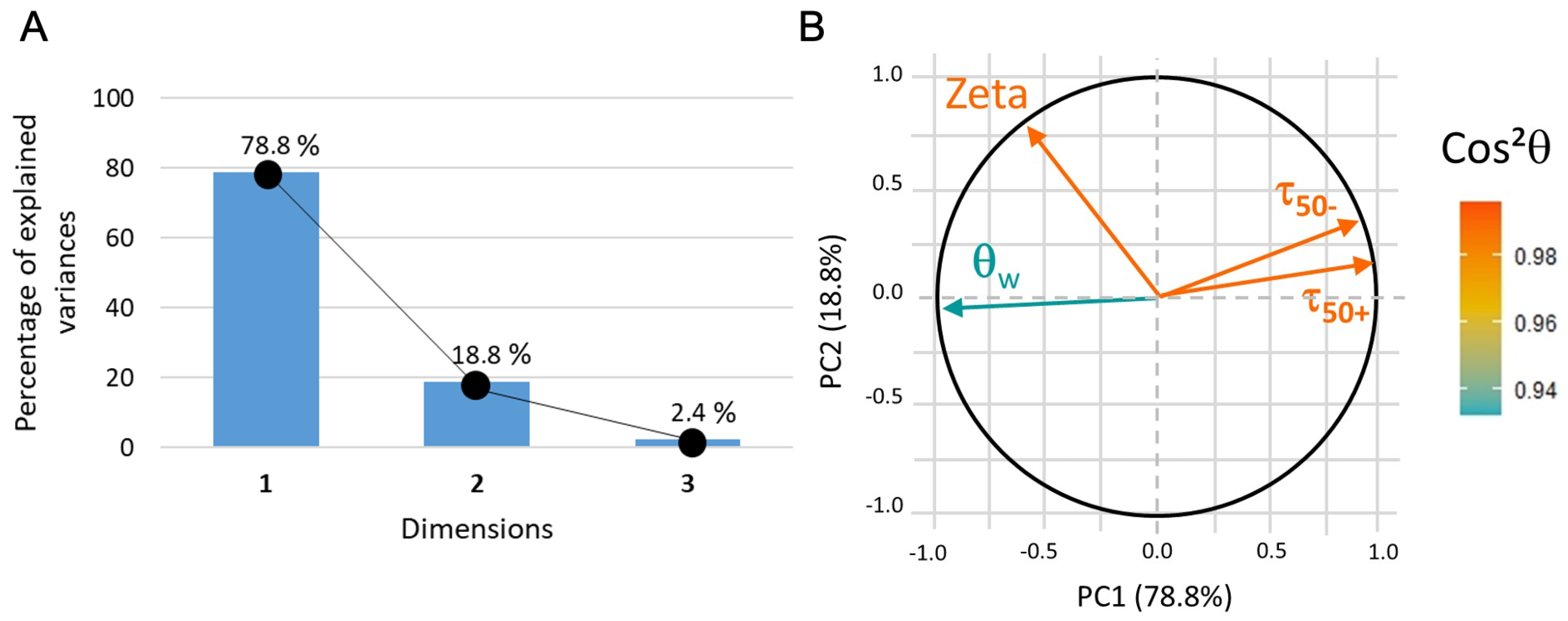
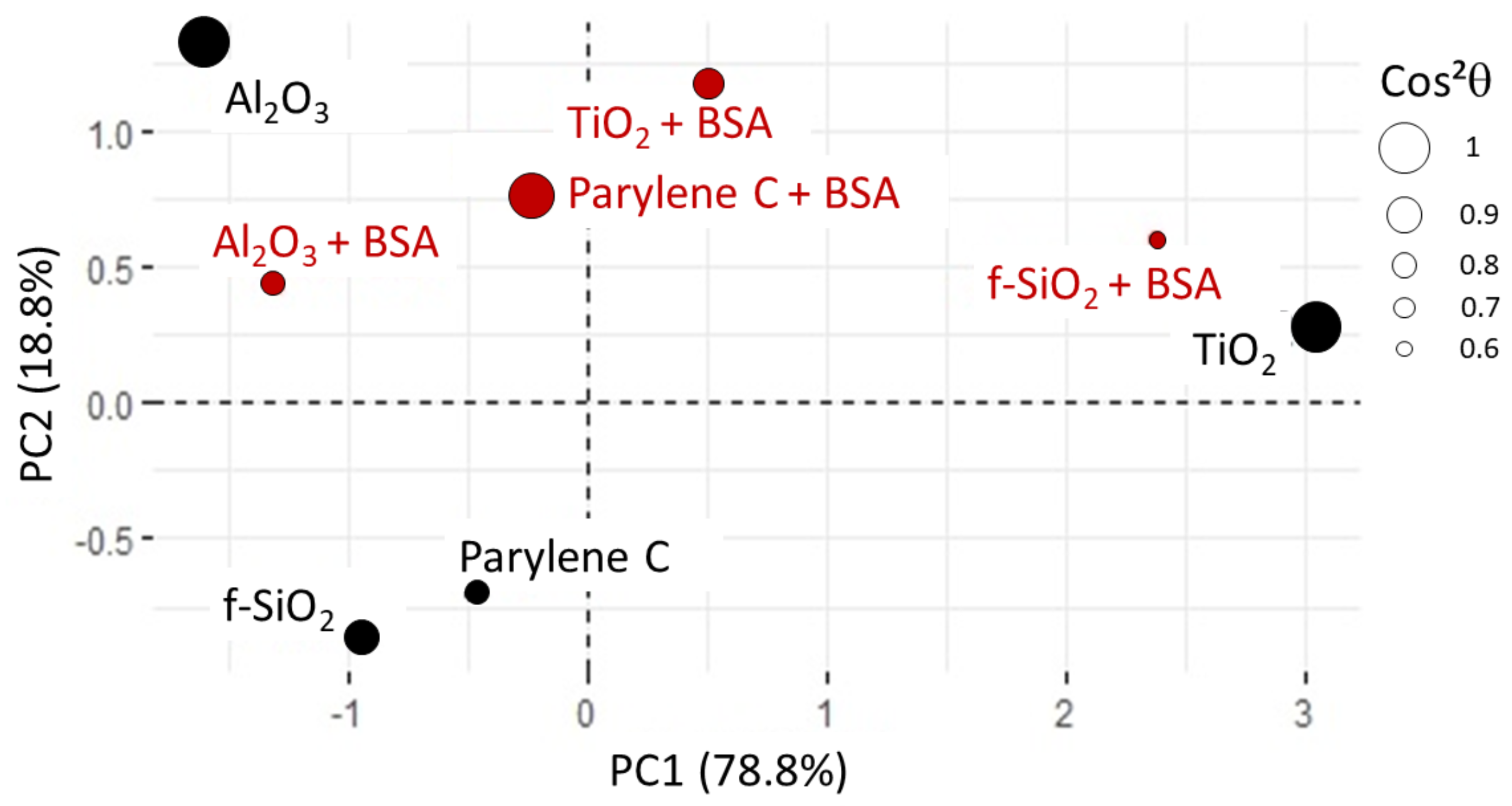
2.10. Principal Component Analysis
- Score plot: The samples are plotted as a function of the new coordinate system and it can help to group samples with similar characteristics. calculated from the coordinates of the samples gives information on the quality of the sample description in the reduced space and thus the reliability of the drawn conclusion: the closer is to one, the better the description;
- Loading plot: The variables are plotted as a function of the principal coordinates. Usually, two or three PCs are sufficient to take into account a large part of the data variability. Vectors that represent each variable should be drawn from the center: if there is, approximately, a difference between the vectors, then the two variables are not correlated, while a difference of means that the two variables are anti-correlated and if the angle is small (approximately –) between the two vectors then they are strongly related.
3. Results
3.1. Roughness and Topography
3.2. Hydrophobicity
3.3. Zeta Potential
3.4. Chemical Composition by XPS
3.5. Microbeads Adhesion to Surfaces
4. Discussion
4.1. Analysis of BSA Adsorption
4.2. Influence of the Conditioning Film on the Adhesion of Functionalized Hydrophobic Polystyrene Microbeads
4.3. Towards the Adsorption of Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| BSA | Bovine Serum Albumin |
| Titanium dioxide | |
| Silica fuse | |
| Alumina | |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Delgado, A.; Briciu-Burghina, C.; Regan, F. Antifouling Strategies for Sensors Used in Water Monitoring: Review and Future Perspectives. Sensors 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Delauney, L.; Compère, C.; Lehaitre, M. Biofouling protection for marine environmental sensors. Ocean. Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef]
- Tendero, C.; Lazar, A.M.; Samélor, D.; Debieu, O.; Constantoudis, V.; Papavieros, G.; Villeneuve, A.; Vahlas, C. Nanocomposite thin film of Ag nanoparticles embedded in amorphous Al2O3 on optical sensors windows: Synthesis, characterization and targeted application towards transparency and anti-biofouling. Surf. Coatings Technol. 2017, 328, 371–377. [Google Scholar] [CrossRef]
- Fletcher, M. Bacterial biofilms and biofouling. Curr. Opin. Biotechnol. 1994, 5, 302–306. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, a.H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control. 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–192. [Google Scholar] [CrossRef]
- Navarro-Villoslada, F.; Orellana, G.; Moreno-Bondi, M.C.; Vick, T.; Driver, M.; Hildebrand, G.; Liefeith, K. Fiber-Optic Luminescent Sensors with Composite Oxygen-Sensitive Layers and Anti-Biofouling Coatings. Anal. Chem. 2001, 73, 5150–5156. [Google Scholar] [CrossRef]
- Whelan, A.; Regan, F. Antifouling strategies for marine and riverine sensors. J. Environ. Monit. 2006, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.A. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci. Total. Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef] [PubMed]
- Gipperth, L. The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. J. Environ. Manag. 2009, 90 (Suppl. S1), S86–S95. [Google Scholar] [CrossRef] [PubMed]
- Uc-Peraza, R.G.; Castro, B.; Fillmann, G. An absurd scenario in 2021: Banned TBT-based antifouling products still available on the market. Sci. Total. Environ. 2022, 805, 150377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Saulou, C.; Despax, B.; Raynaud, P.; Zanna, S.; Marcus, P.; Mercier-Bonin, M. Plasma deposition of organosilicon polymer thin films with embedded nanosilver for prevention of microbial adhesion. Appl. Surf. Sci. 2009, 256, S35–S39. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Barthomeuf, M.; Castel, X.; Le Gendre, L.; Louis, J.; Denis, M.; Pissavin, C. Effect of Titanium Dioxide Film Thickness on Photocatalytic and Bactericidal Activities Against Listeria monocytogenes. Photochem. Photobiol. 2019, 95, 1035–1044. [Google Scholar] [CrossRef]
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 Photocatalytic Membranes with a Hierarchical Mesoporous Multilayer Structure: Synthesis, Characterization, and Multifunction. Adv. Funct. Mater. 2006, 16, 1067–1074. [Google Scholar] [CrossRef]
- Noguchi, T.; Fujishima, A.; Sawunyama, P.; Hashimoto, K. Photocatalytic Degradation of Gaseous Formaldehyde Using TiO2 Film. Environ. Sci. Technol. 1998, 32, 3831–3833. [Google Scholar] [CrossRef]
- Ohko, Y.; Utsumi, Y.; Niwa, C.; Tatsuma, T.; Kobayakawa, K.; Satoh, Y.; Kubota, Y.; Fujishima, A. Self-sterilizing and self-cleaning of silicone catheters coated with TiO(2) photocatalyst thin films: A preclinical work. J. Biomed. Mater. Res. 2001, 58, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y.; Heller, A. Photo-oxidatively self-cleaning transparent titanium dioxide films on soda lime glass: The deleterious effect of sodium contamination and its prevention. J. Mater. Res. 1997, 12, 2759–2766. [Google Scholar] [CrossRef]
- Hölken, I.; Hoppe, M.; Mishra, Y.K.; Gorb, S.N.; Adelung, R.; Baum, M.J. Complex shaped ZnO nano- and microstructure based polymer composites: Mechanically stable and environmentally friendly coatings for potential antifouling applications. Phys. Chem. Chem. Phys. 2016, 18, 7114–7123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, Y.; Jiang, Y.; Ding, Y.; Li, W. Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens. Int. J. Food Sci. Technol. 2009, 44, 2161–2168. [Google Scholar] [CrossRef]
- Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gapeeva, A.; Hölken, I.; Kaps, S.; Adelung, R.; Baum, M. Preventing algae adhesion using lubricant-modified polydimethylsiloxane/polythiourethane nanocomposite. Mater. Des. 2022, 214, 110389. [Google Scholar] [CrossRef]
- Feng, K.; Qiu, H.; Gapeeva, A.; Li, X.; Li, Y.; Kaps, S.; Mishra, Y.; Adelung, R.; Baum, M.; Yu, L. Indole-functionalized polythiourethane/tetrapodal shaped ZnO nanocomposites for eco-friendly marine biofouling control. Prog. Org. Coatings 2023, 185, 107939. [Google Scholar] [CrossRef]
- Klemm, S.; Baum, M.; Qiu, H.; Nan, Z.; Cavalheiro, M.; Teixeira, M.C.; Tendero, C.; Gapeeva, A.; Adelung, R.; Dague, E.; et al. Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy. Nanomaterials 2021, 11, 271. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Lin, L.; Tosatti, S.; Vörös, J.; Textor, M.; Messersmith, P.B. Protein resistance of titanium oxide surfaces modified by biologically inspired mPEG- DOPA. Langmuir 2005, 21, 640–646. [Google Scholar] [CrossRef]
- Kenausis, G.L.; Vörös, J.; Elbert, D.L.; Huang, N.; Hofer, R.; Ruiz-Taylor, L.; Textor, M.; Hubbell, J.A.; Spencer, N.D. Poly(l-lysine)-g-Poly(ethylene glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption. J. Phys. Chem. B 2000, 104, 3298–3309. [Google Scholar] [CrossRef]
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. A Biomimetic Alternative to Poly(ethylene glycol) as an Antifouling Coating: Resistance to Nonspecific Protein Adsorption of Poly(l-lysine)-graft-dextran. Langmuir 2008, 24, 8850–8856. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Ray, P.; Singh, A.V.; Kishore, V.; Singh, S.L. Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances. Coatings 2023, 13, 1095. [Google Scholar] [CrossRef]
- Chelmowski, R.; Köster, S.D.; Kerstan, A.; Prekelt, A.; Grunwald, C.; Winkler, T.; Metzler-Nolte, N.; Terfort, A.; Wöll, C. Peptide-Based SAMs that Resist the Adsorption of Proteins. J. Am. Chem. Soc. 2008, 130, 14952–14953. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mrksich, M.; Whitesides, G.M. Self-assembled monolayers of alkanethiolates presenting tri (propylene sulfoxide) groups resist the adsorption of protein. J. Am. Chem. Soc. 1996, 118, 5136–5137. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Prime, K.L.; Whitesides, G.M. Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164. [Google Scholar] [CrossRef] [PubMed]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo (ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Alpay, P.; Uygun, D.A. Usage of immobilized papain for enzymatic hydrolysis of proteins. J. Mol. Catal. B: Enzym. 2015, 111, 56–63. [Google Scholar] [CrossRef]
- Gogoi, D.; Barman, T.; Choudhury, B.; Khan, M.; Chaudhari, Y.; Dehingia, M.; Pal, A.R.; Bailung, H.; Chutia, J. Immobilization of trypsin on plasma prepared Ag/PPAni nanocomposite film for efficient digestion of protein. Mater. Sci. Eng. C 2014, 43, 237–242. [Google Scholar] [CrossRef]
- Kim, Y.D.; Dordick, J.S.; Clark, D.S. Siloxane-based biocatalytic films and paints for use as reactive coatings. Biotechnol. Bioeng. 2001, 72, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I.; Nam, J.; Hwang, D.S.; Yeon, K.M.; Kim, J. Immobilization and Stabilization of Acylase on Carboxylated Polyaniline Nanofibers for Highly Effective Antifouling Application via Quorum Quenching. ACS Appl. Mater. Interfaces 2017, 9, 15424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wan, D.; Liang, B.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Lysozyme-Coupled Poly(poly(ethylene glycol) methacrylate)—Stainless Steel Hybrids and Their Antifouling and Antibacterial Surfaces. Langmuir 2011, 27, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mercier-Bonin, M.; Ouazzani, K.; Schmitz, P.; Lorthois, S. Study of bioadhesion on a flat plate with a yeast/glass model system. J. Colloid Interface Sci. 2004, 271, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sakiyama, T.; Imamura, K. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Pattanayek, S.K. Effect of surface energy of solid surfaces on the micro- and macroscopic properties of adsorbed BSA and lysozyme. Biophys. Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Zouaghi, S.; Six, T.; Nuns, N.; Simon, P.; Bellayer, S.; Moradi, S.; Hatzikiriakos, S.G.; André, C.; Delaplace, G.; Jimenez, M. Influence of stainless steel surface properties on whey protein fouling under industrial processing conditions. J. Food Eng. 2018, 228, 38–49. [Google Scholar] [CrossRef]
- Coquet, L.; Cosette, P.; Junter, G.A.; Beucher, E.; Saiter, J.M.; Jouenne, T. Adhesion of Yersinia ruckeri to fish farm materials: Influence of cell and material surface properties. Colloids Surfaces B Biointerfaces 2002, 26, 373–378. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, N.; Jo, W.; Jung, W.; Lim, J. Effects of substrates on biofilm formation observed by atomic force microscopy. Ultramicroscopy 2009, 109, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Kalasin, S.; Dabkowski, J.; Nuesslein, K.; Santore, M.M. The role of nano-scale heterogeneous electrostatic interactions in initial bacterial adhesion from flow: A case study with Staphylococcus Aureus. Colloids Surfaces B-Biointerfaces 2010, 76, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S.; Urano, H.; Nagata, K. Adsorption of bovine serum albumin onto metal oxide surfaces. J. Ferment. Bioeng. 1996, 81, 163–167. [Google Scholar] [CrossRef]
- Altankov, G.; Richau, K.; Groth, T. The role of surface zeta potential and substratum chemistry for regulation of dermal fibroblasts interaction. Mater. Werkst. 2003, 34, 1120–1128. [Google Scholar] [CrossRef]
- Guillemot, G.; Lorthois, S.; Schmitz, P.; Mercier-Bonin, M. Evaluating the Adhesion Force Between Saccharomyces Cerevisiae Yeast Cells and Polystyrene From Shear-Flow Induced Detachment Experiments. Chem. Eng. Res. Des. 2007, 85, 800–807. [Google Scholar] [CrossRef]
- Castelain, M.; Rouxhet, P.G.; Pignon, F.; Magnin, A.; Piau, J.M. Single-cell adhesion probed in-situ using optical tweezers: A case study with Saccharomyces cerevisiae. J. Appl. Phys. 2012, 111, 114701. [Google Scholar] [CrossRef]
- Castelain, M.; Pignon, F.; Piau, J.M.; Magnin, A.; Mercier-Bonin, M.; Schmitz, P. Removal forces and adhesion properties of Saccharomyces cerevisiae on glass substrates probed by optical tweezer. J. Chem. Phys. 2007, 127, 135104. [Google Scholar] [CrossRef]
- Talluri, S.N.L.; Winter, R.M.; Salem, D.R. Conditioning film formation and its influence on the initial adhesion and biofilm formation by a cyanobacterium on photobioreactor materials. Biofouling 2020, 36, 183–199. [Google Scholar] [CrossRef]
- Lorite, G.S.; Rodrigues, C.M.; de Souza, A.A.; Kranz, C.; Mizaikoff, B.; Cotta, M.A. The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J. Colloid Interface Sci. 2011, 359, 289–295. [Google Scholar] [CrossRef]
- Jain, A.; Bhosle, N.B. Biochemical composition of the marine conditioning film: Implications for bacterial adhesion. Biofouling 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Garrido, K.D.; Palacios, R.J.S.; Lee, C.; Kang, S. Impact of conditioning film on the initial adhesion of E. coli on polysulfone ultrafiltration membrane. J. Ind. Eng. Chem. 2014, 20, 1438–1443. [Google Scholar] [CrossRef]
- Hwang, G.; Kang, S.; El-Din, M.G.; Liu, Y. Impact of conditioning films on the initial adhesion of Burkholderia cepacia. Colloids Surfaces B Biointerfaces 2012, 91, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liang, J.; Kang, S.; Tong, M.; Liu, Y. The role of conditioning film formation in Pseudomonas aeruginosa PAO1 adhesion to inert surfaces in aquatic environments. Biochem. Eng. J. 2013, 76, 90–98. [Google Scholar] [CrossRef]
- Kerchove, A.J.d.; Elimelech, M. Impact of Alginate Conditioning Film on Deposition Kinetics of Motile and Nonmotile Pseudomonas aeruginosa Strains. Appl. Environ. Microbiol. 2007, 73, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Démonstrateur API Qualité des Rivières Hub’Eau. Available online: https://hubeau.eaufrance.fr/sites/default/files/api/demo/qualriv.htm (accessed on 15 October 2023).
- Triquet, T.; Tendero, C.; Latapie, L.; Manero, M.H.; Richard, R.; Andriantsiferana, C. TiO2 MOCVD coating for photocatalytic degradation of ciprofloxacin using 365 nm UV LEDs - kinetics and mechanisms. J. Environ. Chem. Eng. 2020, 8, 104544. [Google Scholar] [CrossRef]
- Etchepare, P.L.; Baggetto, L.; Vergnes, H.; Samélor, D.; Sadowski, D.; Caussat, B.; Vahlas, C. Process-structure-properties relationship in direct liquid injection chemical vapor deposition of amorphous alumina from aluminum tri-isopropoxide. Phys. Status Solidi C 2015, 12, 944–952. [Google Scholar] [CrossRef]
- Santucci, V.; Maury, F.; Senocq, F. Vapor phase surface functionalization under ultra violet activation of parylene thin films grown by chemical vapor deposition. Thin Solid Film. 2010, 518, 1675–1681. [Google Scholar] [CrossRef][Green Version]
- Mercier-Bonin, M.; Adoue, M.; Zanna, S.; Marcus, P.; Combes, D.; Schmitz, P. Evaluation of adhesion force between functionalized microbeads and protein-coated stainless steel using shear-flow-induced detachment. J. Colloid. Interface Sci. 2009, 338, 73–81. [Google Scholar] [CrossRef]
- Bousse, L.; Mostarshed, S.; Van Der Shoot, B.; de Rooij, N.F.; Gimmel, P.; Göpel, W. Zeta potential measurements of Ta2O5 and SiO2 thin films. J. Colloid Interface Sci. 1991, 147, 22–32. [Google Scholar] [CrossRef]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloids Surfaces B Biointerfaces 2002, 26, 387–395. [Google Scholar] [CrossRef]
- Werner, C.; Korber, H.; Zimmermann, R.; Dukhin, S.; Jacobasch, H.J. Extended electrokinetic characterization of flat solid surfaces. J. Colloid Interface Sci. 1998, 208, 329–346. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media; Taylor & Francis: Oxford, UK, 1994. [Google Scholar]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20; Version 3.4 (Web Version); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003; p. 20899.
- Castelain, M.; Duviau, M.P.; Oxaran, V.; Schmitz, P.; Cocaign-Bousquet, M.; Loubiere, P.; Piard, J.C.; Mercier-Bonin, M. Oligomerized backbone pilin helps piliated Lactococcus lactis to withstand shear flow. Biofouling 2016, 32, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Mozes, N.; Dengis, P.B.; Dufrêne, Y.F.; Gerin, P.A.; Genet, M.J. Application of X-ray photoelectron spectroscopy to microorganisms. Colloids Surfaces B: Biointerfaces 1994, 2, 347–369. [Google Scholar] [CrossRef]
- Wang, C.; Zanna, S.; Frateur, I.; Despax, B.; Raynaud, P.; Mercier-Bonin, M.; Marcus, P. BSA adsorption on a plasma-deposited silver nanocomposite film controls silver release: A QCM and XPS-based modelling. Surf. Coatings Technol. 2016, 307, 1–8. [Google Scholar] [CrossRef]
- Trantidou, T.; Payne, D.J.; Tsiligkiridis, V.; Chang, Y.C.; Toumazou, C.; Prodromakis, T. The dual role of Parylene C in chemical sensing: Acting as an encapsulant and as a sensing membrane for pH monitoring applications. Sensors Actuators B Chem. 2013, 186, 1–8. [Google Scholar] [CrossRef]
- Frateur, I.; Lartundo-Rojas, L.; Méthivier, C.; Galtayries, A.; Marcus, P. Influence of bovine serum albumin in sulphuric acid aqueous solution on the corrosion and the passivation of an iron–chromium alloy. Electrochim. Acta 2006, 51, 1550–1557. [Google Scholar] [CrossRef]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surfaces B Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef]
- Subramani, A.; Huang, X.; Hoek, E.M.V. Direct observation of bacterial deposition onto clean and organic-fouled polyamide membranes. J. Colloid Interface Sci. 2009, 336, 13–20. [Google Scholar] [CrossRef]
- Beragoui, M.; Aguir, C.; Khalfaoui, M.; Enciso, E.; Torralvo, M.J.; Duclaux, L.; Reinert, L.; Vayer, M.; Ben Lamine, A. Bovine serum albumin adsorption onto functionalized polystyrene lattices: A theoretical modeling approach and error analysis. Prog. Theor. Exp. Phys. 2015, 2015, 033J01. [Google Scholar] [CrossRef]
- Larsericsdotter, H.; Oscarsson, S.; Buijs, J. Structure, stability, and orientation of BSA adsorbed to silica. J. Colloid Interface Sci. 2005, 289, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Delivopoulos, E.; Ouberai, M.M.; Coffey, P.D.; Swann, M.J.; Shakesheff, K.M.; Welland, M.E. Serum protein layers on parylene-C and silicon oxide: Effect on cell adhesion. Colloids Surfaces B Biointerfaces 2015, 126, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Scarangella, A.; Soumbo, M.; Villeneuve-Faure, C.; Mlayah, A.; Bonafos, C.; Monje, M.C.; Roques, C.; Makasheva, K. Adsorption properties of BSA and DsRed proteins deposited on thin SiO2 layers: Optically non-absorbing versus absorbing proteins. Nanotechnology 2018, 29, 115101. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Ismail, S.; Pratten, J.; Parkin, I.P. An investigation into bacterial attachment to an elastomeric superhydrophobic surface prepared via aerosol assisted deposition. Thin Solid Film. 2011, 519, 3722–3727. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, W.; Wang, Y.; Zhang, B.; Wang, H.; Lin, C.; Zhang, L. Effect of Superhydrophobic Surface of Titanium on Staphylococcus aureus Adhesion. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. 2013, 110, 5624–5629. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, W.; Weng, Y.; Chen, X.; Cheng, Y.; Zhou, P. Fabrication of PDMS surfaces with micro patterns and the effect of pattern sizes on bacteria adhesion. Food Control 2016, 68, 344–351. [Google Scholar] [CrossRef]
- Thewes, N.; Loskill, P.; Jung, P.; Peisker, H.; Bischoff, M.; Herrmann, M.; Jacobs, K. Hydrophobic interaction governs unspecific adhesion of staphylococci: A single cell force spectroscopy study. Beilstein J. Nanotechnol. 2014, 5, 1501–1512. [Google Scholar] [CrossRef]
- Castelain, M.; Pignon, F.; Piau, J.M.; Magnin, A. The initial single yeast cell adhesion on glass via optical trapping and Derjaguin-Landau-Verwey-Overbeek predictions. J. Chem. Phys. 2008, 128, 135101. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.; Norde, W.; Lyklema, J.; Zehnder, A.J. DLVO and steric contributions to bacterial deposition in media of different ionic strengths. Colloids Surfaces B Biointerfaces 1999, 14, 179–195. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Busscher, H.J.; Mei, H.C.V.D.; Vries, J.D.; Norde, W. Role of lactobacillus cell surface hydrophobicity as probed by AFM in adhesion to surfaces at low and high ionic strength. Colloids Surfaces B Biointerfaces 2005, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Moreno, A.; González-Martıín, M.; Pérez-Giraldo, C.; Bruque, J.; Gómez-García, A. The measurement temperature: An important factor relating physicochemical and adhesive properties of yeast cells to biomaterials. J. Colloid Interface Sci. 2004, 271, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Brant, J. Assessing short-range membrane–colloid interactions using surface energetics. J. Membr. Sci. 2002, 203, 257–273. [Google Scholar] [CrossRef]
- Millsap, K.W.; Bos, R.; Busscher, H.J.; Van Der Mei, H.C. Surface Aggregation ofCandida albicanson Glass in the Absence and Presence of AdheringStreptococcus gordoniiin a Parallel-Plate Flow Chamber: A Surface Thermodynamical Analysis Based on Acid–Base Interactions. J. Colloid Interface Sci. 1999, 212, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Choi, H. Effect of surface hydrophobicity on the adhesion of S. cerevisiae onto modified surfaces by poly(styrene-ran-sulfonic acid) random copolymers. Colloids Surfaces B Biointerfaces 2005, 46, 70–77. [Google Scholar] [CrossRef]
- Bos, R.; Busscher, H.J. Role of acid–base interactions on the adhesion of oral streptococci and actinomyces to hexadecane and chloroform—influence of divalent cations and comparison between free energies of partitioning and free energies obtained by extended DLVO analysis. Colloids Surfaces B Biointerfaces 1999, 14, 169–177. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Boonaert, C.J.P.; Rouxhet, P.G. Surface analysis by X-ray photoelectron spectroscopy in study of bioadhesion and biofilms. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 310, pp. 375–389. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Boonaert, C.J.P.; Rouxhet, P.G. Adhesion of Azospirillum brasilense: Role of proteins at the cell-support interface. Colloids Surfaces B Biointerfaces 1996, 7, 113–128. [Google Scholar] [CrossRef]
- Dewez, J.L.; Doren, A.; Schneider, Y.J.; Rouxhet, P.G. Competitive adsorption of proteins: Key of the relationship between substratum surface properties and adhesion of epithelial cells. Biomaterials 1999, 20, 547–559. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Truong, V.K.; Wang, J.Y.; Berndt, C.C.; Jones, R.T.; Yusuf, I.I.; Peake, I.; Schmidt, H.W.; Fluke, C.; Barnes, D.; et al. Impact of Nanoscale Roughness of Titanium Thin Film Surfaces on Bacterial Retention. Langmuir 2010, 26, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, C.; Bossert, J.; Roth, M.; Jandt, K.D. Physical vapor deposited titanium thin films for biomedical applications: Reproducibility of nanoscale surface roughness and microbial adhesion properties. Appl. Surf. Sci. 2013, 280, 578–589. [Google Scholar] [CrossRef]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traoré, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, Y.; Ge, X.; Leng, Y. Control of bacterial adhesion and growth on honeycomb-like patterned surfaces. Colloids Surfaces B Biointerfaces 2015, 135, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Vadillo-Rodríguez, V.; Guerra-García-Mora, A.I.; Perera-Costa, D.; Gónzalez-Martín, M.L.; Fernández-Calderón, M.C. Bacterial response to spatially organized microtopographic surface patterns with nanometer scale roughness. Colloids Surfaces B Biointerfaces 2018, 169, 340–347. [Google Scholar] [CrossRef]
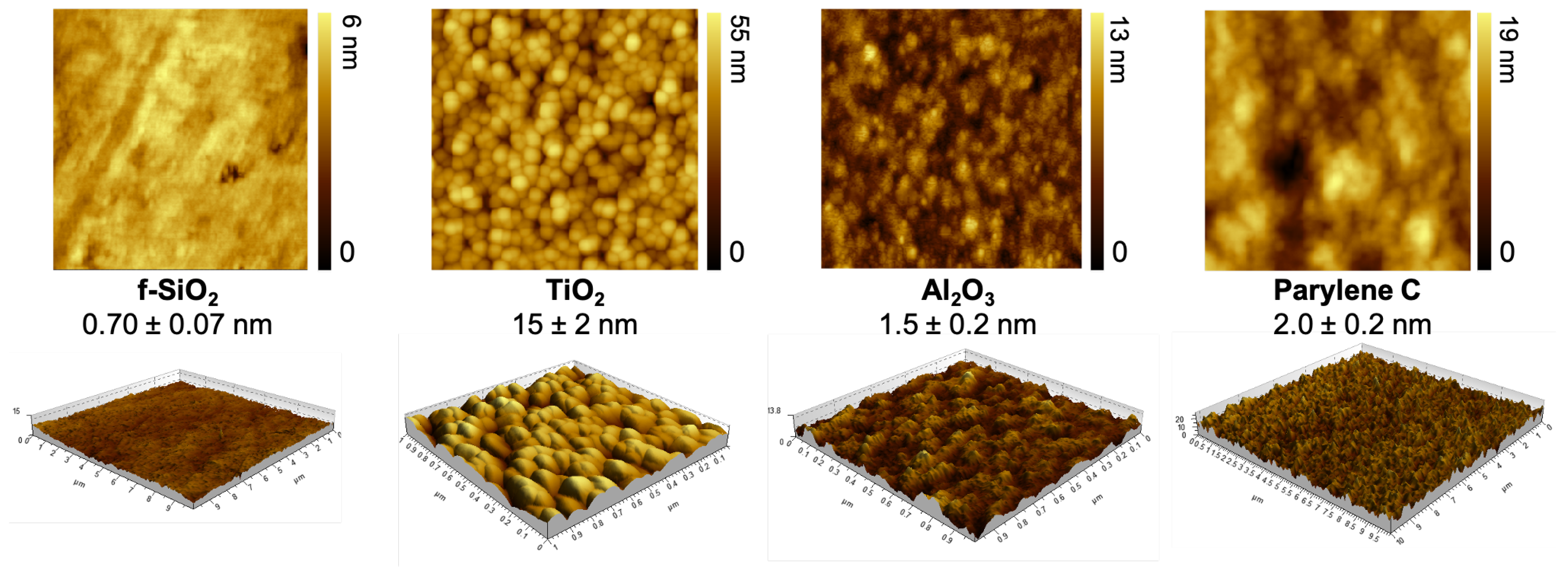


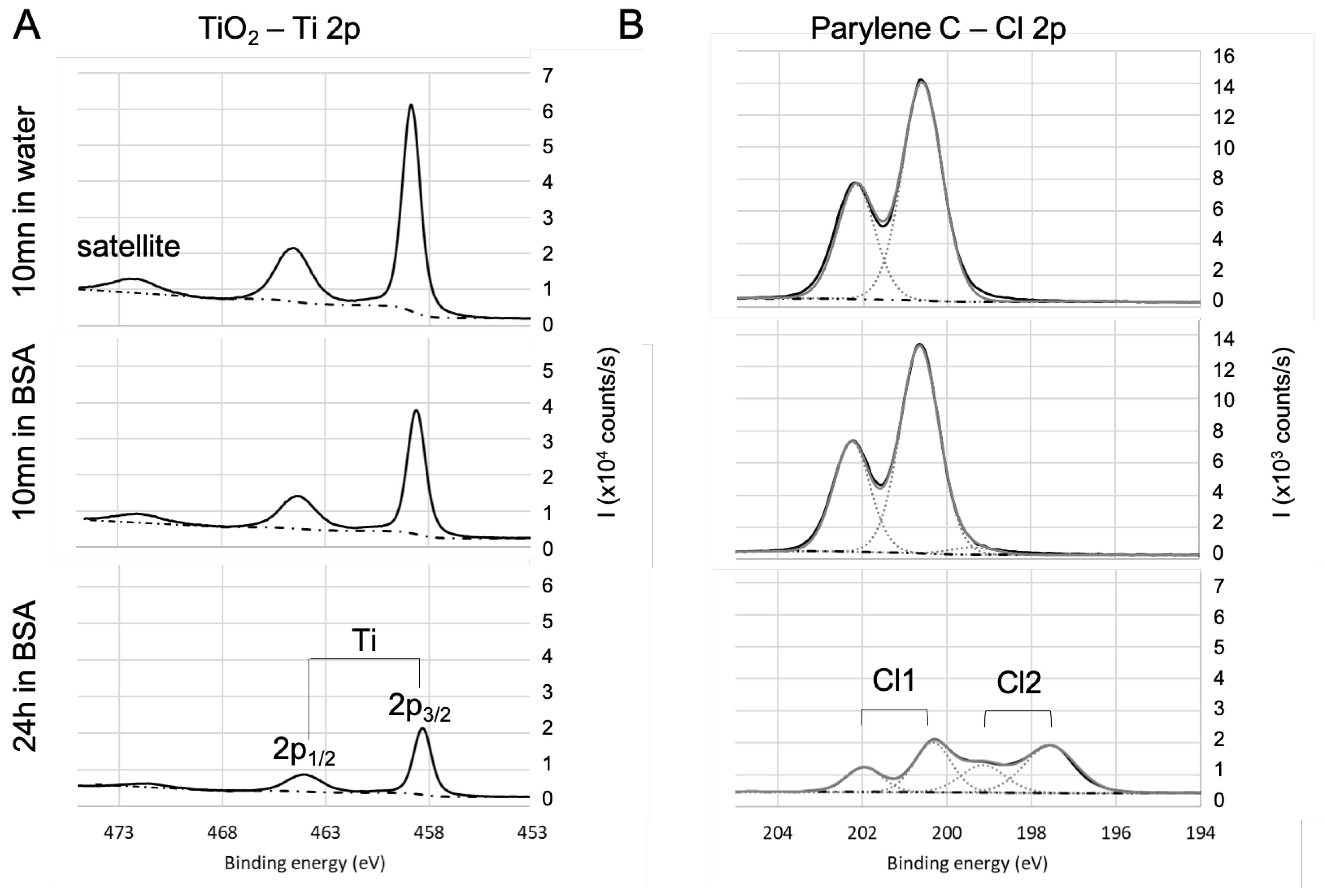
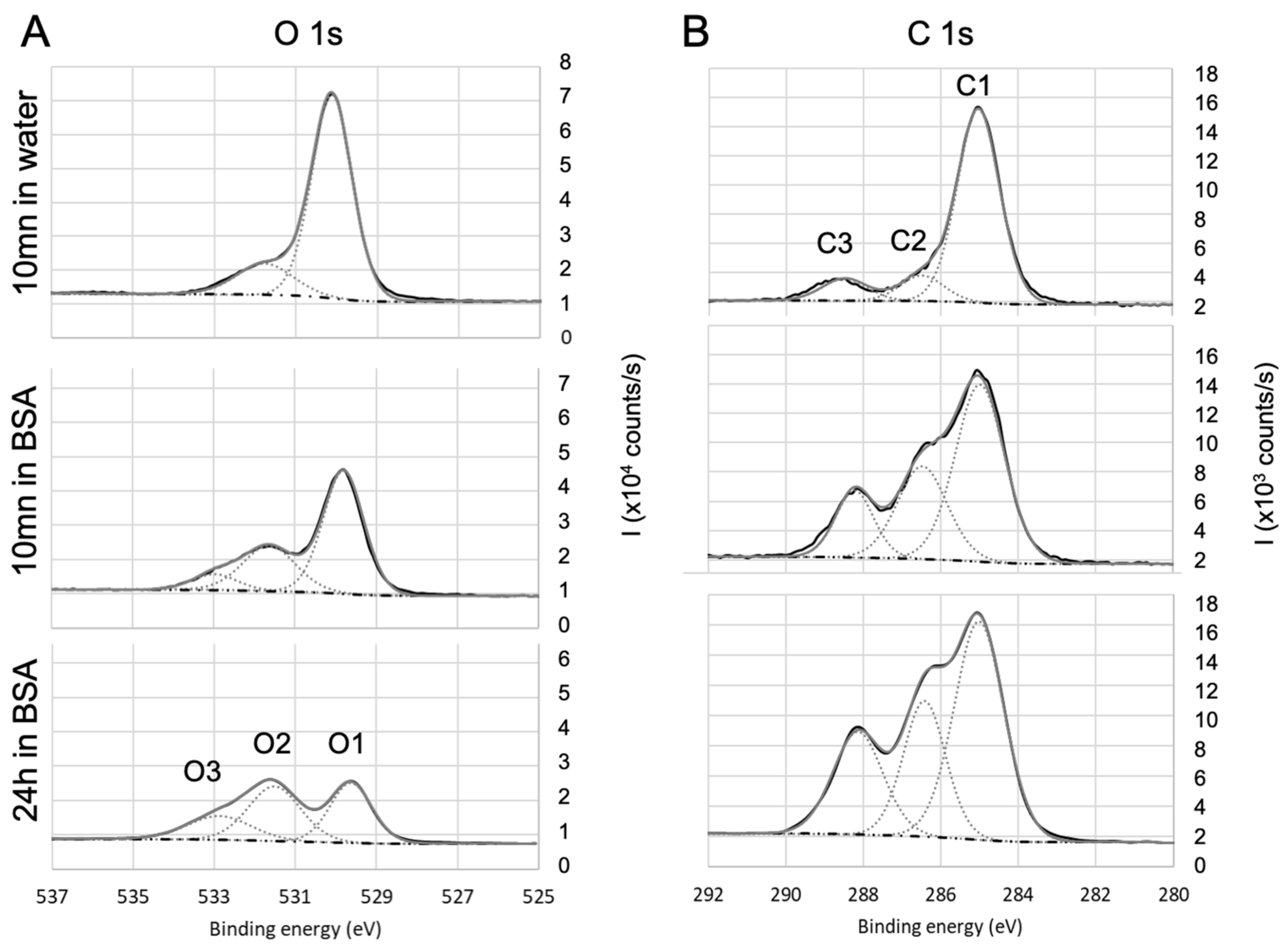
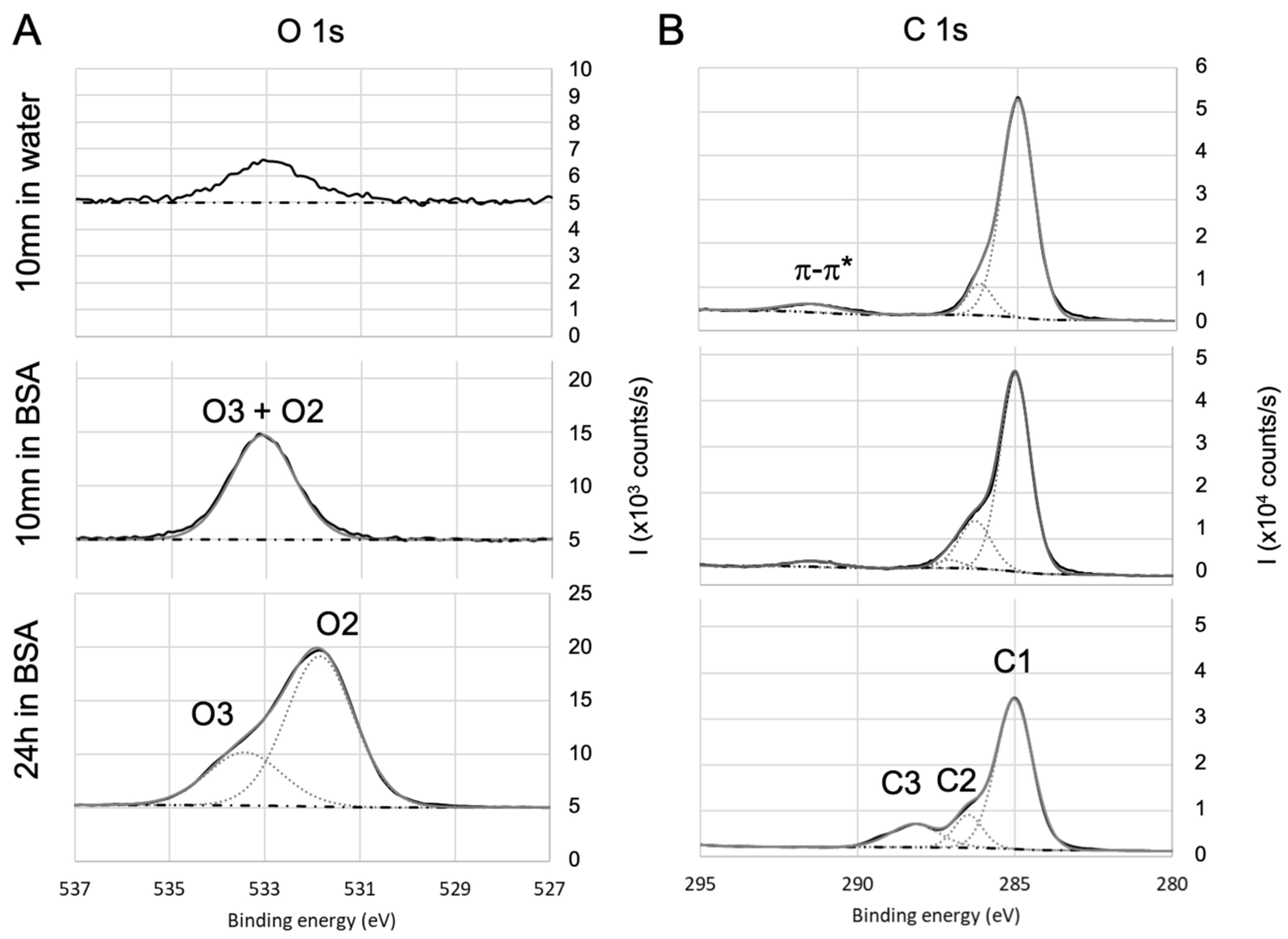
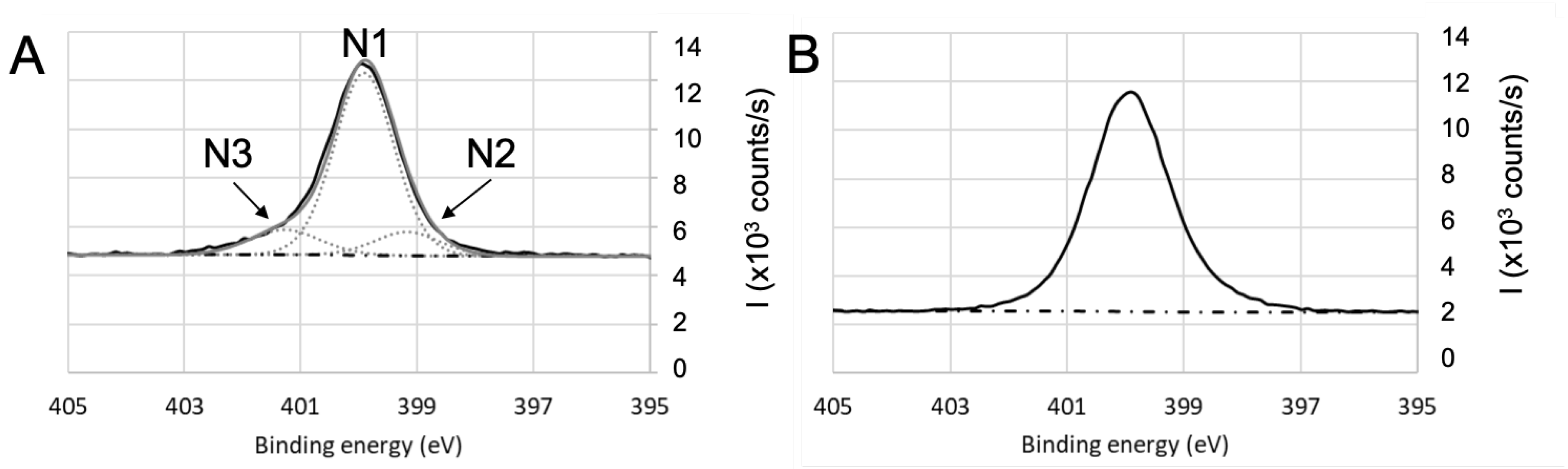
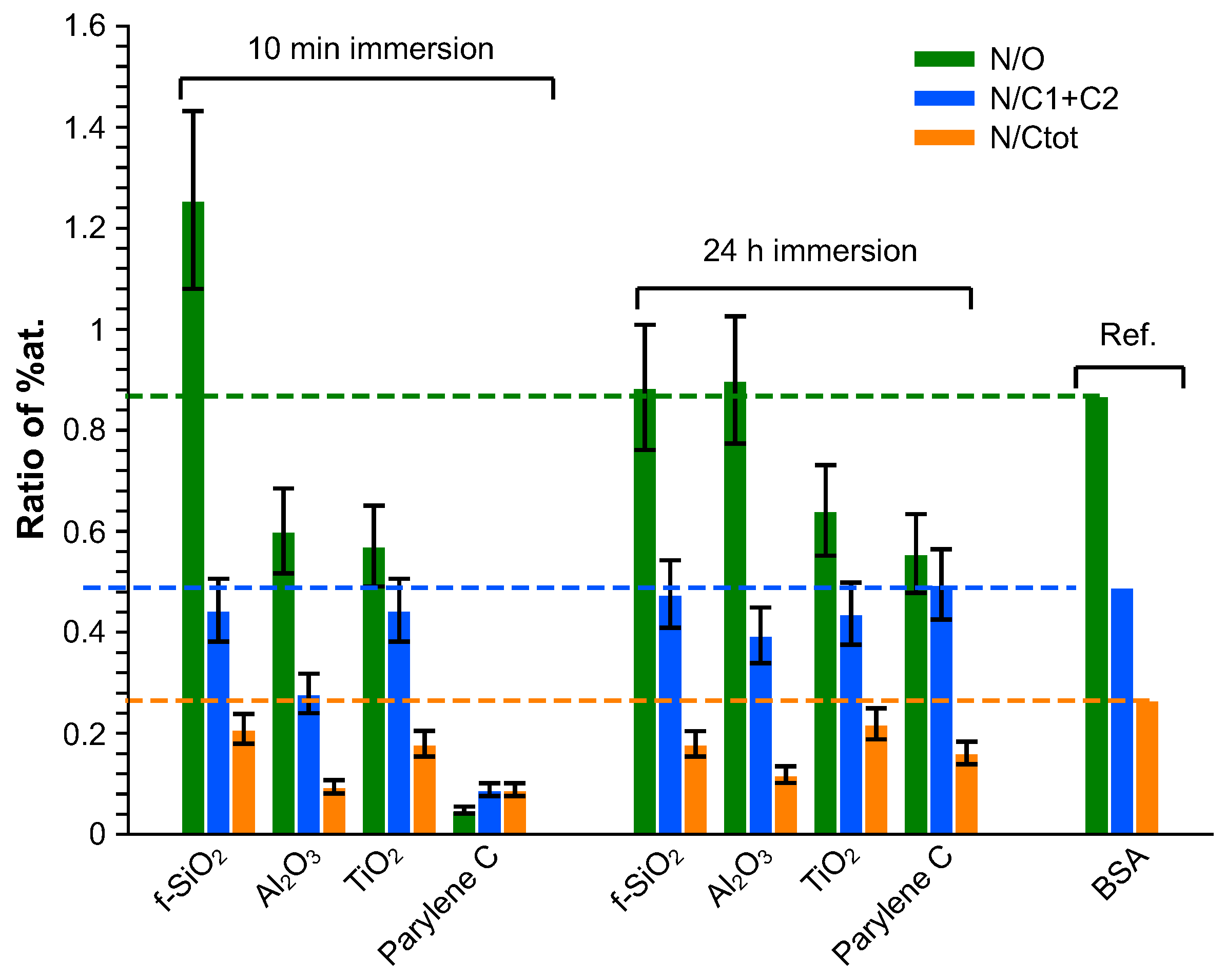
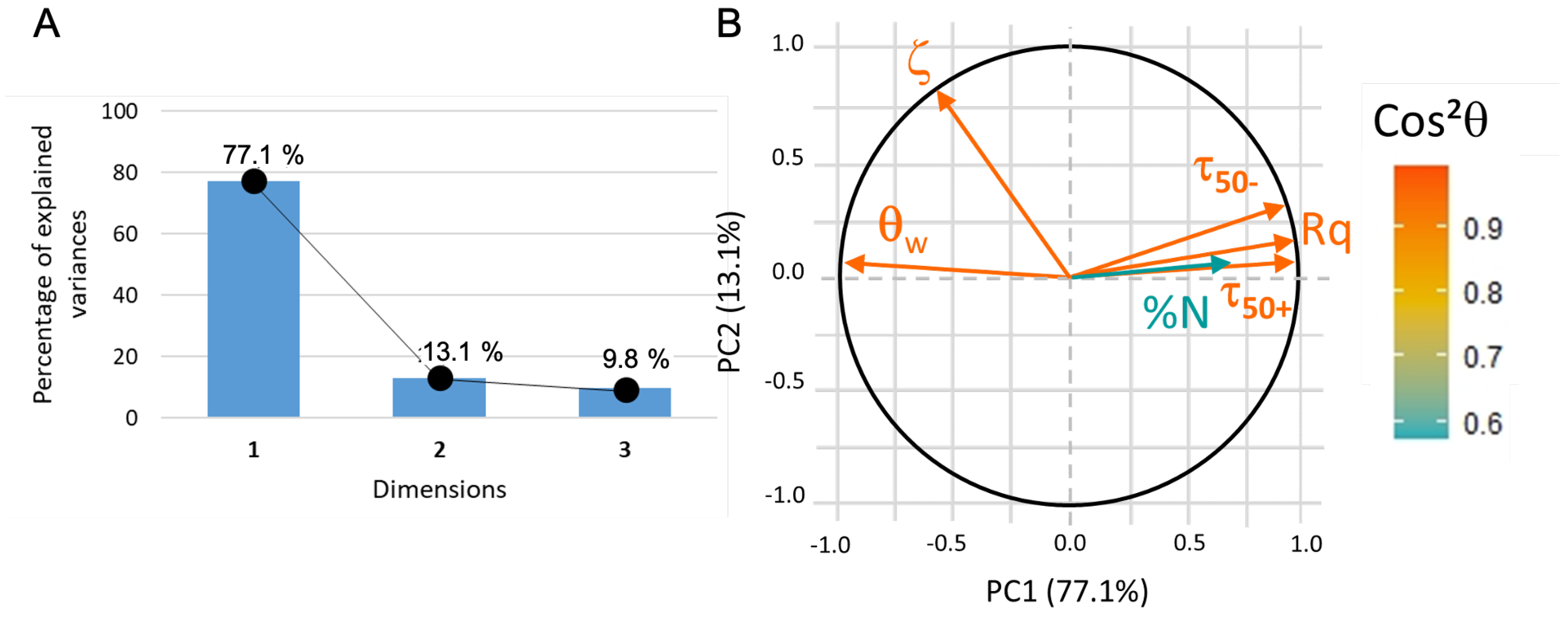
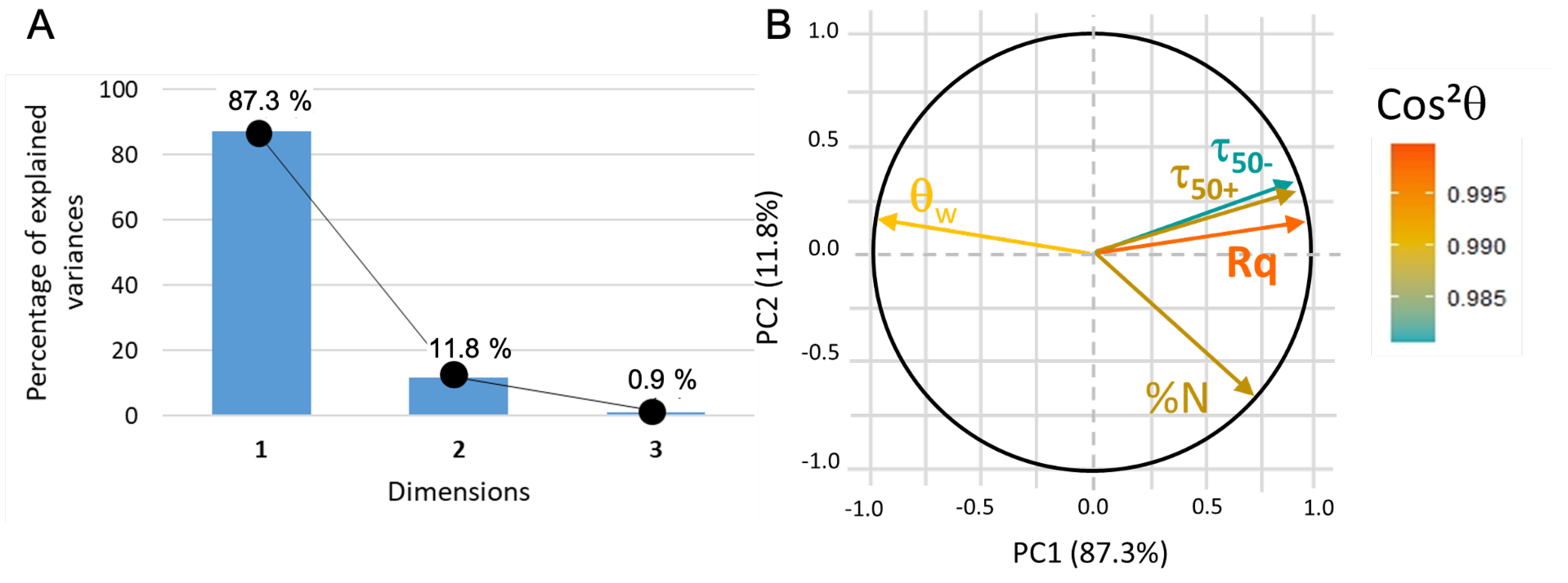

| Coating | Precursor | Deposition Parameters | Reference |
|---|---|---|---|
| Titanium isopropoxide | P = 5 mbar | [66] | |
| d∼300–400 nm | Bubbling at 50 °C under nitrogen flow | T = 400 °C | |
| Aluminium isopropoxide | P = 5 mbar | [67] | |
| d∼300 nm | Direct liquid injection | T = 500 °C | |
| Parylene C | dichloro[2,2]paracyclophane | P = mbar | [68] |
| d∼300 nm | Sublimation at 140 °C then pyrolysis at 670 °C | T room temperature |
| Immersion Time | 0 | 10 min | 24 h |
|---|---|---|---|
| 0.20 ± 0.02 | 6.0 ± 0.2 | 10 ± 1 | |
| 0.45 ± 0.05 | 8.0 ± 0.2 | 11 ± 1 | |
| 0.10 ± 0.02 | 3 ± 1 | 7 ± 1 | |
| Parylene C | 0.20 ± 0.05 | 0.50 ± 0.05 | 9 ± 1 |
| (Pa) | NH(+) | COOH(-) | ||
|---|---|---|---|---|
| bare | +BSA | bare | +BSA | |
| f- | 1.1 ± 0.3 | 23 ± 7 | 5 ± 3 | n/a |
| 17 ± 4 | 9 ± 4 | n/a | n/a | |
| 2 ± 1 | 1.1 ± 0.3 | 24 ± 9 | 9 ± 3 | |
| Parylene C | 5 ± 2 | 3 ± 2 | 22 ± 6 | n/a |
| Surface | (nm) | (mV) | (Pa) | (Pa) | %N (%at.) | |
|---|---|---|---|---|---|---|
| 0.7 ± 0.1 | 63 ± 3 | 34 ± 1 | 1.1 ± 0.3 | 5 ± 3 | 6.0 ± 0.2 | |
| 15 ± 2 | 9 ± 4 | −39 ± 1 | 17 ± 4 | 80 ± 3 | 8.0 ± 0.2 | |
| 1.5 ± 0.2 | 81 ± 4 | −7 ± 1 | 2 ± 1 | 24 ± 9 | 3 ± 1 | |
| Parylene C | 2.0 ± 0.2 | 77 ± 3 | −36 ± 1 | 5 ± 2 | 22 ± 6 | 0.50 ± 0.05 |
| PCA3 | Surface | (mV) | (Pa) | (Pa) | |
|---|---|---|---|---|---|
| 1st step | 63 ± 3 | −34 ± 1 | 1.1 ± 0.3 | 5 ± 3 | |
| 9 ± 4 | −39 ± 1 | 17 ± 4 | 80 ± 3 | ||
| 81 ± 4 | −7 ± 1 | 2 ± 1 | 24 ± 9 | ||
| Parylene C | 77 ± 3 | −36 ± 1 | 5 ± 2 | 22 ± 6 | |
| 2nd step + BSA | 66 ± 4 | −36 ± 1 | 23 ± 7 | 80 ± 3 | |
| 62 ± 4 | −20 ± 1 | 9 ± 4 | 65 ± 3 | ||
| 60 ± 4 | −16 ± 1 | 1.1 ± 0.3 | 9 ± 3 | ||
| Parylene C | 59 ± 6 | −20 ± 1 | 3 ± 2 | 50 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, Z.; Floquet, P.; Combes, D.; Tendero, C.; Castelain, M. Surface Conditioning Effects on Submerged Optical Sensors: A Comparative Study of Fused Silica, Titanium Dioxide, Aluminum Oxide, and Parylene C. Sensors 2023, 23, 9546. https://doi.org/10.3390/s23239546
Nan Z, Floquet P, Combes D, Tendero C, Castelain M. Surface Conditioning Effects on Submerged Optical Sensors: A Comparative Study of Fused Silica, Titanium Dioxide, Aluminum Oxide, and Parylene C. Sensors. 2023; 23(23):9546. https://doi.org/10.3390/s23239546
Chicago/Turabian StyleNan, Zibin, Pascal Floquet, Didier Combes, Claire Tendero, and Mickaël Castelain. 2023. "Surface Conditioning Effects on Submerged Optical Sensors: A Comparative Study of Fused Silica, Titanium Dioxide, Aluminum Oxide, and Parylene C" Sensors 23, no. 23: 9546. https://doi.org/10.3390/s23239546
APA StyleNan, Z., Floquet, P., Combes, D., Tendero, C., & Castelain, M. (2023). Surface Conditioning Effects on Submerged Optical Sensors: A Comparative Study of Fused Silica, Titanium Dioxide, Aluminum Oxide, and Parylene C. Sensors, 23(23), 9546. https://doi.org/10.3390/s23239546







