TRCCBP: Transformer Network for Radar-Based Contactless Continuous Blood Pressure Monitoring
Abstract
:1. Introduction
- 1.
- We present a heartbeat signal-guided single-beat pulse wave extraction method that differs from existing denoising methods. It effectively extracts pure pulse-wave signals and provides a basis for blood pressure estimation.
- 2.
- We propose a transformer-based blood pressure estimation network suitable for processing continuous temporal radar signals. It can automatically capture appropriate time-domain characteristics of radar signals and map them to BP.
- 3.
- We have established an IR-UWB radar signal dataset for blood pressure measurement captured in an indoor environment and a real medical situation, which includes radar signal data and corresponding BP values from 36 persons in total. The proposed TRCCBP source codes and radar signal dataset are available at https://github.com/bupt-uwb/TRCCBP (accessed on 11 October 2023).
2. The Proposed TRCCBP Method
2.1. Signal Model
2.2. Heartbeat Signal-Guided Single-Beat Pulse Wave Extraction
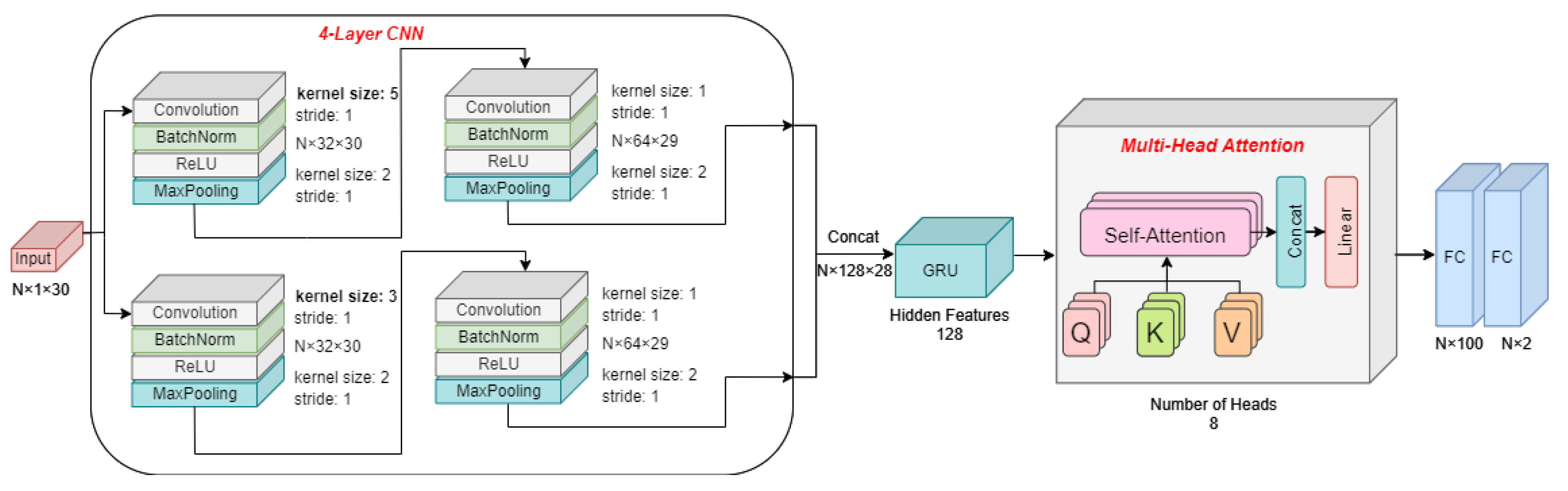
2.3. Transformer Network-Based Blood Pressure Estimation Network
3. Experimental Setting and Dataset
- A.
- Relaxation: The participant was asked to breathe regularly during 30 s for data collection.
- B.
- Apnea: During 30 s for data collection, the participant was asked to hold their breath for 10 s, then breathe regularly for 10 s and hold their breath again for 10 s.
- C.
- Post-exercise: Before data collection, the participant was asked to run for 30 s to produce a hypertension state and then breathe rapidly during 30 s for data collection.
4. Experimental Results and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTT | Pulse Transit Time |
| VMD | Variational Mode Decomposition |
| FC | Fully Connected |
| IR-UWB | Impulse Radio Ultra-Wideband |
| CNN | Convolutional Neural Network |
| BN | BatchNormalization |
| SNR | Signal-to-Noise Ratio |
| MSE | Mean Square Error |
| LSTM | Long Short-Term Memory |
| GRU | Gated Recurrent Unit |
| BP | Blood Pressure |
| DBP | Diastolic Blood Pressure |
| SBP | Systolic Blood Pressure |
References
- Man, P.K.; Cheung, K.L.; Sangsiri, N.; Shek, W.J.; Wong, K.L.; Chin, J.W.; Chan, T.T.; So, R.H.Y. Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring. Healthcare 2022, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Al Fahoum, A.S.; Abu Al-Haija, A.O.; Alshraideh, H.A. Identification of Coronary Artery Diseases Using Photoplethysmography Signals and Practical Feature Selection Process. Bioengineering 2023, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Neha; Sardana, H.; Kanwade, R.; Tewary, S. Arrhythmia detection and classification using ECG and PPG techniques: A review. Phys. Eng. Sci. Med. 2021, 44, 1027–1048. [Google Scholar]
- Ganti, V.G.; Carek, A.M.; Nevius, B.N.; Heller, J.A.; Etemadi, M.; Inan, O.T. Wearable Cuff-Less Blood Pressure Estimation at Home via Pulse Transit Time. IEEE J. Biomed. Health Inform. 2021, 25, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Herranz Olazabal, J.; Wieringa, F.; Hermeling, E.; Van Hoof, C. Comparing Remote Speckle Plethysmography and Finger-Clip Photoplethysmography with Non-Invasive Finger Arterial Pressure Pulse Waves, Regarding Morphology and Arrival Time. Bioengineering 2023, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Mösch, L.; Barz, I.; Müller, A.; Pereira, C.B.; Moormann, D.; Czaplik, M.; Follmann, A. For Heart Rate Assessments from Drone Footage in Disaster Scenarios. Bioengineering 2023, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Sugita, N.; Yoshizawa, M.; Abe, M.; Tanaka, A.; Homma, N.; Yambe, T. Contactless technique for measuring blood-pressure variability from one region in video plethysmography. J. Med. Biol. Eng. 2019, 39, 76–85. [Google Scholar] [CrossRef]
- Fan, X.; Ye, Q.; Yang, X.; Choudhury, S.D. Robust blood pressure estimation using an RGB camera. J. Ambient Intell. Humaniz. Comput. 2020, 11, 4329–4336. [Google Scholar] [CrossRef]
- Rong, M.; Li, K. A blood pressure prediction method based on imaging photoplethysmography in combination with machine learning. Biomed. Signal Process. Control 2021, 64, 102328. [Google Scholar] [CrossRef]
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H.U.; Lee, J.Y.; Wang, X.; Xie, Z.; Kang, D.; Wang, H.; et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl. Acad. Sci. USA 2018, 115, 11144–11149. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.U.; Rwei, A.Y.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.C.; Xie, Z.; Liu, C.; Carlini, A.; Kim, D.H.; et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, B.; Guo, Y. Non-Contact Calibration-Free Blood Pressure Estimation Method Using Dual Radar. In Proceedings of the 2022 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Suzhou, China, 16–18 May 2022; pp. 186–188. [Google Scholar]
- Kuwahara, M.; Yavari, E.; Boric-Lubecke, O. Non-Invasive, Continuous, Pulse Pressure Monitoring Method. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6574–6577. [Google Scholar] [CrossRef]
- Tang, M.C.; Liao, C.M.; Wang, F.K.; Horng, T.S. Noncontact Pulse Transit Time Measurement Using a Single-Frequency Continuous-Wave Radar. In Proceedings of the 2018 IEEE/MTT-S International Microwave Symposium-IMS, Philadelphia, PA, USA, 10–15 June 2018; pp. 1409–1412. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, X.; Hong, H.; Li, Y.; Zhu, X.; Li, C. Non-contact Beat-to-beat Blood Pressure Measurement Using Continuous Wave Doppler Radar. In Proceedings of the 2018 IEEE/MTT-S International Microwave Symposium-IMS, Philadelphia, PA, USA, 10–15 June 2018; pp. 1413–1415. [Google Scholar] [CrossRef]
- Ohata, T.; Ishibashi, K.; Sun, G. Non-Contact Blood Pressure Measurement Scheme Using Doppler Radar. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 778–781. [Google Scholar] [CrossRef]
- Schrumpf, F.; Frenzel, P.; Aust, C.; Osterhoff, G.; Fuchs, M. Assessment of non-invasive blood pressure prediction from ppg and rppg signals using deep learning. Sensors 2021, 21, 6022. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.U.; Lim, K.M. Combined deep CNN–LSTM network-based multitasking learning architecture for noninvasive continuous blood pressure estimation using difference in ECG-PPG features. Sci. Rep. 2021, 11, 13539. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.F.; Chiu, L.W.; Wu, Y.C.; Lai, C.C.; Chu, P.H. Contactless Blood Pressure Measurement via Remote Photoplethysmography with Synthetic Data Generation Using Generative Adversarial Network. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 2130–2138. [Google Scholar]
- Ishizaka, S.; Yamamoto, K.; Ohtsuki, T. Non-contact Blood Pressure Measurement using Doppler Radar based on Waveform Analysis by LSTM. In Proceedings of the ICC 2021-IEEE International Conference on Communications, Montreal, QC, Canada, 14–23 June 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, W. Wave Height Estimation From X-Band Radar Data Using Variational Mode Decomposition. IEEE Geosci. Remote Sens. Lett. 2022, 19, 1505405. [Google Scholar] [CrossRef]







| Demographics | Gender | Age | Height (cm) | Weight (kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | >24 | ≤24 | >175 | ≤175 | >70 | ≤70 | |
| Num | 20 | 11 | 10 | 21 | 16 | 15 | 18 | 13 |
| Methods (MAE) | A | B | C | D | E |
|---|---|---|---|---|---|
| SBP (mmHg) | 4.73 | 5.19 | 6.49 | 6.96 | 8.05 |
| DBP (mmHg) | 4.49 | 4.82 | 5.21 | 5.54 | 6.43 |
| Dataset 1 | Dataset 2 | Dataset 3 | |||||
|---|---|---|---|---|---|---|---|
| Method | TRCCBP | T.Ohata | TRCCBP | T.Ohata | TRCCBP | T.Ohata | |
| SBP(mmHg) | MAE | 4.73 | 13.53 | 5.97 | 14.14 | 7.75 | 17.20 |
| STD | 5.52 | 12.64 | 6.31 | 13.82 | 6.40 | 15.20 | |
| DBP(mmHg) | MAE | 4.49 | 16.85 | 5.88 | 15.97 | 6.85 | 11.88 |
| STD | 5.25 | 16.55 | 6.12 | 16.35 | 6.35 | 10.98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhang, J.; Mu, W.; Wang, K.; Li, L.; Zhang, L. TRCCBP: Transformer Network for Radar-Based Contactless Continuous Blood Pressure Monitoring. Sensors 2023, 23, 9680. https://doi.org/10.3390/s23249680
Jiang X, Zhang J, Mu W, Wang K, Li L, Zhang L. TRCCBP: Transformer Network for Radar-Based Contactless Continuous Blood Pressure Monitoring. Sensors. 2023; 23(24):9680. https://doi.org/10.3390/s23249680
Chicago/Turabian StyleJiang, Xikang, Jinhui Zhang, Wenyao Mu, Kun Wang, Lei Li, and Lin Zhang. 2023. "TRCCBP: Transformer Network for Radar-Based Contactless Continuous Blood Pressure Monitoring" Sensors 23, no. 24: 9680. https://doi.org/10.3390/s23249680
APA StyleJiang, X., Zhang, J., Mu, W., Wang, K., Li, L., & Zhang, L. (2023). TRCCBP: Transformer Network for Radar-Based Contactless Continuous Blood Pressure Monitoring. Sensors, 23(24), 9680. https://doi.org/10.3390/s23249680






