Microfluidic Chip with Fiber-Tip Sensors for Synchronously Monitoring Concentration and Temperature of Glucose Solutions
Abstract
1. Introduction
2. Principles and Fabrication
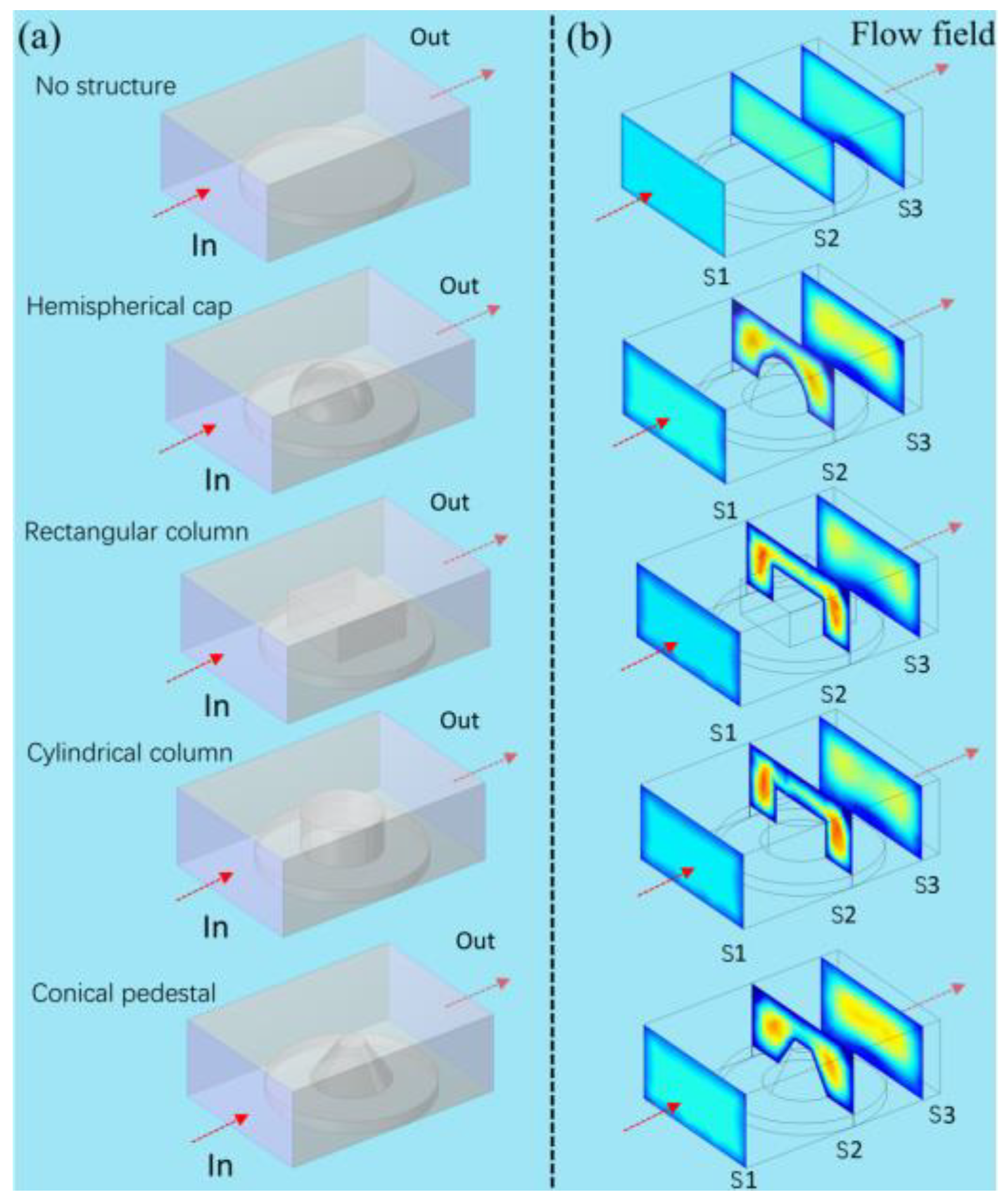
2.1. Principles
2.2. Fabrication
3. Results and Discussion
4. Performance Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavaniol, C.; Cesar, W.; Descroix, S.; Viovy, J.L. Flowmetering for microfluidics. Lab Chip 2022, 22, 3603–3617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, X.; Zuo, Y.; Hu, X.; Shi, Y.; Liang, L.; Yang, Y. Optofluidics: The interaction between light and flowing liquids in integrated devices. Opto-Electron. Adv. 2019, 2, 5–14. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Surdo, S.; Merlo, S.; Carpignano, F.; Strambini, L.M.; Trono, C.; Giannetti, A.; Barillaro, G. Optofluidic microsystems with integrated vertical one-dimensional photonic crystals for chemical analysis. Lab Chip 2012, 12, 4403–4415. [Google Scholar] [CrossRef]
- Tang, J.; Cao, X.; Qiu, G.; de Mello, A.; Wang, J. Optical-switch-enabled microfluidics for sensitive multichannel colorimetric analysis. Anal. Chem. 2021, 93, 6784–6791. [Google Scholar] [CrossRef]
- Agnihotri, S.N.; Raveshi, M.R.; Bhardwaj, R.; Neild, A. Microfluidic valves for selective on-chip droplet splitting at multiple sites. Langmuir 2020, 36, 1138–1146. [Google Scholar] [CrossRef]
- Rodriguez-Trujillo, R.; Castillo-Fernandez, O.; Garrido, M.; Arundell, M.; Valencia, A.; Gomila, G. High-speed particle detection in a micro-Coulter counter with two-dimensional adjustable aperture. Biosens. Bioelectron. 2008, 24, 290–296. [Google Scholar] [CrossRef]
- Yan, S.C.; Liu, Z.Y.; Li, C.; Ge, S.J.; Xu, F.; Lu, Y.Q. “Hot-wire” microfluidic flowmeter based on a microfiber coupler. Opt. Lett. 2016, 41, 5680–5683. [Google Scholar] [CrossRef]
- Li, J.; de Ávila, B.E.F.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, 4. [Google Scholar] [CrossRef]
- Ma, Z.C.; Zhang, Y.L.; Han, B.; Hu, X.Y.; Li, C.H.; Chen, Q.D.; Sun, H.B. Femtosecond laser programmed artificial musculoskeletal systems. Nat. Commun. 2020, 11, 4536. [Google Scholar] [CrossRef]
- Wei, S.; Yu-Qing, C.; Guo-An, L.; Zhang, M.; Zhang, H.Y.; Yue-Rong, W.; Ping, H. Organs-on-chips and its applications. Chin. J. Anal. Chem. 2016, 44, 533–541. [Google Scholar]
- Wikswo, J.P.; Curtis, E.L.; Eagleton, Z.E.; Evans, B.C.; Kole, A.; Hofmeister, L.H.; Matloff, W.J. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013, 13, 3496–3511. [Google Scholar] [CrossRef]
- Zhao, Y.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Multi-organs-on-chips: Towards long-term biomedical investigations. Molecules 2019, 24, 675. [Google Scholar] [CrossRef] [PubMed]
- Palaninathan, V.; Kumar, V.; Maekawa, T.; Liepmann, D.; Paulmurugan, R.; Eswara, J.R.; Kumar, D.S. Multi-organ on a chip for personalized precision medicine. Mrs. Commun. 2018, 8, 652–667. [Google Scholar] [CrossRef]
- Ni, X.; Wang, M.; Guo, D.; Hao, H.; Zhu, J. A hybrid Mach–Zehnder interferometer for refractive index and temperature measurement. IEEE Photonic. Tech. Lett. 2016, 28, 1850–1853. [Google Scholar] [CrossRef]
- Zhao, J.R.; Huang, X.G.; He, W.X.; Chen, J.H. High-resolution and temperature-insensitive fiber optic refractive index sensor based on Fresnel reflection modulated by Fabry–Perot interference. J. Lightw. Technol. 2010, 28, 2799–2803. [Google Scholar] [CrossRef]
- Zhou, X.; Li, S.; Li, X.; Yan, X.; Zhang, X.; Wang, F.; Cheng, T. High-sensitivity SPR temperature sensor based on hollow-core fiber. IEEE T. Instrum. Meas. 2020, 69, 8494–8499. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber. Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Liu, Y.G.; Liu, X.; Zhang, T.; Zhang, W. Integrated FPI-FBG composite all-fiber sensor for simultaneous measurement of liquid refractive index and temperature. Opt. Laser. Eng. 2018, 111, 167–171. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Luo, J.; Chen, Y.; Fu, C.; Xiong, C.; Wang, Y. Torsion, refractive index, and temperature sensors based on an improved helical long period fiber grating. J. Lightw. Technol. 2020, 38, 2504–2510. [Google Scholar] [CrossRef]
- Zhong, N.; Wu, Y.; Wang, Z.; Chang, H.; Zhong, D.; Xu, Y.; Huang, L. Monitoring microalgal biofilm growth and phenol degradation with fiber-optic sensors. Anal. Chem. 2019, 91, 15155–15162. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Huang, T.; Yuan, Z.; Yang, M.; Huang, Y.; Xiao, P.; Guan, B.O. Ultrasensitive optofluidic interferometer for online monitoring of photocatalytic reactions. J. Lightw. Technol. 2019, 37, 5435–5441. [Google Scholar] [CrossRef]
- Zhu, J.M.; Shi, Y.; Zhu, X.Q.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Hanc, X.T. Optofluidic marine phosphate detection with enhanced absorption using a Fabry–Pérot resonator. Lab Chip 2017, 17, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. Glucose metabolism and hyperglycemia. Am. J. Clin. Nutr. 2008, 87, 217–222. [Google Scholar] [CrossRef]
- Brufsky, A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J. Med. Virol. 2020, 92, 770–775. [Google Scholar] [CrossRef]
- Yu, H.; Chong, Y.; Zhang, P.; Ma, J.; Li, D. D-shaped fiber SPR sensor with a composite nanostructure of MoS2-graphene for glucose detection. Talanta 2020, 219, 121324. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Singh, R.; Zhang, W.; Marques, C.; Xie, Y.; Kumar, S. Graphene Oxide/Multiwalled Carbon Nanotubes Assisted Serial Quadruple Tapered Structure-Based LSPR Sensor for Glucose Detection. IEEE Sens. J. 2022, 22, 16904–16911. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, V.K. Review on recent experimental SPR/LSPR based fiber optic analyte sensors. Opt. Fiber. Technol. 2021, 64, 102580. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Gong, D.; Hu, W.; Yang, M. A SPR glucose sensor based on immobilized glucose oxidases and silica mesocellular foams. IEEE Sens. J. 2018, 18, 2229–2235. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Qu, S.; Li, Y. Fiber-optic sensor tip for measuring temperature and liquid refractive index. Opt. Eng. 2014, 53116110. [Google Scholar] [CrossRef]
- Hou, L.; Li, Y.; Fu, Y.; Yang, J.; Xu, W.; Song, X.; Li, J.; Liu, Y.; Ran, L. Ultra-Sensitive Optical Fiber Humidity Sensor via Au-Film-Assisted Polyvinyl Alcohol Micro-Cavity and Vernier Effect. IEEE T. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Yeh, Y.L. Real-time measurement of glucose concentration and average refractive index using a laser interferometer. Opt. Laser. Eng. 2008, 46, 666–670. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Jiang, H. Quantitative reconstruction of refractive index distribution and imaging of glucose concentration by using diffusing light. Appl. Opt. 2006, 45, 8360–8365. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express. 2008, 16, 1020–1028. [Google Scholar] [CrossRef]
- Wu, C. S-shaped long period fiber grating glucose concentration biosensor based on immobilized glucose oxidase. Optik 2020, 203, 163960. [Google Scholar] [CrossRef]
- Sridevi, S.; Vasu, K.S.; Sampath, S.; Asokan, S.; Sood, A.K. Optical detection of glucose and glycated hemoglobin using etched fiber Bragg gratings coated with functionalized reduced graphene oxide. J. Biophotonics 2016, 9, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Badmos, A.A.; Sun, Q.; Sun, Z.; Zhang, J.; Yan, Z.; Lutsyk, P.; Rozhin, A.; Zhang, L. Enzyme-functionalized thin-cladding long-period fiber grating in transition mode at dispersion turning point for sugar-level and glucose detection. J. Biomed. Opt. 2017, 22, 27003. [Google Scholar] [CrossRef]
- Lidiya, A.E.; Raja, R.V.J.; Pham, V.D.; Ngo, Q.M.; Vigneswaran, D. Detecting hemoglobin content blood glucose using surface plasmon resonance in D-shaped photonic crystal fiber. Opt. Fiber Technol. 2019, 50, 132–138. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, X.; Gong, D.; Liu, F.; Hu, W.; Cai, W.; Huang, J.; Yang, M. Investigation for terminal reflection optical fiber SPR glucose sensor and glucose sensitive membrane with immobilized GODs. Opt. Express. 2017, 25, 3884–3898. [Google Scholar] [CrossRef]
- Novais, S.; Ferreira, C.I.A.; Ferreira, M.S.; Pinto, J.L. Optical fiber tip sensor for the measurement of glucose aqueous solutions. IEEE Photon. J. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Wu, C.; Chiang, C. Glucose sensor using U-shaped optical fiber probe with gold nanoparticles and glucose oxidase. Sensors 2018, 18, 1217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, H.; Gan, L.; Liu, Q.; Yan, Z.; Liu, D.; Sun, Q. Immobilized optical fiber microprobe for selective and high sensitiveglucose detection. Sens. Actuators B 2018, 255, 3004–3010. [Google Scholar] [CrossRef]






| Sensor Principle | Core Structure | Detection Limit (M) | Range (mM) | Sensitivity | Type | Reference |
|---|---|---|---|---|---|---|
| Mode filter | LFBG | 0.139 | 0–10 | 0.64 dB/(g/L) | Transmission | [35] |
| FBG | 1 × 10−9 | 0–10 | − | Reflection | [36] | |
| TFG | 0.02 | 0–150 | 1.33 nm/(g/L) | Transmission | [37] | |
| SPR | D-type PCF | – | 0–100 | 0.83 nm/(g/L) | Transmission | [38] |
| MMF | 7.89 × 10−4 | 0–80 | 14 nm/(g/L) | Transmission | [39] | |
| Mode interference | MMF+SMF | 0.189 | 0–450 | 0.0267 nm/(g/L) | Transmission | [40] |
| U-shape Fiber | 0.011 | 1–5 | 3.123 nm/(g/L) | Transmission | [41] | |
| Single Cone SMF | 0.278 | 0–300 | 1.74 nm/(g/L) | Reflection | [42] | |
| Polymer-cavity FP | 2.8 × 10−4 | 0–6.6 | −0.678 dB/(g/L) | Reflection | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Liu, Y.; Li, Y.; Li, J.; Meng, S. Microfluidic Chip with Fiber-Tip Sensors for Synchronously Monitoring Concentration and Temperature of Glucose Solutions. Sensors 2023, 23, 2478. https://doi.org/10.3390/s23052478
Qu J, Liu Y, Li Y, Li J, Meng S. Microfluidic Chip with Fiber-Tip Sensors for Synchronously Monitoring Concentration and Temperature of Glucose Solutions. Sensors. 2023; 23(5):2478. https://doi.org/10.3390/s23052478
Chicago/Turabian StyleQu, Jian, Yi Liu, Yan Li, Jinjian Li, and Songhe Meng. 2023. "Microfluidic Chip with Fiber-Tip Sensors for Synchronously Monitoring Concentration and Temperature of Glucose Solutions" Sensors 23, no. 5: 2478. https://doi.org/10.3390/s23052478
APA StyleQu, J., Liu, Y., Li, Y., Li, J., & Meng, S. (2023). Microfluidic Chip with Fiber-Tip Sensors for Synchronously Monitoring Concentration and Temperature of Glucose Solutions. Sensors, 23(5), 2478. https://doi.org/10.3390/s23052478





