Colorimetric Sensors for Chemical and Biological Sensing Applications
Abstract
:1. Introduction
2. Discussion
2.1. Classifications of Colorimetric Sensors
2.1.1. Graphene and Its Derivatives-Based Colorimetric Sensors

2.1.2. Metal and Metal Oxide Nanoparticles Colorimetric Sensors
Metal and Metal Oxide Nanoparticles Based on Nanozymes-like Characteristics
Metal Nanoparticles Based on Localized Surface Plasmon Resonance

2.1.3. DNA Nanomaterial-Based Colorimetric Sensors
G-Quadruplex-Based Colorimetric Sensors
DNA Tetrahedron-Based Colorimetric Sensors
2.1.4. Other Types of Colorimetric Sensors
2.2. Summary on the Colorimetric Sensors for Chemical and Biological Sensing Applications
2.2.1. Detection of Metallic and Non-Metallic Ions
2.2.2. Detection of Proteins
2.2.3. Detection of Small Molecules
2.2.4. Detection of Gas
2.2.5. Detection of Virus or Bacteria
2.2.6. Detection of DNA/RNA
3. Conclusions—Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, Y.; Lin, H.; Chen, Y.; Liu, Z.; Zhuang, X.; Tian, C.; Fu, X.; Chen, L. Label-free exonuclease I-assisted signal amplification colorimetric sensor for highly sensitive detection of kanamycin. Food Chem. 2021, 347, 128988–128993. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, B.; Wei, G. Two-Dimensional Material-Based Colorimetric Biosensors: A Review. Biosensors 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pu, H.; Sun, D.W. DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021, 61, 2277–2296. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.; Panacek, D.; Jayaramulu, K.; Pykal, M.; Frebort, I.; Kolar, M.; Hajduch, M.; Zboril, R.; Otyepka, M. Human virus detection with graphene-based materials. Biosens. Bioelectron. 2020, 166, 112436–112456. [Google Scholar] [CrossRef] [PubMed]
- Siripongpreda, T.; Siralertmukul, K.; Rodthongkum, N. Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide. Food Chem. 2020, 329, 127165–127171. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Neeroli Kizhakayil, R. Graphene-Rhodamine Nanoprobe for Colorimetric and Fluorimetric Hg2+ Ion Assay. ACS Appl. Mater. Interfaces 2016, 8, 14125–14132. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Mehdinia, A.; Jabbari, A. Seed-mediated grown silver nanoparticles as a colorimetric sensor for detection of ascorbic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 180, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Xu, Z.; Liu, D. Multichannel Stimulus-Responsive Nanoprobes for H2O2 Sensing in Diverse Biological Milieus. Anal. Chem. 2020, 92, 12639–12646. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.H.; Jeong, S.M.; Son, K.Y.; Lim, S.K. Colorimetric hydrogen gas sensor based on PdO/metal oxides hybrid nanoparticles. Talanta 2018, 188, 356–364. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Liu, S.; Zheng, L.; Bu, Y.; Deng, H.; Chen, R.; Peng, H.; Lin, X.; Chen, W. Colorimetric acid phosphatase sensor based on MoO3 nanozyme. Anal. Chim. Acta 2020, 1105, 162–168. [Google Scholar] [CrossRef]
- Thanomsak, S.; Insombat, C.; Chaiyo, P.; Tuntulani, T.; Janrungroatsakul, W. Fabrication of a paper-based sensor from graphene quantum dots coated with a polymeric membrane for the determination of gold(III) ions. Anal. Methods 2021, 13, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Kazemzad, M. Quantum dots based sensitive nanosensors for detection of antibiotics in natural products: A review. Sci. Total Environ. 2022, 810, 151997–152019. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; He, N.; Li, Z. Recent progresses in DNA nanostructure-based biosensors for detection of tumor markers. Biosens. Bioelectron. 2018, 109, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Liu, S.; Yang, X.; He, X.; Huang, J.; Wang, K. DNA tetrahedron nanostructures for biological applications: Biosensors and drug delivery. Analyst 2017, 142, 3322–3332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Jabbari, A. Intrinsic peroxidase-like activity of graphene nanoribbons for label-free colorimetric detection of dopamine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111034–111044. [Google Scholar] [CrossRef]
- Qiu, J.; Li, Z.; Miao, L.; Wang, H.; Zhang, Y.; Wu, S.; Zhang, Y.; Li, X.; Wu, A. Colorimetric detection of Ba2+, Cd2+ and Pb2+ based on a multifunctionalized Au NP sensor. Analyst 2019, 144, 5081–5089. [Google Scholar] [CrossRef]
- Yin, B.; Zheng, W.; Dong, M.; Yu, W.; Chen, Y.; Joo, S.W.; Jiang, X. Enzyme-mediated competitive colorimetric sensor based on Au@Ag bimetallic nanoparticles for highly sensitive detection of disease biomarkers. Analyst 2017, 142, 2954–2960. [Google Scholar] [CrossRef]
- Ji, G.; Tian, J.; Xing, F.; Feng, Y. Optical Biosensor Based on Graphene and Its Derivatives for Detecting Biomolecules. Int. J. Mol. Sci. 2022, 23, 10838. [Google Scholar] [CrossRef]
- Gao, X.G.; Cheng, L.X.; Jiang, W.S.; Li, X.K.; Xing, F. Graphene and its Derivatives-Based Optical Sensors. Front. Chem. 2021, 9, 615164–615177. [Google Scholar] [CrossRef]
- Chouhan, A.; Mungse, H.P.; Khatri, O.P. Surface chemistry of graphene and graphene oxide: A versatile route for their dispersion and tribological applications. Adv. Colloid. Interface Sci. 2020, 283, 102215–102242. [Google Scholar] [CrossRef] [PubMed]
- Danial, W.H.; Md Bahri, N.F.; Abdul Majid, Z. Preparation, Marriage Chemistry and Applications of Graphene Quantum Dots-Nanocellulose Composite: A Brief Review. Molecules 2021, 26, 6158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, A.; Wei, G. Graphene-based aptasensors: From molecule-interface interactions to sensor design and biomedical diagnostics. Analyst 2018, 143, 1526–1543. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme Cofactor-Resembling Fe–N Single Site Embedded Graphene as Nanozymes to Selectively Detect H2O2 with High Sensitivity. Adv. Funct. Mater. 2019, 30, 1905410–1905419. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. Engl. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuators B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Liang, A.; Li, C.; Li, D.; Luo, Y.; Wen, G.; Jiang, Z. A facile and sensitive peptide-modulating graphene oxide nanoribbon catalytic nanoplasmon analytical platform for human chorionic gonadotropin. Int. J. Nanomed. 2017, 12, 8725–8734. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef]
- Tian, H.; Liu, J.; Guo, J.; Cao, L.; He, J. (L)-Cysteine functionalized graphene oxide nanoarchitectonics: A metal-free Hg2+ nanosensor with peroxidase-like activity boosted by competitive adsorption. Talanta 2022, 242, 123320–123328. [Google Scholar] [CrossRef]
- Hai, X.; Li, Y.; Yu, K.; Yue, S.; Li, Y.; Song, W.; Bi, S.; Zhang, X. Synergistic in-situ growth of silver nanoparticles with nanozyme activity for dual-mode biosensing and cancer theranostics. Chin. Chem. Lett. 2021, 32, 1215–1219. [Google Scholar] [CrossRef]
- Zhu, X.; Li, T.; Hai, X.; Bi, S. A nanozyme-based colorimetric sensor array as electronic tongue for thiols discrimination and disease identification. Biosens. Bioelectron. 2022, 213, 114438–114446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, K.; Zhang, H.; Zeng, L.; Zhou, Y.; Jing, T. Preparation of molecularly imprinted polymers on hemin-graphene surface for recognition of high molecular weight protein. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105–112, 110141–110148. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, J.; Liu, W.E.; Guo, Y.; Wu, G.; Qi, W.; Lu, X. Enhanced His@AuNCs oxidase-like activity by reduced graphene oxide and its application for colorimetric and electrochemical detection of nitrite. Anal. Bioanal. Chem. 2019, 411, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600. [Google Scholar] [CrossRef]
- Qi, Y.; Song, D.; Chen, Y. Colorimetric oligonucleotide-based sensor for ultra-low Hg2+ in contaminated environmental medium: Convenience, sensitivity and mechanism. Sci. Total Environ. 2021, 766, 142579–142588. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174–101184. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ramezani, M.; Darroudi, M. Bio-sensing applications of cerium oxide nanoparticles: Advantages and disadvantages. Biosens. Bioelectron. 2017, 96, 33–43. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic nanoparticles in developing electrochemical sensors for pharmaceutical and biomedical applications. Talanta 2021, 226, 1222108. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Peng, J.; Liu, G.; Yuan, D.; Feng, S.; Zhou, T. A flow-batch manipulated Ag NPs based SPR sensor for colorimetric detection of copper ions (Cu2+) in water samples. Talanta 2017, 167, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Vinita; Prakash, R. Quick colorimetric determination of choline in milk and serum based on the use of MoS2 nanosheets as a highly active enzyme mimetic. Mikrochim. Acta 2018, 185, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, Y.; Sun, Y.; Lu, Y.; Tian, E.; Lan, J.; Li, J.; Ye, W.; Zhang, H. Colorimetric determination of Hg2+ in environmental water based on the Hg2+-stimulated peroxidase mimetic activity of MoS2-Au composites. J. Colloid Interface Sci. 2019, 537, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.; Sicwetsha, S.; Mashazi, P. Nanomagnet-Silica Nanoparticles Decorated with Au@Pd for Enhanced Peroxidase-like Activity and Colorimetric Glucose Sensing. ACS Appl. Mater. Interfaces 2020, 12, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, K.; Arthi, C.; Pavithra, A.J.; Poovizhi, P.; Antinate, S.S.; Hikku, G.S.; Jeyasubramanian, K.; Murugesan, R. Bioconjugated gold nanoparticles as an efficient colorimetric sensor for cancer diagnostics. Photodiagnosis Photodyn. Ther. 2020, 30, 101699–101708. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Roy, P.; Karmodak, N.; Jemmis, E.D.; Mugesh, G. Nanoisozymes: Crystal Facet-Dependent Enzyme-Mimetic Activity of V2O5 Nanomaterials. Angew. Chem. Int. Ed. Engl. 2018, 57, 4510–4515. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, Y.; Chen, S.; Zhou, Z.; Tao, W.; Li, C.; Feng, Y.; Wang, F. Co-N-C single-atom nanozymes with oxidase-like activity for highly sensitive detection of biothiols. Anal. Bioanal. Chem. 2022, 414, 1857–1865. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, D.; Jiang, Y.; Ni, P.; Zhang, C.; Wang, B.; Yang, F.; Lu, Y.; Sun, J. Logically Regulating Peroxidase-like Activity of Gold Nanoclusters for Sensing Phosphate-Containing Metabolites and Alkaline Phosphatase Activity. Anal. Chem. 2019, 91, 15017–15024. [Google Scholar] [CrossRef]
- Jiang, C.; Bai, Z.; Yuan, F.; Ruan, Z.; Wang, W. A colorimetric sensor based on Glutathione-AgNPs as peroxidase mimetics for the sensitive detection of Thiamine (Vitamin B1). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120348–120355. [Google Scholar] [CrossRef]
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic interactions between peroxidase-mimic silver NanoZymes and chlorpyrifos-specific aptamers enable highly-specific pesticide sensing in river water. Anal. Chim. Acta 2019, 1083, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Nguyen, Q.H.; Kim, M.I. Highly Sensitive Fluorescent Detection of Acetylcholine Based on the Enhanced Peroxidase-like Activity of Histidine Coated Magnetic Nanoparticles. Nanomaterials 2021, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, J.; Chung, H.; Choi, Y.-K.; Hashkavayi, A.B.; Zhou, Y.; Park, K.S. Pyrophosphate-Enhanced Oxidase Activity of Cerium Oxide Nanoparticles for Colorimetric Detection of Nucleic Acids. Sensors 2021, 21, 7567. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-S.; Chang, T.-C.; Wang, S.-C. Gold nanoparticle-based colorimetric methods to determine protein contents in artificial urine using membrane micro-concentrators and mobile phone camera. Sens. Actuators B Chem. 2017, 239, 9–16. [Google Scholar] [CrossRef]
- Yue, G.; Su, S.; Li, N.; Shuai, M.; Lai, X.; Astruc, D.; Zhao, P. Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coord. Chem. Rev. 2016, 311, 75–84. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Yang, J.; Bai, Y. Colorimetric sensing of selenocystine using gold nanoparticles. Anal. Biochem. 2017, 535, 19–24. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, Y.; Huang, C.; Wang, Z.; Liu, L. In Situ Functionalization of Silver Nanoparticles by Gallic Acid as a Colorimetric Sensor for Simple Sensitive Determination of Melamine in Milk. ACS Omega 2021, 6, 23630–23635. [Google Scholar] [CrossRef]
- Ma, G.; Cao, J.; Hu, G.; Zhu, L.; Chen, H.; Zhang, X.; Liu, J.; Ji, J.; Liu, X.; Lu, C. Porous chitosan/partially reduced graphene oxide/diatomite composite as an efficient adsorbent for quantitative colorimetric detection of pesticides in a complex matrix. Analyst 2021, 146, 4576–4584. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, Y.; Guo, X.; Gao, Y.; Han, Y.; Sun, J.; Han, L. A rapid and ultrasensitive colorimetric biosensor based on aptamer functionalized Au nanoparticles for detection of saxitoxin. RSC Adv. 2020, 10, 15293–15298. [Google Scholar] [CrossRef] [Green Version]
- Abolghasemi-Fakhri, Z.; Amjadi, M. Gold nanostar@graphene quantum dot as a new colorimetric sensing platform for detection of cysteine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120010–120016. [Google Scholar] [CrossRef] [PubMed]
- Salari, R.; Hallaj, T. A dual colorimetric and fluorometric sensor based on N, P-CDs and shape transformation of AgNPrs for the determination of 6-mercaptopurine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120104–120111. [Google Scholar] [CrossRef] [PubMed]
- Zandi, A.; Amjadi, M.; Hallaj, T. Plasmon-enhanced fluorimetric and colorimetric dual sensor based on fluorescein/Ag nanoprisms for sensitive determination of mancozeb. Food Chem. 2022, 369, 130967–130972. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, J.; Li, X.; Weng, G.J.; Li, J.J.; Zhao, J.W. Surface etching-dependent geometry tailoring and multi-spectral information of Au@AuAg yolk-shell nanostructure with asymmetrical pyramidal core: The application in Co2+ determination. J. Colloid Interface Sci. 2022, 625, 340–353. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, J. Review of recent progress on DNA-based biosensors for Pb2+ detection. Anal. Chim. Acta 2021, 1147, 124–143. [Google Scholar] [CrossRef]
- Ye, T.; Gao, H.; Zhang, Q.; Yan, C.; Yu, Y.; Fei, Y.; Gao, L.; Zhou, X.; Shao, Y. Polarity inversion sensitized G-quadruplex metal sensors with K+ tolerance. Biosens. Bioelectron. 2019, 145, 111703–111707. [Google Scholar] [CrossRef]
- Ma, G.; Yu, Z.; Zhou, W.; Li, Y.; Fan, L.; Li, X. Investigation of Na+ and K+ Competitively Binding with a G-Quadruplex and Discovery of a Stable K+-Na+-Quadruplex. J. Phys. Chem. B 2019, 123, 5405–5411. [Google Scholar] [CrossRef]
- Fu, X.; Lin, H.; Qi, J.; Li, F.; Chen, Y.; Li, B.; Chen, L. A tetrahedral DNA nanostructure functionalized paper-based platform for ultrasensitive colorimetric mercury detection. Sens. Actuators B Chem. 2022, 362, 131830–131836. [Google Scholar] [CrossRef]
- Zhou, X.H.; Kong, D.M.; Shen, H.X. G-quadruplex-hemin DNAzyme-amplified colorimetric detection of Ag+ ion. Anal. Chim. Acta 2010, 678, 124–127. [Google Scholar] [CrossRef]
- Chen, J.; Pan, J.; Chen, S. A naked-eye colorimetric sensor for Hg2+ monitoring with cascade signal amplification based on target-induced conjunction of split DNAzyme fragments. Chem. Commun. 2017, 53, 10224–10227. [Google Scholar] [CrossRef]
- Li, D.; Ling, S.; Cheng, X.; Yang, Z.; Lv, B. Development of a DNAzyme-based colorimetric biosensor assay for dual detection of Cd2+ and Hg2+. Anal. Bioanal. Chem. 2021, 413, 7081–7091. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.; Cai, D.; Jiang, J.; Zhao, P.; Huang, Y.; Sang, G. Enzyme-free and label-free ultra-sensitive colorimetric detection of Pb2+ using molecular beacon and DNAzyme based amplification strategy. Biosens. Bioelectron. 2016, 80, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, J.; Shi, H.; Luo, X.; Xiao, L.; Zhou, C.; Guo, Y.; Xiao, D. A rapid and colorimetric biosensor based on GR-5 DNAzyme and self-replicating catalyzed hairpin assembly for lead detection. Anal. Methods 2020, 12, 2215–2220. [Google Scholar] [CrossRef]
- Ali, M.M.; Wolfe, M.; Tram, K.; Gu, J.; Filipe, C.D.M.; Li, Y.; Brennan, J.D. A DNAzyme-Based Colorimetric Paper Sensor for Helicobacter pylori. Angew. Chem. Int. Ed. Engl. 2019, 58, 9907–9911. [Google Scholar] [CrossRef]
- Ida, J.; Kuzuya, A.; Choong, Y.S.; Lim, T.S. An intermolecular-split G-quadruplex DNAzyme sensor for dengue virus detection. RSC Adv. 2020, 10, 33040–33051. [Google Scholar] [CrossRef]
- Ma, X.; Miao, P. Silver nanoparticle@DNA tetrahedron-based colorimetric detection of HIV-related DNA with cascade strand displacement amplification. J. Mater. Chem. B 2019, 7, 2608–2612. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, G.; Deng, Z.; Tang, Z.; Zhang, H.; Khusbu, F.Y.; Wu, K.; Chen, M.; Ma, C. A novel label-free colorimetric detection of l-histidine using Cu2+-modulated G-quadruplex-based DNAzymes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 195–200. [Google Scholar] [CrossRef]
- Lin, X.; Yu, C.; Lin, H.; Wang, C.; Su, J.; Cheng, J.; Kankala, R.K.; Zhou, S.F. Self-Assembly of Functional Nucleic Acid-Based Colorimetric Competition Assay for the Detection of Immunoglobulin E. Sensors 2019, 19, 2224. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chu, H.; Xu, X.; Tian, J.; Wu, Y.; Xu, W.; Tian, H.; Zhu, L. Rapid label-free colorimetric dual-functional aptasensor for β-lactoglobulin detection based on a rational tailoring strategy. Biosens. Bioelectron. 2022, 208, 114223–114229. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q.; Jin, Y.; Li, B. G-triplex/hemin DNAzyme: An ideal signal generator for isothermal exponential amplification reaction-based biosensing platform. Anal. Chim. Acta 2019, 1079, 139–145. [Google Scholar] [CrossRef]
- Sun, H.; Chen, H.; Zhang, X.; Liu, Y.; Guan, A.; Li, Q.; Yang, Q.; Shi, Y.; Xu, S.; Tang, Y. Colorimetric detection of sodium ion in serum based on the G-quadruplex conformation related DNAzyme activity. Anal. Chim. Acta 2016, 912, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, H.; Wu, G.; Sun, N.; Meng, L.; Li, Y.; Liu, Z.; Diao, A. A dual-channel detection of mercuric ions using a label free G-quadruplex-based DNAzyme molecule. Analyst 2016, 141, 3997–4000. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Song, C.; Shi, Q.; Liu, H.; Li, S.; Liu, R.; Liu, S.; Lv, C. Amplified colorimetric sensor for detecting radon by its daughter lead based on the free-fixed auto-assembly structure of Duplex-hemin/G-quadruplex. J. Pharm. Biomed. Anal. 2018, 159, 459–465. [Google Scholar] [CrossRef]
- Yu, X.; Lin, Y.; Wang, X.; Xu, L.; Wang, Z.; Fu, F. Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Mikrochim. Acta 2018, 185, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Kim, S.U.; Mun, H.; Choi, C.; Shim, W.B.; Kim, M.G. Colorimetric Detection of Norovirus in Oyster Samples through DNAzyme as a Signaling Probe. J. Agric. Food Chem. 2018, 66, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, Z.; Wang, X.; Zhang, W.; Xu, D.; Sun, X.; Guo, Y.; Xu, S.; Li, F. Construction of a dual-model aptasensor based on G-quadruplexes generated via rolling circle amplification for visual/sensitive detection of kanamycin. Sci. Total Environ. 2022, 839, 156276–156283. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.; Huang, P.-J.J.; Liu, J. G-Quadruplex DNA for Fluorescent and Colorimetric Detection of Thallium(I). ACS Sens. 2015, 1, 137–143. [Google Scholar] [CrossRef]
- Kang, S.; Oh, J.; Han, M.S. A colorimetric sensor for hydrogen sulfide detection using direct inhibition of active site in G-quadruplex DNAzyme. Dye. Pigment. 2017, 139, 187–192. [Google Scholar] [CrossRef]
- Wu, C.; Gao, G.; Zhai, K.; Xu, L.; Zhang, D. A visual Hg2+ detection strategy based on distance as readout by G-quadruplex DNAzyme on microfluidic paper. Food Chem. 2020, 331, 127208–127212. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Cheng, M.; Mergny, J.L.; Lin, Q.; Zhou, J.; Ju, H. Highly active G-quadruplex/hemin DNAzyme for sensitive colorimetric determination of lead(II). Mikrochim. Acta 2019, 186, 786–793. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.-K.; Lee, S.; Yoon, S.; Kim, W.-K. Graphene oxide-based NET strategy for enhanced colorimetric sensing of miRNA. Sens. Actuators B Chem. 2019, 282, 861–867. [Google Scholar] [CrossRef]
- Lee, J.; Na, H.K.; Lee, S.; Kim, W.K. Advanced graphene oxide-based paper sensor for colorimetric detection of miRNA. Mikrochim. Acta 2021, 189, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Berry, R.M.; Turberfield, A.J. The single-step synthesis of a DNA tetrahedron. Chem. Commun. 2004, 12, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Schaap, I.A.; Tardin, C.F.; Erben, C.M.; Berry, R.M.; Schmidt, C.F.; Turberfield, A.J. Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science 2005, 310, 1661–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, H.; Zuo, X.; Zhu, D.; Huang, Q.; Fan, C. Functional DNA nanostructures for theranostic applications. Acc. Chem. Res. 2014, 47, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wen, Y.; Wang, L.; Zhou, C.; Li, Q.; Xu, L.; Li, L.; Shi, J.; Lal, R.; Ren, S.; et al. PCR-Free Colorimetric DNA Hybridization Detection Using a 3D DNA Nanostructured Reporter Probe. ACS Appl. Mater. Interfaces 2017, 9, 38281–38287. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Yang, S.; Zheng, C.; Zhu, L.; Sun, S.; Guo, M.; Hu, X.; Huang, X.; Wang, L.; Shen, Z. DNAzyme-based sensing probe protected by DNA tetrahedron from nuclease degradation for the detection of lead ions. Talanta 2021, 233, 122543–122550. [Google Scholar] [CrossRef]
- Pei, H.; Liang, L.; Yao, G.; Li, J.; Huang, Q.; Fan, C. Reconfigurable Three-Dimensional DNA Nanostructures for the Construction of Intracellular Logic Sensors. Angew. Chem. Int. Ed. 2012, 124, 9154–9158. [Google Scholar] [CrossRef]
- Mitri, F.; De Iacovo, A.; De Santis, S.; Quarta, D.; Giansante, C.; Orsini, M.; Colace, L. Optical gas sensor based on the combination of a QD photoluminescent probe and a QD photodetector. Nanotechnology 2022, 33, 475501–475510. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Naberezhnykh, G.A.; Khomenko, V.A.; Amosov, A.V.; Nepomnyaschiy, A.V.; Solov’eva, T.F.; Chistyulin, D.K.; Tutov, M.V.; Kulchin, Y.N.; Novikova, O.D. In situ-Synthesized cadmium sulfide quantum dots in pore-forming protein and polysaccharide matrices for optical biosensing applications. Colloids Surf. B Biointerfaces 2022, 217, 112607–112616. [Google Scholar] [CrossRef]
- Ou, P.; Wu, J.; Lin, Y.; Tan, X.; Wu, Y.; Chen, Z.; Wei, F.; Huang, K. Flexible photoelectrochemical sensor for highly sensitive chloramphenicol detection based on M-TiO2-CdTe QDs/CdS QDs composite. Anal. Bioanal. Chem. 2022, 414, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, M.; Su, W.; Wu, B.; Yang, Z.; Wang, X.; Qiao, B.; Pei, H.; Tu, J.; Chen, D.; et al. Photoelectrochemical Enzyme Biosensor Based on TiO2 Nanorod/TiO2 Quantum Dot/Polydopamine/Glucose Oxidase Composites with Strong Visible-Light Response. Langmuir 2022, 38, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, L.; Wu, Y.; Di, J. A cathodic photoelectrochemical sensor for chromium(VI) based on the use of PbS quantum dot semiconductors on an ITO electrode. Mikrochim. Acta 2018, 185, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, L.; Niu, S.; Ding, C.; Luo, X. Ratiometric electrogenerated chemiluminescence sensor based on a designed anti-fouling peptide for the detection of carcinoembryonic antigen. Anal. Chim. Acta 2020, 1136, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, L.; Wang, X.; Wang, Y.; Li, J.; Luo, C. CdTe quantum dots@luminol as signal amplification system for chrysoidine with chemiluminescence-chitosan/graphene oxide-magnetite-molecularly imprinting sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 535–541. [Google Scholar] [CrossRef]
- Yin, H.; Truskewycz, A.; Cole, I.S. Quantum dot (QD)-based probes for multiplexed determination of heavy metal ions. Mikrochim. Acta 2020, 187, 336–360. [Google Scholar] [CrossRef]
- Li, J.; Shan, X.; Jiang, D.; Wang, Y.; Wang, W.; Chen, Z. A novel electrochemiluminescence sensor based on resonance energy transfer from MoS2QDs@g-C3N4 to NH2-SiO2@PTCA for glutathione assay. Analyst 2020, 145, 7616–7622. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, X.; Zhang, Q.; Liang, Z.; Ma, Q.; Su, X. A novel high efficient electrochemiluminescence sensor based on reductive Cu(I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosens. Bioelectron. 2020, 160, 112217–112223. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Yao, Y.; Yang, B.; Tian, T.; Miao, Y.; Liu, B. An electrochemiluminescence sensor for 17β-estradiol detection based on resonance energy transfer in α-FeOOH@CdS/Ag NCs. Talanta 2021, 221, 121479–121485. [Google Scholar] [CrossRef]
- Wei, Z.; Li, H.; Liu, S.; Wang, W.; Chen, H.; Xiao, L.; Ren, C.; Chen, X. Carbon Dots as Fluorescent/Colorimetric Probes for Real-Time Detection of Hypochlorite and Ascorbic Acid in Cells and Body Fluid. Anal. Chem. 2019, 91, 15477–15483. [Google Scholar] [CrossRef]
- Mostafapour, S.; Mohamadi Gharaghani, F.; Hemmateenejad, B. Converting electronic nose into opto-electronic nose by mixing MoS2 quantum dots with organic reagents: Application to recognition of aldehydes and ketones and determination of formaldehyde in milk. Anal. Chim. Acta 2021, 1170, 338654–338662. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, M.; Li, Z.; Tong, P.; Chen, Q.; Li, G.; Chen, J.; Zhang, L. A novel sensitive colorimetric sensor for Cu2+ based on in situ formation of fluorescent quantum dots with photocatalytic activity. Biosens. Bioelectron. 2017, 89, 866–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Jia, H.; Wamer, W.G.; Zheng, Z.; Li, P.; Callahan, J.H.; Yin, J.-J. Predicting and identifying reactive oxygen species and electrons for photocatalytic metal sulfide micro–nano structures. J. Catal. 2014, 320, 97–105. [Google Scholar] [CrossRef]
- Li, H.; Bai, H.; Lv, Q.; Wang, W.; Wang, Z.; Wei, H.; Zhang, Q. A new colorimetric sensor for visible detection of Cu(II) based on photoreductive ability of quantum dots. Anal. Chim. Acta 2018, 1021, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Xu, J.J.; Chen, H.Y. Selective detection of trace amount of Cu2+ using semiconductor nanoparticles in photoelectrochemical analysis. Nanoscale 2010, 2, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, D.; Wu, Y.; Di, J. Photoelectrochemical determination of Cu2+ ions based on assembly of Au/ZnS nanoparticles. Sens. Actuators B Chem. 2016, 235, 432–438. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Sherazee, M.; Srinivasan, S.; Rajabzadeh, A.R. Positively Charged Gold Quantum Dots: An Nanozymatic “Off-On” Sensor for Thiocyanate Detection. Foods 2022, 11, 1189. [Google Scholar] [CrossRef]
- Hayat, A.; Haider, W.; Raza, Y.; Marty, J.L. Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 2015, 143, 157–161. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Q.; Chen, J.; Huang, L.; Zhang, H.; Rong, K.; Dong, S. Biomimetic design for enhancing the peroxidase mimicking activity of hemin. Nanoscale 2019, 11, 12603–12609. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, T.; Huang, J.; Chen, Y.; Zhong, J.; Guo, L.; Fu, F. Boron- and Phenyl-Doped Graphitic Carbon Nitride (g-C3N4) Nanosheets for Colorimetric Detection of Hydrogen Peroxide in Soaked Foods. J. Nanosci. Nanotechnol. 2019, 19, 4220–4227. [Google Scholar] [CrossRef]
- Wu, S.; Huang, H.; Feng, X.; Du, C.; Song, W. Facile visual colorimetric sensor based on iron carbide nanoparticles encapsulated in porous nitrogen-rich graphene. Talanta 2017, 167, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Liu, Q.; Yu, S. Peroxidase mimetic activity of porphyrin modified ZnFe2O4/reduced graphene oxide and its application for colorimetric detection of H2O2 and glutathione. Colloids Surf. B Biointerfaces 2019, 181, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, H.; Huang, Y.; Jiang, H.; Yang, N.; Shahzad, S.A.; Meng, L.; Yu, C. Silver nanoparticles decorated and tetraphenylethene probe doped silica nanoparticles: A colorimetric and fluorometric sensor for sensitive and selective detection and intracellular imaging of hydrogen peroxide. Biosens. Bioelectron. 2018, 121, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Shen, J.; Li, C.; Zhao, Q.; Fodjo, E.K.; Zhang, L.; Chen, S.; Fan, Q.; Wang, L. Tetrahedral DNA-directed core-satellite assembly as SERS sensor for mercury ions at the single-particle level. Analyst 2022, 147, 1866–1872. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, P.; Xu, Y.; Liu, X.; Kong, D. Self-paired dumbbell DNA -assisted simple preparation of stable circular DNAzyme and its application in Pb2+ sensor. Anal. Chim. Acta 2021, 1175, 338733–338740. [Google Scholar] [CrossRef]
- Kaneko, N.; Horii, K.; Akitomi, J.; Kato, S.; Shiratori, I.; Waga, I. An Aptamer-Based Biosensor for Direct, Label-Free Detection of Melamine in Raw Milk. Sensors 2018, 18, 3227. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Li, B.; Guo, S.; Zhou, Z.; Zhou, M.; Wang, E.; Dong, S. G-Quadruplex-based DNAzyme for colorimetric detection of cocaine: Using magnetic nanoparticles as the separation and amplification element. Analyst 2011, 136, 493–497. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, H.; Zhang, C.; Qu, L.; Yu, L. A novel molecularly imprinted sensor based on PtCu bimetallic nanoparticle deposited on PSS functionalized graphene with peroxidase-like activity for selective determination of puerarin. Talanta 2020, 210, 120621–120628. [Google Scholar] [CrossRef]
- Arshad, A.; Wang, H.; Bai, X.; Jiang, R.; Xu, S.; Wang, L. Colorimetric paper sensor for sensitive detection of explosive nitroaromatics based on Au@Ag nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 16–22. [Google Scholar] [CrossRef]
- Chen, C.; Li, N.; Lan, J.; Ji, X.; He, Z. A label-free colorimetric platform for DNA via target-catalyzed hairpin assembly and the peroxidase-like catalytic of graphene/Au-NPs hybrids. Anal. Chim. Acta 2016, 902, 154–159. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Lv, Q.; Wang, C.; Liang, J.; Zhou, Z.; Li, G. Colorimetric biosensor for visual determination of Golgi protein 73 based on reduced graphene oxide-carboxymethyl chitosan-Hemin/platinum@palladium nanozyme with peroxidase-like activity. Mikrochim. Acta 2022, 189, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Mehdinia, A.; Niroumand, R.; Jabbari, A. Enhanced LSPR performance of graphene nanoribbons-silver nanoparticles hybrid as a colorimetric sensor for sequential detection of dopamine and glutathione. Anal. Chim. Acta 2020, 1120, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhu, A.; Xu, Q.; Chen, Y.; Xu, J.; Weng, J. Colorimetric Biosensor for Detection of Cancer Biomarker by Au Nanoparticle-Decorated Bi2Se3 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 6931–6940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Li, M.; Gao, P.; Zhou, Y.; Zhang, G.; Shuang, S.; Dong, C. Colorimetric sensor for cysteine in human urine based on novel gold nanoparticles. Talanta 2016, 161, 520–527. [Google Scholar] [CrossRef]
- Khoris, I.M.; Takemura, K.; Lee, J.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Enhanced colorimetric detection of norovirus using in-situ growth of Ag shell on Au NPs. Biosens. Bioelectron. 2019, 126, 425–432. [Google Scholar] [CrossRef]
- Behzadifar, S.; Hosseini, M.; Mohammadnejad, J.; Asiabanha, M. A new colorimetric assay for sensitive detection of glucose-6-phosphate dehydrogenase deficiency based on silver nanoparticles. Nanotechnology 2021, 33, 055502–055512. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Q.; Wen, Y.; Bian, X.; Tao, Q.; Liu, G.; Yan, J. A competitive colorimetric aptasensor for simple and sensitive detection of kanamycin based on terminal deoxynucleotidyl transferase-mediated signal amplification strategy. Food Chem. 2022, 377, 132072–132079. [Google Scholar] [CrossRef]
- Xie, N.; Huang, J.; Yang, X.; Yang, Y.; Quan, K.; Ou, M.; Fang, H.; Wang, K. Competition-Mediated FRET-Switching DNA Tetrahedron Molecular Beacon for Intracellular Molecular Detection. ACS Sens. 2016, 1, 1445–1452. [Google Scholar] [CrossRef]
- Naorungroj, S.; Teengam, P.; Vilaivan, T.; Chailapakul, O. Paper-based DNA sensor enabling colorimetric assay integrated with smartphone for human papillomavirus detection. New J. Chem. 2021, 45, 6960–6967. [Google Scholar] [CrossRef]
- Su, L.; Zhang, X.; Su, Y.; Liu, B. A simple colorimetric method based on “on-off-on” mode for detection of H2S and Hg2+in water. Anal. Sci. 2022, 38, 1407–1416. [Google Scholar] [CrossRef]
- Baruah, U.; Chowdhury, D. Functionalized graphene oxide quantum dot-PVA hydrogel: A colorimetric sensor for Fe2+, Co2+ and Cu2+ ions. Nanotechnology 2016, 27, 145501–145516. [Google Scholar] [CrossRef]
- Shi, X.; Gu, W.; Zhang, C.; Zhao, L.; Peng, W.; Xian, Y. A label-free colorimetric sensor for Pb2+ detection based on the acceleration of gold leaching by graphene oxide. Dalton Trans. 2015, 44, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Keshipour, S.; Ahour, F. New water-soluble colorimetric pH and metal ione sensor based on graphene quantum dot modified with alizarine red S. Sci. Rep. 2020, 10, 14185. [Google Scholar] [CrossRef] [PubMed]
- He, S.B.; Chen, F.Q.; Xiu, L.F.; Peng, H.P.; Deng, H.H.; Liu, A.L.; Chen, W.; Hong, G.L. Highly sensitive colorimetric sensor for detection of iodine ions using carboxylated chitosan-coated palladium nanozyme. Anal. Bioanal. Chem. 2020, 412, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Duan, W.; Cao, S.; Zeng, T.; Zhao, T.; Huang, J.; Lu, X.; Zeng, J. Highly Specific Colorimetric Probe for Fluoride by Triggering the Intrinsic Catalytic Activity of a AgPt-Fe3O4 Hybrid Nanozyme Encapsulated in SiO2 Shells. Environ. Sci. Technol. 2022, 56, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
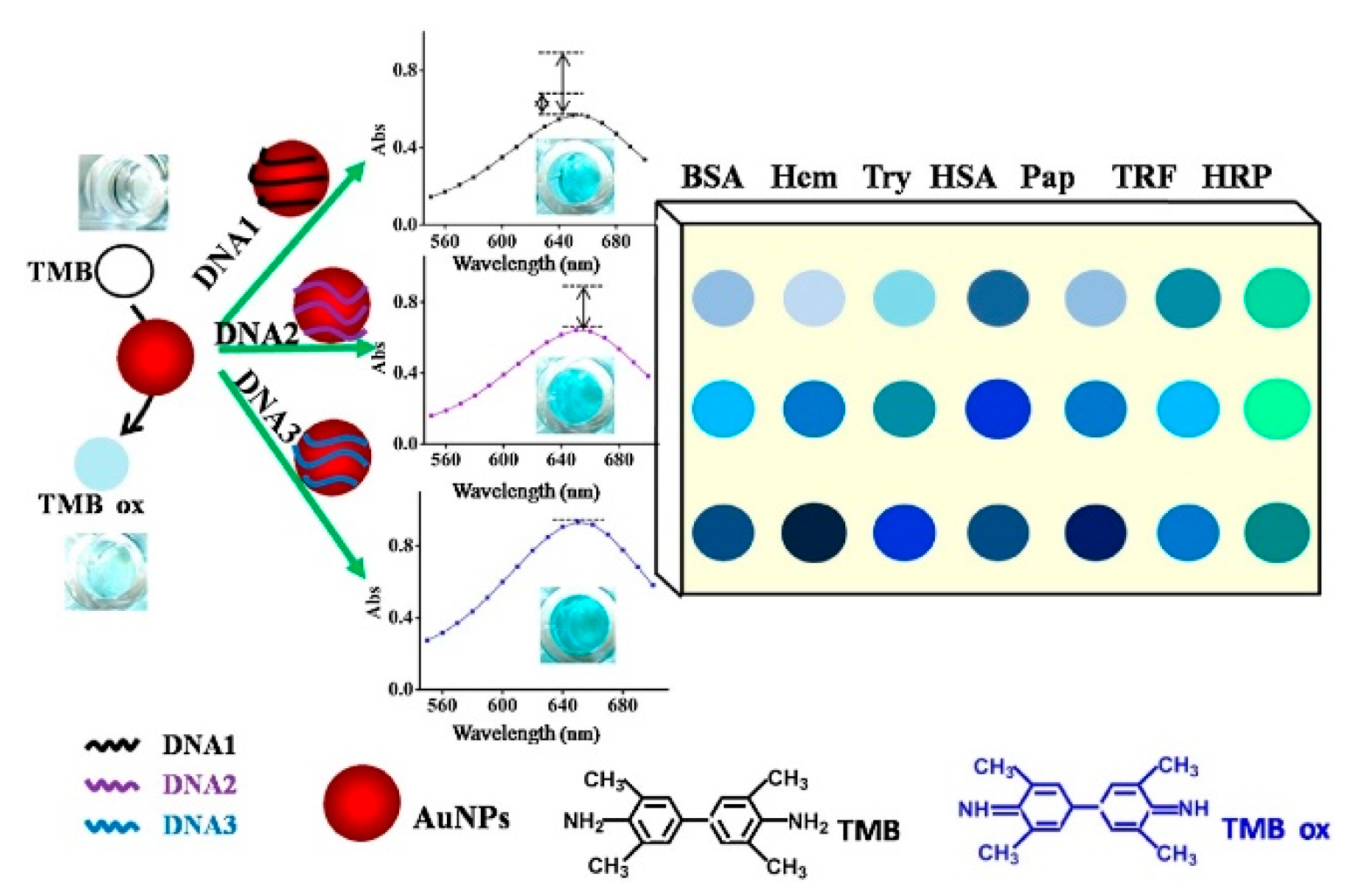
- Yang, J.; Lu, Y.; Ao, L.; Wang, F.; Jing, W.; Zhang, S.; Liu, Y. Colorimetric sensor array for proteins discrimination based on the tunable peroxidase-like activity of AuNPs-DNA conjugates. Sens. Actuators B Chem. 2017, 245, 66–73. [Google Scholar] [CrossRef]
- Jia, F.; Liu, Q.; Wei, W.; Chen, Z. Colorimetric sensor assay for discrimination of proteins based on exonuclease I-triggered aggregation of DNA-functionalized gold nanoparticles. Analyst 2019, 144, 4865–4870. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Zhao, Y.; Wang, J. Fluorescent and colorimetric dual-response sensor based on copper (II)-decorated graphitic carbon nitride nanosheets for detection of toxic organophosphorus. Food Chem. 2021, 345, 128560–128568. [Google Scholar] [CrossRef]
- Bi, J.; Tian, C.; Zhang, G.L.; Hao, H.; Hou, H.M. Detection of Histamine Based on Gold Nanoparticles with Dual Sensor System of Colorimetric and Fluorescence. Foods 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wu, X.; Zhuang, S.; Zhang, Y.; Ding, Y.; Zhou, X. Colorimetric Biosensor Based on Magnetic Enzyme and Gold Nanorods for Visual Detection of Fish Freshness. Biosensors 2022, 12, 135. [Google Scholar] [CrossRef]
- Wang, C.; Lei, S.; Li, X.; Guo, S.; Cui, P.; Wei, X.; Liu, W.; Liu, H. A Reduced GO-Graphene Hybrid Gas Sensor for Ultra-Low Concentration Ammonia Detection. Sensors 2018, 18, 3147. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.; Xu, Z.; Duan, X.; Yang, J.; Wang, F.; Li, Z. High-Performance Colorimetric Room-Temperature NO2 Sensing Using Spin-Coated Graphene/Polyelectrolyte Reflecting Film. ACS Appl. Mater. Interfaces 2019, 11, 32390–32397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shang, Y.; Yu, R.; Wang, Y.; Feng, F.; Guo, Q.; Xing, J.; Tian, Z.; Zeng, J.; Yan, Z. Cu2O induced Au nanochains for highly sensitive dual-mode detection of hydrogen sulfide. J. Hazard. Mater. 2022, 436, 129144–129153. [Google Scholar] [CrossRef] [PubMed]
- Khoris, I.M.; Chowdhury, A.D.; Li, T.C.; Suzuki, T.; Park, E.Y. Advancement of capture immunoassay for real-time monitoring of hepatitis E virus-infected monkey. Anal. Chim. Acta 2020, 1110, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Ding, Z.; Li, X.; Dong, Y.; Fu, T.; Wu, Y. Colorimetric Sensor Array Based on Wulff-Type Boronate Functionalized AgNPs at Various pH for Bacteria Identification. Anal. Chem. 2019, 91, 12134–12137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, T.; Wang, H.N.; Vo-Dinh, T. Smartphone-Based Device for Colorimetric Detection of MicroRNA Biomarkers Using Nanoparticle-Based Assay. Sensors 2021, 21, 8044. [Google Scholar] [CrossRef]









| Materials | Analytes | Symbolic Parameters | Ref. |
|---|---|---|---|
| Graphene nanoribbons | Dopamine | This colorimetric sensor had a good linear in the concentration range of 0.1–50 μM with a LOD of 0.035 μM. | [16] |
| Heteroatom-doped graphene | Pesticides | The nanozyme sensor arrays exhibited linear response of five pesticides in the range of 5 to 500 μM. | [28] |
| L-cysteine functionalized graphene oxide | Hg2+ | The colorimetric sensor was developed with CGO as an oxidase mimic for determination of Hg2+ with linear ranges of 0–200 μg/mL, and the LOD was 7.6 μg/mL. | [29] |
| Metal ions-TPA@GQDs | Thiols | — | [31] |
| Hemin–graphene nanosheets | Thyroglobulin | The gray intensity of the proposed paper-based sensor proved to be proportional to the concentration of thyroglobulin in the range of 5–100 ng/mL with a LOD of 1 ng/mL. | [32] |
| His@AuNCs/RGO | Nitrite | Under the optimized conditions, the proposed sensor showed a good linear correlation with nitrite concentration in the range of 10–500 μM with a LOD of 2 μM. | [33] |
| G/Fe3O4-Au NPs | Pb2+ | Sensitive detection of Pb2+ was performed in the range of 1 to 300 ng/mL with the LOD was 0.63 ng/mL. | [34] |
| Oligonucleotide-GO/Au NPs | Hg2+ | The absorbance value at the wavelength of 655 nm was linearly related with the concentration of Hg2+ in the range between 5.2 × 10−9 M and 1.2 × 10−7 M, and the LOD was 3.8 × 10−10 M. | [35] |
| Analytes | Probe | Ref. |
|---|---|---|
| Cu2+ | Ag nanoparticles | [40] |
| L-histidine | Cu2+-modulated G-quadruplex | [77] |
| Hg2+ | G-quadruplex | [82] |
| Glucose | Iron carbide nanoparticles | [121] |
| H2O2 and glutathione | Porphyrin modified ZnFe2O4/reduced graphene oxide | [122] |
| H2O2 | Ag@TPE-SiO2 nanoparticles | [123] |
| Hg2+ | Tetrahedral DNA | [124] |
| Pb2+ | Dumbbell DNA | [125] |
| Melamine | Aptamer–DNAzyme conjunction | [126] |
| Cocaine | G-quadruplex | [127] |
| Puerarin | PtCu bimetallic nanoparticles deposited on PSS functionalized graphene | [128] |
| Explosive nitroaromatics | Au@Ag nanoparticles | [129] |
| DNA | Graphene/Au nanoparticles | [130] |
| Golgi protein 73 | Reduced graphene oxide-carboxymethyl-hemin/platinum@palladium | [131] |
| Dopamine and glutathione | Graphene nanoribbons-silver nanoparticles | [132] |
| α-fetoprotein and prostate-specific antigen | Au/Bi2Se3 | [133] |
| Cysteine | Pectinase protected Au nanoparticles | [134] |
| Norovirus | Ag/Au nanoparticles | [135] |
| Glucose-6-phosphate dehydrogenase | Ag nanoparticles | [136] |
| Kanamycin | Aptamer@terminal deoxynucleotidyl transferase | [137] |
| mRNA | DNA tetrahedron molecular beacon | [138] |
| HPV | Dextrin-stabilized Au nanoparticles | [139] |
| H2S and Hg2+ | MnO2/multi-wall carbon nanotubes composite | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Feng, J.; Hu, G.; Zhang, E.; Yu, H.-H. Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors 2023, 23, 2749. https://doi.org/10.3390/s23052749
Wu Y, Feng J, Hu G, Zhang E, Yu H-H. Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors. 2023; 23(5):2749. https://doi.org/10.3390/s23052749
Chicago/Turabian StyleWu, Yu, Jing Feng, Guang Hu, En Zhang, and Huan-Huan Yu. 2023. "Colorimetric Sensors for Chemical and Biological Sensing Applications" Sensors 23, no. 5: 2749. https://doi.org/10.3390/s23052749
APA StyleWu, Y., Feng, J., Hu, G., Zhang, E., & Yu, H.-H. (2023). Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors, 23(5), 2749. https://doi.org/10.3390/s23052749






