CochleRob: Parallel-Serial Robot to Position a Magnetic Actuator around a Patient’s Head for Intracochlear Microrobot Navigation
Abstract
1. Introduction
2. Design Specifications
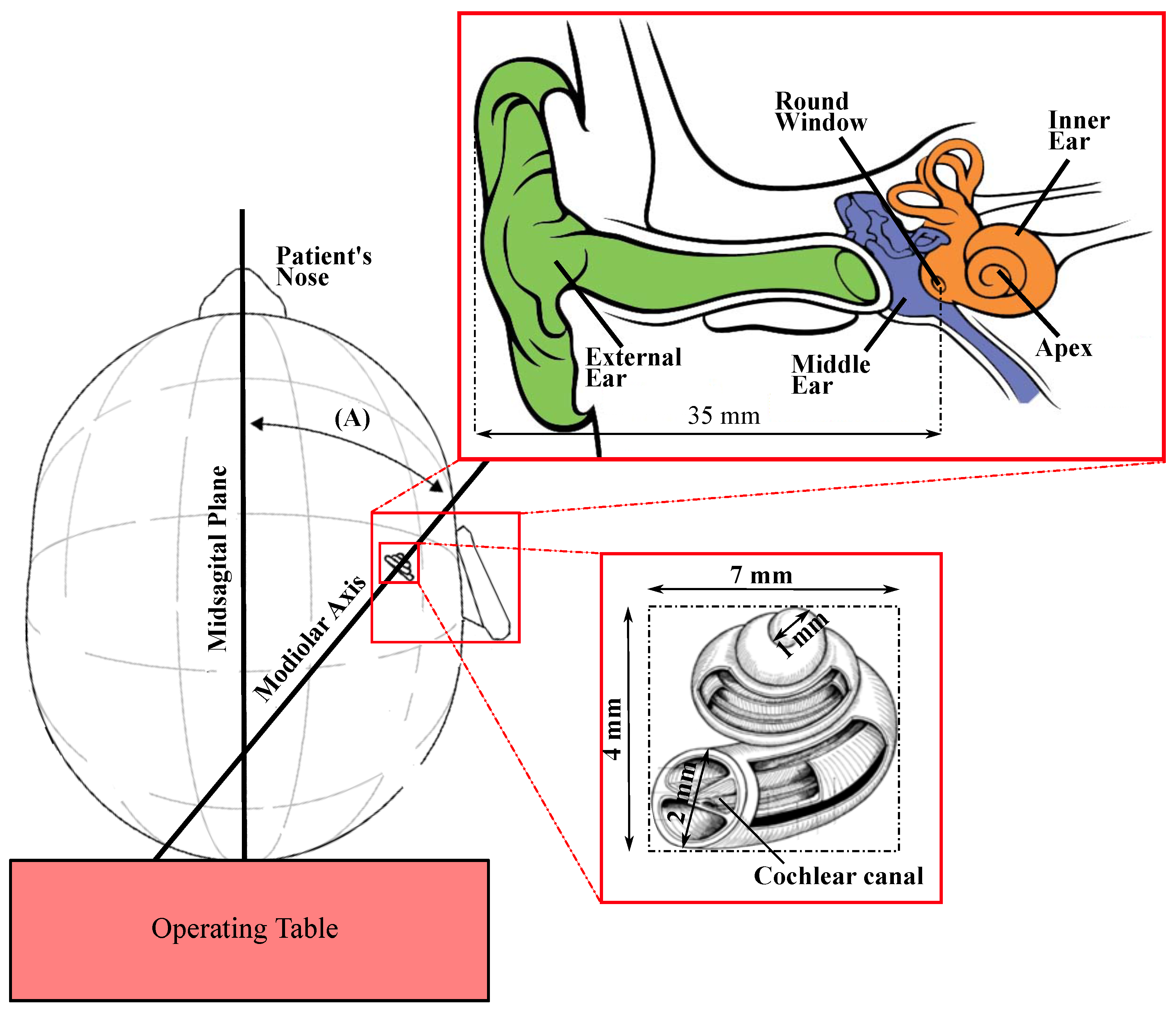
2.1. Anatomical Specifications
2.2. Magnetic Actuator Specifications
3. Robot Design
3.1. Optimal Robot Degrees of Freedom and Workspace
3.2. From Specifications to the Shape of the Robot
3.3. Mechanical Design Description
3.4. CochleRob Joints
- The movement of the three kinematic chains of the delta structure is controlled by three joints, represented by the angles and . These angles represent the angle between the forearm (14) and the relative horizontal plane. The different dimensions and joints of the delta part is illustrated in Figure 8. This part can be simplified if each chain (segments and ) is translated toward the axis by a distance . The equivalent configuration is illustrated in Figure 9, with .
- The fourth joint is represented with the angle . It is the rotation of the slider (3).
- The fifth joint is represented with the angle . It is the rotation of the arc (2). See Figure 10.
4. Forward Kinematics Model
4.1. Modeling
- Serial part, in which the joints are and .
- Parallel structure, for which the joints are , , and .
4.2. Validation of the FKM Model
5. Inverse Kinematics Model
5.1. Modeling
5.2. Numerical Validation of the IKM Model
6. The Dynamic Model
- is the torque generated by motor i (electromagnetic torque).
- is the necessary torque provided by motor i to make the robot steady in front of the gravity affect: we call it the gravity torque.
- is the torque provided by each motor i, when the different organs perform specific accelerations in gravity-free space: we call it the acceleration torque.
6.1. The Dynamic Model of the Delta Structure
- : the mass of the forearm .
- : the mass of the parallel sticks .
- : the mass of the nacelle.
- the center of mass of each parallel sticks is the midpoint of the segment .
- the center of mass of each forearm is at a distance of from the corresponding joint .
6.2. The Dynamic Model of the Serial Structure
6.3. Validation of the Dynamic Model
7. The State Model
8. Controller
8.1. Control Architecture
- As discussed and validated in the dynamic model section, the velocity and acceleration of the various Delta structures have no effect on the motors’ torques because of the high-reduction and the slowness required during the operation. Thus, the friction is also neglected since it is proportional to the velicty.
- In case we want to consider friction of the motors rotors, the Equation (39) takes the following form:With () being the friction of each motor.
8.2. Trajectory, Validation by Simulation
9. Prototyping and Experimental Validation
10. Conclusions and Perspectives
- Our work introduces a new approach for intracochlear drug administration for the treatment of hearing loss through the development of the hybrid parallel–serial robot, CochleRob. This robot has a compact, precise, and rigid structure with five degrees of freedom that meets the requirements of positioning a magnetic actuator within the inner ear, including the necessary workspace and degrees of freedom.
- CochleRob reduces the risk of cochlear damage by the introduction of electrode arrays or catheters inside the cochlea. Drugs are delivered remotely, without the need for catheter or CI insertion.
- Through the validation of mathematical models, including kinematics, dynamics, and control laws, we have demonstrated the feasibility and effectiveness of our proposed solution. The results obtained from simulations were highly satisfactory, supporting the potential of CochleRob as a promising solution for the safe treatment of hearing loss.
- The proposed robot has been successfully prototyped and its components were manufactured using the additive manufacturing process. The robot was effectively controlled using Labview software, further demonstrating its viability as a solution for treating hearing loss. The motors chosen for the robot met the necessary specifications for torque and volume.
- It is important to consider the potential movement of the patient. Currently, it is possible to perform the procedure with a stabilized head using a face mask, a headband, or even anesthesia; however, this solution may not always be feasible or desirable. To address this issue, the head position can be tracked and fed back to the controller in real-time, enabling the adjustment of the assigned trajectory and reducing the risk of error. Several potential solutions for tracking the head position can be investigated, including deep neural network-based visual tracking using face landmark detection, among others.
- In order to expand the volume of the workspace and potentially increase compactness, it is worth conducting a thorough analysis of the workspace of the Delta structure based on its lengths, , , and L (the distance between and the nacelle center ). The distances, and , are doomed to be invariable (or variable in a very short range) by the constraints on the motors’ lengths and the end-effector.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Computer Aided Design |
| RWM | Round Window Membrane |
| CI | Cochlear Implant |
| SPMNP | SuperParamagnetic NanoParticles |
| LSC | Lateral Semicircular Canal |
| DoF | Degrees-of-Freedom |
| RCM | Remote Center of Motion |
| FKM | Forward Kinematics Model |
| IKM | Inverse Kinematics Model |
| PID | Proportional Integral Derivative |
References
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef]
- Pile, J.; Simaan, N. Modeling, design, and evaluation of a parallel robot for cochlear implant surgery. IEEE/ASME Trans. Mechatron. 2014, 19, 1746–1755. [Google Scholar] [CrossRef]
- Zhao, Y.; Labadie, R.F.; Dawant, B.M.; Noble Sr, J.H. Validation of cochlear implant electrode localization techniques. In Medical Imaging 2018: Image-Guided Procedures, Robotic Interventions, and Modeling; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10576, p. 105761U. [Google Scholar]
- Noble, J.H.; Hedley-Williams, A.J.; Sunderhaus, L.; Dawant, B.M.; Labadie, R.F.; Camarata, S.M.; Gifford, R.H. Initial results with image-guided cochlear implant programming in children. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2016, 37, e63. [Google Scholar] [CrossRef]
- Caversaccio, M.; Wimmer, W.; Anso, J.; Mantokoudis, G.; Gerber, N.; Rathgeb, C.; Schneider, D.; Hermann, J.; Wagner, F.; Scheidegger, O.; et al. Robotic middle ear access for cochlear implantation: First in man. PLoS ONE 2019, 14, e0220543. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.; Caversaccio, M.; Weber, S. Robotics in ear, nose and throat (ent) surgery. In Medical Robotics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 159–184. [Google Scholar]
- Gao, X.; Wang, Y.; Chen, K.; Grady, B.P.; Dormer, K.J.; Kopke, R.D. Magnetic assisted transport of plga nanoparticles through a human round window membrane model. J. Nanotechnol. Eng. Med. 2010, 1, 031010. [Google Scholar] [CrossRef]
- Juhn, S. Barrier systems in the inner ear. Acta Oto-Laryngol. 1988, 105, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sterkers, O.; Ferrary, E.; Amiel, C. Production of inner ear fluids. Physiol. Rev. 1988, 68, 1083–1128. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-S.; Park, J.-R.; Boo, S.-H.; Jo, J.-M.; Park, K.-W.; Lee, W.-Y.; Ahn, J.-G.; Kang, M.-K.; Park, B.-G.; Lee, H. Clinical efficacy of initial intratympanic steroid treatment on sudden sensorineural hearing loss with diabetes. Otolaryngol. Head Neck Surg. 2009, 141, 572–578. [Google Scholar] [CrossRef]
- McCall, A.A.; Swan, E.E.L.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug delivery for treatment of inner ear disease: Current state of knowledge. Ear Hear. 2010, 31, 156. [Google Scholar] [CrossRef]
- Goycoolea, M.V.; Lundman, L. Round window membrane. structure function and permeability: A review. Microsc. Res. Tech. 1997, 36, 201–211. [Google Scholar] [CrossRef]
- Alzamil, K.S.; Linthicum, F.H., Jr. Extraneous round window membranes and plugs: Possible effect on intratympanic therapy. Ann. Otol. Rhinol. Laryngol. 2000, 109, 30–32. [Google Scholar] [CrossRef]
- Plontke, S.K.; Salt, A.N. Local drug delivery to the inner ear: Principles, practice, and future challenges. Hear. Res. 2018, 368, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Miroir, M.; Kazmitcheff, G.; Ferrary, E.; Sterkers, O.; Grayeli, A.B. Super paramagnetic nanoparticles delivery through a microcatheter by solenoids. In Proceedings of the 2010 IEEE International Conference on Nano/Molecular Medicine and Engineering, Hong Kong, China, 5–9 December 2010; pp. 153–157. [Google Scholar]
- Scheper, V.; Hessler, R.; Hütten, M.; Wilk, M.; Jolly, C.; Lenarz, T.; Paasche, G. Local inner ear application of dexamethasone in cochlear implant models is safe for auditory neurons and increases the neuroprotective effect of chronic electrical stimulation. PLoS ONE 2017, 12, e0183820. [Google Scholar] [CrossRef]
- Riojas, K.E.; Narasimhan, N.; Morrel, W.G.; Mitchell, J.; Bruns, T.; Webster, R.J., III; Labadie, R.F. A new manual insertion tool for minimally invasive, image-guided cochlear implant surgery. In Medical Imaging 2019: Image-Guided Procedures, Robotic Interventions, and Modeling; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 10951, p. 109510J. [Google Scholar]
- Hoskison, E.; Mitchell, S.; Coulson, C. Systematic review: Radiological and histological evidence of cochlear implant insertion trauma in adult patients. Cochlear Implant. Int. 2017, 18, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Pile, J.; Simaan, N. Characterization of friction and speed effects and methods for detection of cochlear implant electrode tip fold-over. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 4409–4414. [Google Scholar]
- Clark, J.R.; Leon, L.; Warren, F.M.; Abbott, J.J. Magnetic guidance of cochlear implants: Proof-of-concept and initial feasibility study. J. Med. Dev. 2012, 6, 035002. [Google Scholar] [CrossRef]
- Clark, J.R.; Leon, L.; Warren, F.M.; Abbott, J.J. Investigation of magnetic guidance of cochlear implants. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 1321–1326. [Google Scholar]
- Leon, L.; Cavilla, M.S.; Doran, M.B.; Warren, F.M.; Abbott, J.J. Scala-tympani phantom with cochleostomy and round-window openings for cochlear-implant insertion experiments. J. Med. Dev. 2014, 8, 041010. [Google Scholar] [CrossRef]
- Zhang, J.; Bhattacharyya, S.; Simaan, N. Model and parameter identification of friction during robotic insertion of cochlear-implant electrode arrays. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 3859–3864. [Google Scholar]
- Gawęcki, W.; Balcerowiak, A.; Podlawska, P.; Borowska, P.; Gibasiewicz, R.; Szyfter, W.; Wierzbicka, M. Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study. J. Clin. Med. 2022, 11, 7045. [Google Scholar] [CrossRef]
- Vittoria, S.; Lahlou, G.; Torres, R.; Daoudi, H.; Mosnier, I.; Mazalaigue, S.; Ferrary, E.; Nguyen, Y.; Sterkers, O. Robot-based assistance in middle ear surgery and cochlear implantation: First clinical report. Eur. Arch. Otorhinolaryngol. 2021, 278, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mom, T.; Puechmaille, M.; Yagoubi, M.E.; Lère, A.; Petersen, J.-E.; Bécaud, J.; Saroul, N.; Gilain, L.; Mirafzal, S.; Chabrot, P. Robotized Cochlear Implantation under Fluoroscopy: A Preliminary Series. J. Clin. Med. 2023, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T. Cochlear implant–state of the art. Laryngo-Rhino-Otologie 2017, 96, S123–S151. [Google Scholar] [CrossRef]
- Federspil, P.A.; Plinkert, P.K. Restoring hearing with active hearing (Wiederherstellung des Gehörs mit aktiven Hörimplantaten). De Gruyter 2004, 49, 78–82. [Google Scholar] [CrossRef]
- Leterme, G.; Guigou, C.; Oudot, A.; Collin, B.; Boudon, J.; Millot, N.; Geissler, A.; Belharet, K.; Grayeli, A.B. Superparamagnetic nanoparticle delivery to the cochlea through round window by external magnetic field: Feasibility and toxicity. Surg. Innov. 2019, 26, 646–655. [Google Scholar] [CrossRef]
- Abbes, M.; Belharet, K.; Mekki, H.; Poisson, G. Permanent magnets based actuator for microrobots navigation. In Proceedings of the 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), IEEE, Macau, China, 3–8 November 2019; pp. 7062–7067. [Google Scholar]
- Schuknecht, H.F.; Gacek, M.R. Cochlear pathology in presbycusis. Ann. OfOtology Rhinol. Laryngol. 1993, 102 (Suppl. 1), 1–16. [Google Scholar] [CrossRef]
- Shin, K.-J.; Lee, J.-Y.; Kim, J.-N.; Yoo, J.-Y.; Shin, C.; Song, W.-C.; Koh, K.-S. Quantitative analysis of the cochlea using three-dimensional reconstruction based on microcomputed tomographic images. Anat. Rec. 2013, 296, 1083–1088. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.-A.; Cohen, L.T.; Clark, G.M. Cochlear view: Postoperative radiography for cochlear implantation. Otol. Neurotol. 2000, 21, 49–56. [Google Scholar]
- Singla, A.; Sahni, D.; Gupta, A.; Loukas, M.; Aggarwal, A. Surgical anatomy of round window and its implications for cochlear implantation. Clin. Anat. 2014, 27, 331–336. [Google Scholar] [CrossRef]
- Amokrane, W.; Belharet, K.; Souissi, M.; Grayeli, A.B.; Ferreira, A. Design and prototyping of a magnetic actuator based permanent magnets for microbead navigation in viscous environment. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 395–400. [Google Scholar]
- Amokrane, W.; Belharet, K.; Souissi, M.; Grayeli, A.B.; Ferreira, A. Macro-micro manipulation platform for inner ear drug delivery. Robot. Auton. Syst. 2018, 107, 10–19. [Google Scholar] [CrossRef]
- Aksungur, S. Remote center of motion (rcm) mechanisms for surgical operations. Int. J. Appl. Math. Electron. Comput. 2015, 3, 119–126. [Google Scholar] [CrossRef]
- Abbes, M.; Souissi, M.; Belharet, K.; Mekki, H.; Poisson, G. A novel remote-center-of-motion serial manipulator for inner ear drug delivery. In Proceedings of the 2018 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Auckland, New Zealand, 9–12 July 2018. [Google Scholar]
- Molaei, A.; Abedloo, E.; Taghirad, H.D.; Marvi, Z. Kinematic and workspace analysis of diamond: An innovative eye surgery robot. In Proceedings of the 2015 23rd Iranian Conference on Electrical Engineering, Tehran, Iran, 10–14 May 2015; pp. 882–887. [Google Scholar]
- Al Bassit, L. Structures Mécaniques à Modules Sphériques Optimisées Pour un Robot Médical de Télé-échographie Mobile. Ph.D. Thesis, Université d’Orléans, Eugene, OR, USA, 2005. [Google Scholar]
- Codourey, A. Dynamic modelling and mass matrix evaluation of the delta parallel robot for axes decoupling control. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, IROS’96, Osaka, Japan, 8 November 1996; Volume 3, pp. 1211–1218. [Google Scholar]
- Clavel, R. Conception d’un Robot Parallèle Rapide à 4 Degrés de Liberté. Ph.D. Thesis, Swiss Federal Institute of Technology Lausanne, Lausanne, Switzerland, 1991. [Google Scholar]
- Codourey, A. Contribution à la Commande des Robots Rapides et Précis. Ph.D. Thesis, Swiss Federal Institute of Technology Lausanne, Lausanne, Switzerland, 1991. [Google Scholar]
- Bolton, W. Cascade Control. In Instrumentation and Control Systems, 2nd ed.; Newnes: Oxford, UK, 2015; Chapter 13.5; pp. 281–302. [Google Scholar]



































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadour, H.; Bozorg Grayeli, A.; Poisson, G.; Belharet, K. CochleRob: Parallel-Serial Robot to Position a Magnetic Actuator around a Patient’s Head for Intracochlear Microrobot Navigation. Sensors 2023, 23, 2973. https://doi.org/10.3390/s23062973
Nadour H, Bozorg Grayeli A, Poisson G, Belharet K. CochleRob: Parallel-Serial Robot to Position a Magnetic Actuator around a Patient’s Head for Intracochlear Microrobot Navigation. Sensors. 2023; 23(6):2973. https://doi.org/10.3390/s23062973
Chicago/Turabian StyleNadour, Housseyne, Alexis Bozorg Grayeli, Gérard Poisson, and Karim Belharet. 2023. "CochleRob: Parallel-Serial Robot to Position a Magnetic Actuator around a Patient’s Head for Intracochlear Microrobot Navigation" Sensors 23, no. 6: 2973. https://doi.org/10.3390/s23062973
APA StyleNadour, H., Bozorg Grayeli, A., Poisson, G., & Belharet, K. (2023). CochleRob: Parallel-Serial Robot to Position a Magnetic Actuator around a Patient’s Head for Intracochlear Microrobot Navigation. Sensors, 23(6), 2973. https://doi.org/10.3390/s23062973




