Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology
Abstract
1. Introduction
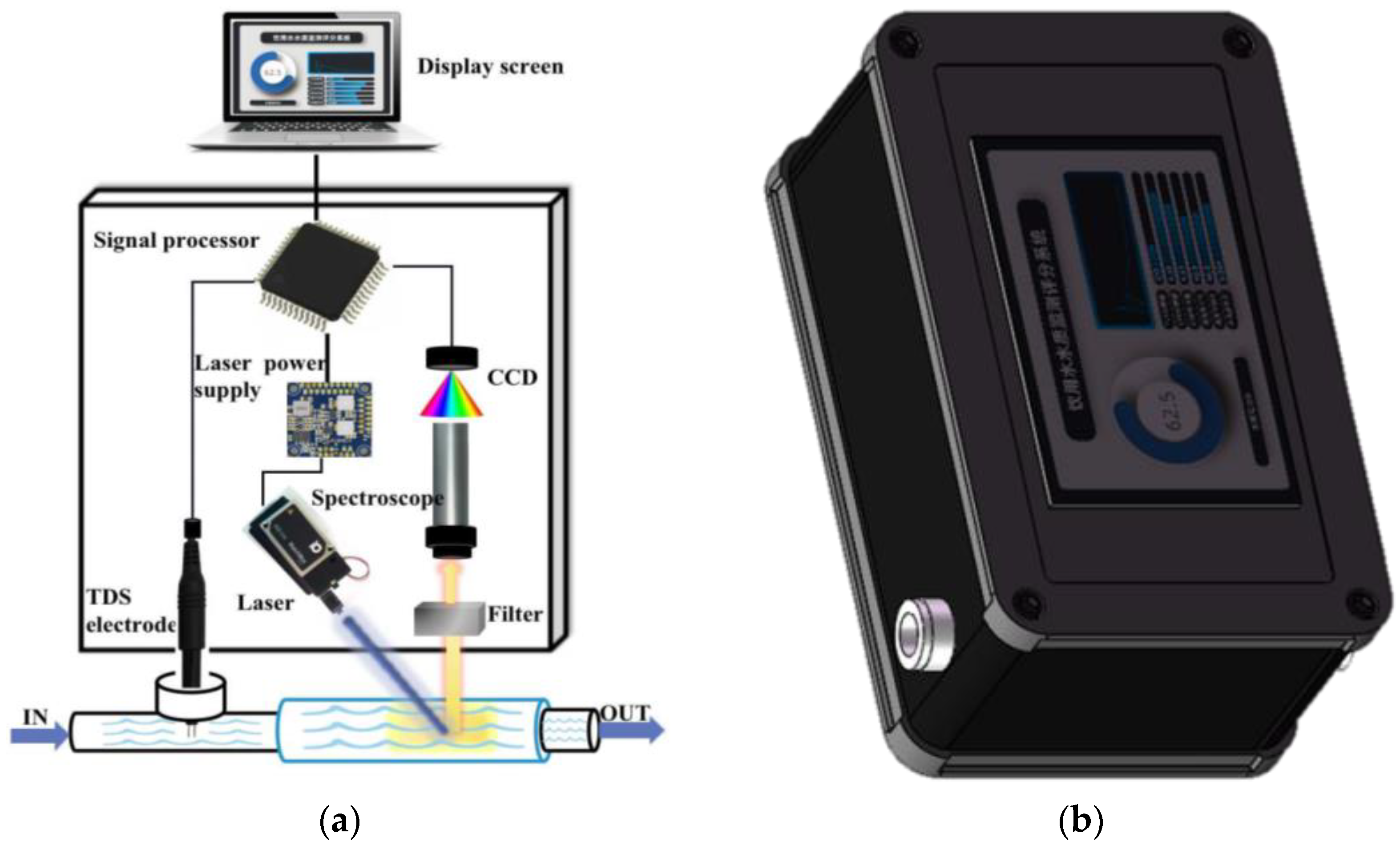
2. Instrument Structure and Detection Principle
2.1. Detection Standard
2.2. Instrument Structure
2.3. Detection Principle
2.3.1. Laser Spectroscopy Detection
2.3.2. TDS Detection
2.3.3. Water Quality Evaluation Score
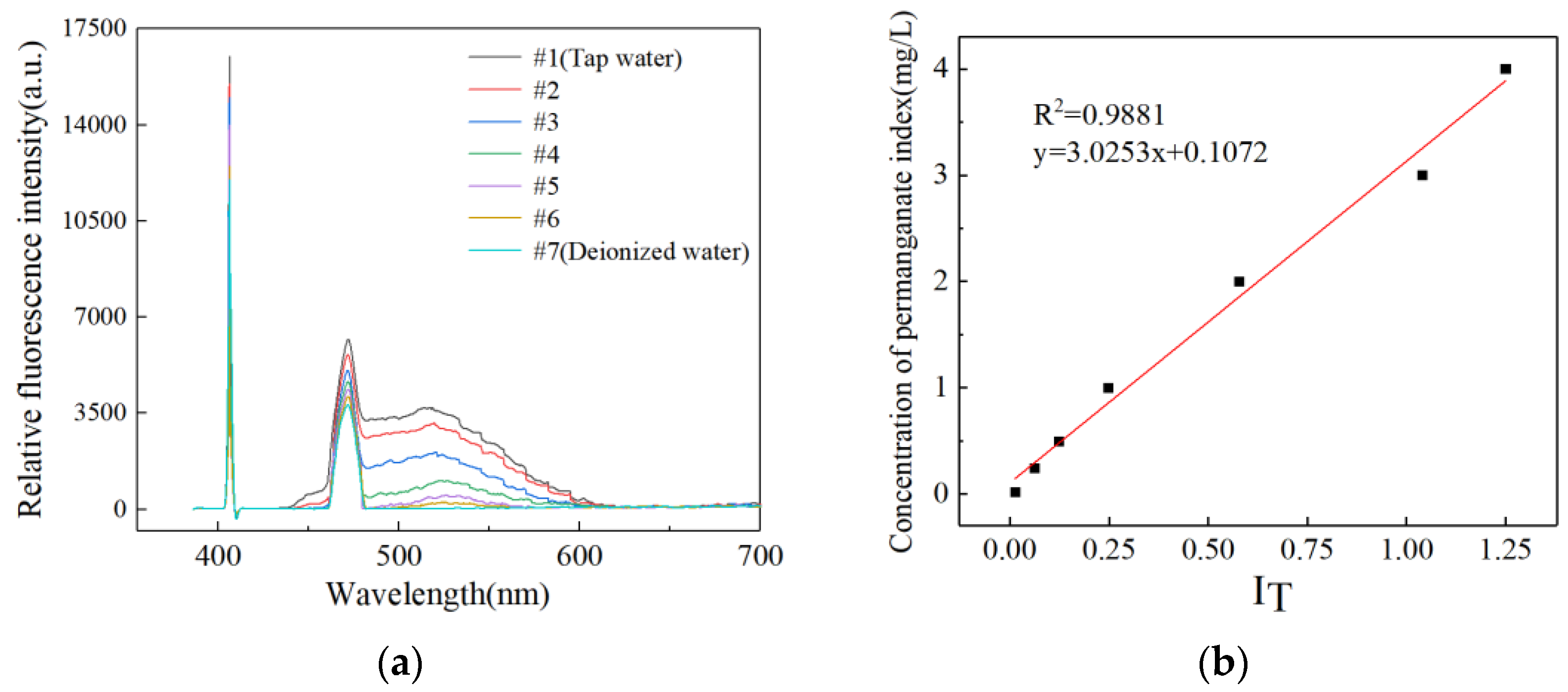
3. Experimental Measurement Results
3.1. Preliminary Experiment
3.2. Practical Application Experiment
4. Conclusions
- (1)
- The small-scale laser spectrometer can be used to detect the organic matter in water with high sensitivity, which makes up for the shortcoming that the TDS sensor is unfit for measuring organic matter in water. It makes the rapid detection of drinking water quality possible.
- (2)
- The TDS value exceeds the standard, which indicates that the water quality is poor and unfit for drinking. In fact, the TDS value of tap water in China rarely exceeds 500 mg/L, which indicates that TDS meets the water quality standard even without water purification. In addition, the TDS value is not the smaller the better, because the water contains a certain concentration of ions, such as Ca and Mg ions, that are beneficial to the human body. If the TDS parameter measured by the conductivity method is lower than a certain value, it can be considered that the inorganic matter in the water meets the standard.
- (3)
- Since TDS mainly reflects inorganic salt content, its value measured by the conductance method cannot reflect water quality accurately. We adopt high-sensitivity laser spectroscopy technology to detect organic matter and give a percentage value to evaluate the water quality. If the water quality score is 100 points, the concentration of organic matter in the water is 0 mg/L, such as in deionized water. A score above 90 indicates that the water has less organic matter, making it suitable for human consumption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDS | Total Dissolved Solids |
| SOC | Synthetic Organic Compounds |
| COD | Chemical Oxygen Demand |
| TOC | Total Organic Carbon, |
| DOM | Dissolved Organic Matter |
| ORP | Oxidation Reduction Potential |
| EC | Electrical Conductivity |
| PLSR | Partial Least Squares Regression |
| MCL | Maximum Contaminant Levels |
| EPA | Environmental Protection Agency |
| LFRR | Laser Fluorescence–Raman ratio |
References
- Koppanen, M.; Kesti, T.; Kokko, M.; Rintala, J.; Palmroth, M. An online flow-imaging particle counter and conventional water quality sensors detect drinking water contamination in the presence of normal water quality fluctuations. Water Res. 2022, 213, 118149. [Google Scholar] [PubMed]
- Sinitsa, S.; Sochen, N.; Mendlovic, D.; Borisover, M.; Lew, B.; Sela-Saldinger, S.; Vladimir, Y.; Nadia, B.; Yulia, K.; Lavi, R.; et al. Optical sensor system for early warning of inflow organic matter breach in large-scale irrigation systems and water treatment systems. IEEE Sens. J. 2022, 22, 1680–1691. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, S.; Ghosh, R.; Hasan, M.N.; Bera, A.; Roy, L.; Bhattacharya, N.; Halder, A.; Chattopadhyay, A.; Mukhopadhyay, S.; et al. A portable spectroscopic instrument for multiplexed monitoring of acute water toxicity: Design, testing, and evaluation. Rev. Sci. Instrum. 2022, 93, 115105. [Google Scholar] [CrossRef]
- Chang, W.L.; Sun, I.M.; Tsai, J.A.; Meng, H.F.; Zan, H.W.; Chen, L.Y.; Lu, C.J. Rapid quality test for drinking water by vertical-channel organic semiconductor gas sensor. Anal. Chim. Acta 2022, 1206, 339729. [Google Scholar] [CrossRef]
- Selvi, A.G.; Vibhithra, S.; John, C.S.A. IoT Based Water Quality Monitoring System for Smart Cities. J. Trend Sci. Res. Dev. 2021, 5, 986–991. [Google Scholar]
- Punit, K.; Karunesh, K.G.; Raj, K.G.; Panchariya, P.C. Towards the Green Analytics: Design and Development of Sustainable Drinking Water Quality Monitoring System for Shekhawati Region in Rajasthan. MAPAN 2021, 36, 1–15. [Google Scholar]
- Alam, A.U.; Clyne, D.; Deen, M.J. A Low-Cost Multi-Parameter Water Quality Monitoring System. Sensors 2021, 21, 3775. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.R.; Nagalakshmi, K.; Suresh, V.M.; Vaishnavee, V. Potnet: Online Potable Water Quality Monitoring Network for Overhead Water Tanks in Rural Water Supply Schemes in India. Int. J. Sens. Wirel. Commun. Control 2021, 11, 872–887. [Google Scholar]
- Sandip, K.R.; Preeta, S. Application of machine learning for real-time evaluation of salinity (or TDS) in drinking water using photonic sensors. Drink. Water Eng. Sci. 2016, 9, 37–45. [Google Scholar]
- Shi, Z.; Chow, C.W.K.; Fabris, R.; Liu, J.; Jin, B. Applications of Online UV-Vis Spectrophotometer for Drinking Water Quality Monitoring and Process Control: A Review. Sensors 2022, 22, 2987. [Google Scholar] [CrossRef]
- Junchen, L.; Ye, Y.; Zheng, D.; Guangyu, Z.; Sune, S. Short-range remote sensing of water quality by a handheld fluorosensor system. Appl. Opt. 2020, 59, C1–C7. [Google Scholar]
- Carstea, E.M.; Popa, C.L.; Baker, A.; Bridgeman, J. In situ fluorescence measurements of dissolved organic matter: A review. Sci. Total Environ. 2020, 699, 134361. [Google Scholar] [CrossRef]
- Sorensen, J.P.R.; Vivanco, A.; Ascott, M.J.; Gooddy, D.C.; Lapworth, D.J.; Read, D.S.; Rushworth, C.M.; Bucknall, J.; Herbert, K.; Karapanos, I.; et al. Online fluorescence spectroscopy for the real-time evaluation of the microbial quality of drinking water. Water Res. 2018, 137, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Zhang, W.; Yu, S.; Wang, X.; Gao, N. New advances in fluorescence excitation-emission matrix spectroscopy for the characterization of dissolved organic matter in drinking water treatment: A review. Chem. Eng. J. 2020, 381, 122676. [Google Scholar] [CrossRef]
- Bridgeman, J.; Baker, A.; Brown, D.; Boxall, J.B. Portable LED fluorescence instrumentation for the rapid assessment of potable water quality. Sci. Total Environ. 2015, 524, 338–346. [Google Scholar] [CrossRef]
- Haskell, D.; Heo, J.; Park, J.; Dong, C. Hydrogeochemical Evaluation of Groundwater Quality Parameters for Ogallala Aquifer in the Southern High Plains Region, USA. Int. J. Environ. Res. Public Health 2022, 19, 8453. [Google Scholar] [CrossRef] [PubMed]
- Xiaohua, C.; Zhaoshuo, T.; Fenghao, S.; Qingcao, L.; Zongjie, B.; Hao, C.; Zihao, C. Research on chemical oxygen demand based on laser Fluorescence-Raman spectroscopy. Front. Phys. 2022, 10, 1116. [Google Scholar] [CrossRef]
- Zhaoshuo, T.; Zongjie, B.; Yiwei, W.; Hongyan, Z. Rapid evaluation method based on DOM for water quality by microlaser fluorescence spectrometer. Appl. Phys. B Lasers Opt. 2020, 126, 205–219. [Google Scholar]
- Sun, B.; Meng, X.; Niu, Y.; Li, J.; Zou, N. Application of instrumental method for determination of water environmental permanganate index. Environ. Prot. Oil Gas Fields 2022, 32, 45–49. [Google Scholar]


| Detection Parameter | Drinking Water Standard | Standard Detection Method | Detection Method in This Paper | Remarks |
|---|---|---|---|---|
| TDS | ≤1000 mg/L 1 (≤500 mg/L 2) | Gravimetric method | Conductance method | Reflect inorganic content |
| Permanganate index | ≤3 mg/L | Chemical analysis | Laser spectroscopy | Reflect organic content |
| Sample Number | Permanganate Index (mg/L) | TDS (mg/L) | Water Quality Rating (Points) | ||
|---|---|---|---|---|---|
| Laser Spectroscopy (mg/L) | Chemical Analysis (mg/L) | Relative Error (%) | |||
| #1 | 4 | 4.15 | 3.75 | 125 | 59.9 |
| #2 | 2.97 | 3.12 | 5.05 | 92 | 68.8 |
| #3 | 2.01 | 2.04 | 1.5 | 66 | 78.2 |
| #4 | 0.97 | 0.99 | 2 | 30 | 87.9 |
| #5 | 0.48 | 0.51 | 6.25 | 17 | 93.1 |
| #6 | 0.24 | 0.26 | 8.33 | 8 | 95.4 |
| #7 | 0.04 | 0 | / | 0 | 100 |
| Water Sample | Permanganate Index (mg/L) | TDS (mg/L) | Water Quality Scores |
|---|---|---|---|
| Tap water | 4 | 125 | 59.9 |
| Primary filtered water | 1.41 | 138 | 81.2 |
| Secondary filtered water | 0.23 | 156 | 94.8 |
| Water Sample | Laser Spectroscopy (mg/L) | Chemical Analysis (mg/L) | Relative Error (%) |
|---|---|---|---|
| Tap water | 4 | 4.15 | 3.75 |
| Primary filtered water | 1.41 | 1.53 | 8.51 |
| Secondary filtered water | 0.23 | 0.25 | 8.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Chen, H.; Ding, Q.; Che, X.; Bi, Z.; Wang, L. Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology. Sensors 2023, 23, 2985. https://doi.org/10.3390/s23062985
Tian Z, Chen H, Ding Q, Che X, Bi Z, Wang L. Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology. Sensors. 2023; 23(6):2985. https://doi.org/10.3390/s23062985
Chicago/Turabian StyleTian, Zhaoshuo, Hao Chen, Qiping Ding, Xiaohua Che, Zongjie Bi, and Ling Wang. 2023. "Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology" Sensors 23, no. 6: 2985. https://doi.org/10.3390/s23062985
APA StyleTian, Z., Chen, H., Ding, Q., Che, X., Bi, Z., & Wang, L. (2023). Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology. Sensors, 23(6), 2985. https://doi.org/10.3390/s23062985







