Dynamic Vascular Imaging Using Active Breast Thermography
Abstract
1. Introduction
2. Method
2.1. Goal
2.2. Approach
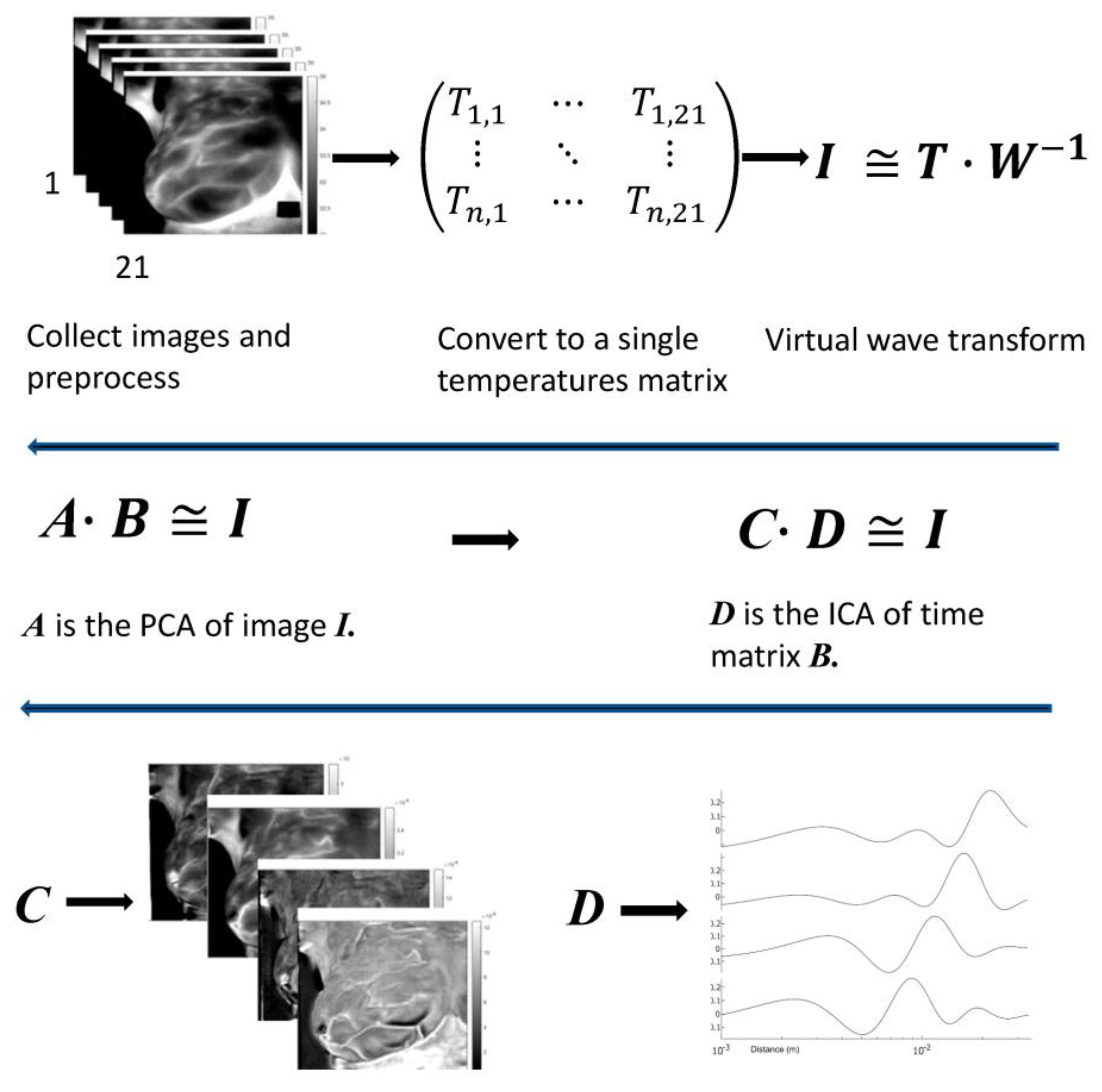
2.3. Mathematical Analysis
3. Results
3.1. Data
3.2. Data Preparation
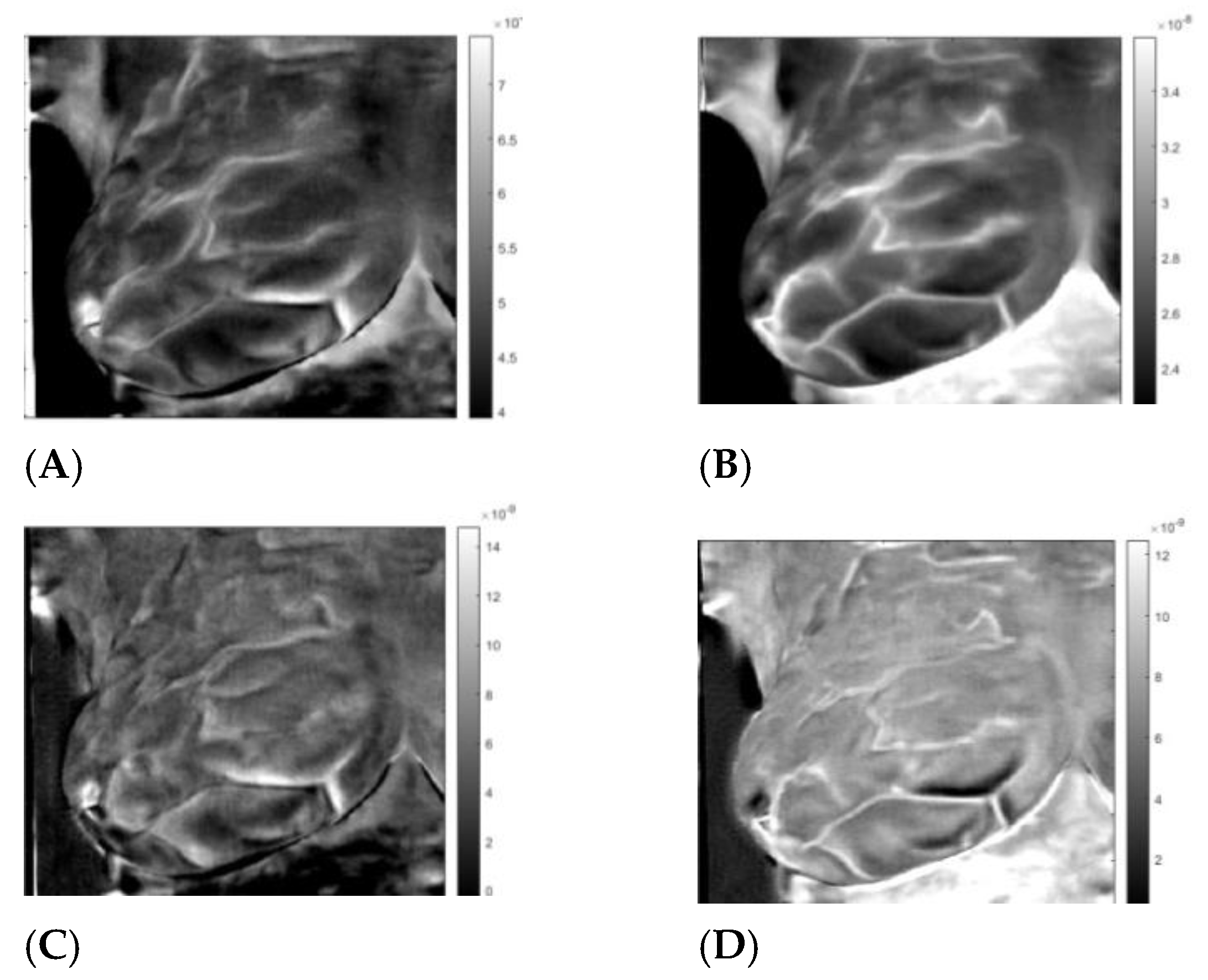
3.3. Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Virtual Wave Transform (VWT)
Appendix A.1. Virtual Wave
Appendix A.2. Modified Truncated Singular Value Decomposition
Appendix B. Matrix Factorization
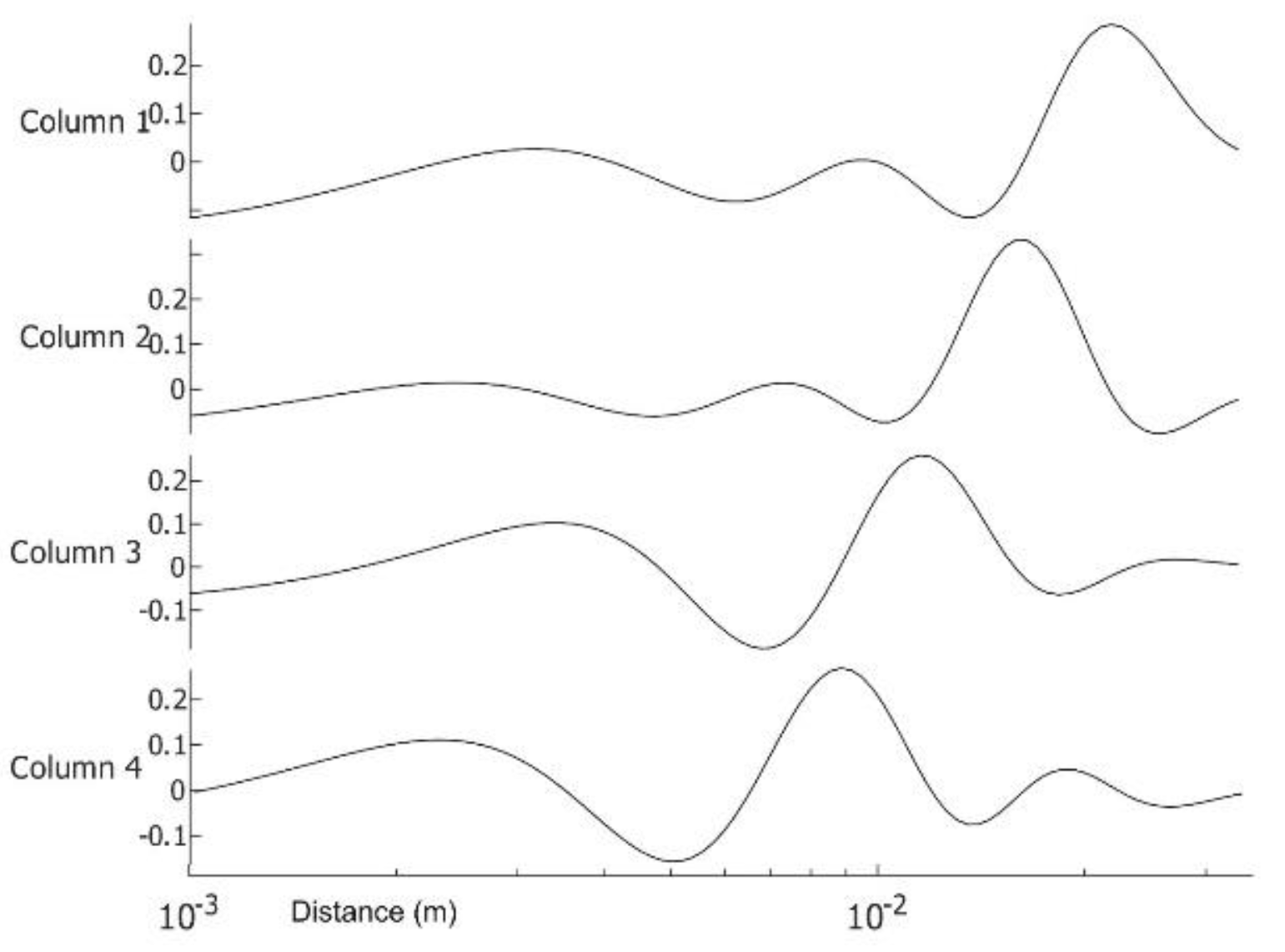
Appendix C. Excitation Extraction
Appendix D. Image Registration
References
- Ginsburg, O.; Yip, C.; Brooks, A.; Cabanes, A.; Caleffi, M.; Yataco, J.A.D.; Gyawali, B.; McCormack, V.; de Anderson, M.M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235, ISSN 1350-4495. [Google Scholar] [CrossRef]
- Flir Boson original equipment manufecturer IR Camera. Available online: https://www.flir.com/products/boson/ (accessed on 28 January 2023).
- FDA Safety Communication, FDA Warns Thermography Should Not Be Used in Place of Mammography to Detect, Diagnose, or Screen for Breast Cancer. 2019. Available online: www.fda.gov (accessed on 28 January 2023).
- Kubicka, A.; Matczak, K.; Łabieniec-Watała, M. More Than Meets the Eye Regarding Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 9507. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Gautherie, M.; Gros, C.M. Breast thermography and cancer risk prediction. Cancer 1980, 45, 51–56. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, L.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. Generation and thermal simulation of a digital model of the female breast in prone position. J. Eng. Sci. Med. Diagn. Ther. 2018, 1, 041006. [Google Scholar] [CrossRef]
- Ng, E.Y.-K.; Sudharsan, N.M. Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer 2004, 4, 17–26. [Google Scholar] [CrossRef]
- Yousef, H.; Ramezanpour Ahangar, E.; Varacallo, M. Physiology, Thermal Regulation; StatPearls: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK499843/ (accessed on 28 January 2023).
- Ohashi, Y.; Uchida, I. Applying dynamic thermography in the diagnosis of breast cancer. IEEE Eng. Med. Biol. Mag. 2000, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, J.; Gershenson, M. Use of equivalent wave field transform in evaluating dynamic thermal tomography of infrared breast images. In Proceedings of SPIE 11004, Thermosense: Thermal Infrared Applications XLI; SPIE: Bellingham, WA, USA, 2019; XLI, 1100404. [Google Scholar] [CrossRef]
- Gershenson, J.P.; Gershenson, M. Early results for equivalent wavefield transform as a direct solution to the inverse problem for active infrared thermography and potential for perfusion information to differentiate healthy versus cancerous breast tissue. In Proceedings of the SPIE Medical Imaging, Houston, TX, USA, 15–20 February 2020; Volume 11312, p. 113125E. [Google Scholar] [CrossRef]
- Burgholzer, P.; Thor, M.; Gruber, J.; Mayr, G. Three-dimensional thermographic imaging using a virtual wave concept. J. Appl. Phys. 2017, 121, 105102. [Google Scholar] [CrossRef]
- Yousefi, B.; Akbari, H.; Maldague, X. Detecting Vasodilation as Potential Diagnostic Biomarker in Breast Cancer Using Deep Learning-Driven Thermomics. Biosensors 2020, 10, 164. [Google Scholar] [CrossRef]
- Vigil, N.; Barry, M.; Amini, A.; Akhloufi, M.; Maldague, X.P.V.; Ma, L.; Ren, L.; Yousefi, B. Dual-Intended Deep Learning Model for Breast Cancer Diagnosis in Ultrasound Imaging. Cancers 2022, 14, 2663. [Google Scholar] [CrossRef]
- Yousefi, B.; Akbari, H.; Hershman, M.; Kawakita, S.; Fernandes, H.C.; Ibarra-Castanedo, C.; Ahadian, S.; Maldague, X.P.V. SPAER: Sparse Deep Convolutional Autoencoder Model to Extract Low Dimensional Imaging Biomarkers for Early Detection of Breast Cancer Using Dynamic Thermography. Appl. Sci. 2021, 11, 3248. [Google Scholar] [CrossRef]
- Mukhmetov, O.; Mashekova, A.; Zhao, Y.; Ng, E.Y.K.; Midlenko, A.; Fok, S.; Teh, S.L. Inverse thermal modeling and experimental validation for breast tumor detection by using highly personalized surface thermal patterns and geometry of the breast. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 3777–3791. [Google Scholar] [CrossRef]
- Lozano, A., 3rd; Hayes, J.C.; Compton, L.M.; Azarnoosh, J.; Hassanipour, F. Determining the thermal characteristics of breast cancer based on high-resolution infrared imaging, 3D breast scans, and magnetic resonance imaging. Sci. Rep. 2020, 10, 10105. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, J.-L.; Recinella, A.N.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. An inverse heat transfer approach for patient-specific breast cancer detection and tumor localization using surface thermal images in the prone position. Infrared Phys. Technol. 2020, 105, 103202. [Google Scholar] [CrossRef]
- Hellgren, R.J.; Sundbom, A.E.; Czene, K.; Izhaky, D.; Hall, P.; Dickman, P.W. Does three-dimensional functional infrared imaging improve breast cancer detection based on digital mammography in women with dense breasts. Eur. Radiol. 2019, 29, 6227–6235. [Google Scholar] [CrossRef]
- Roslidar, R.; Rahman, A.; Muharar, R.; Syahputra, M.R.; Arnia, F.; Syukri, M.; Pradhan, B.; Munadi, K. A Review on Recent Progress in Thermal Imaging and Deep Learning Approaches for Breast Cancer Detection. IEEE Access 2020, 8, 116176–116194. [Google Scholar] [CrossRef]
- Plaza, D.; Baic, A.; Lange, B.; Brzęk, A.; Ślosarek, K.; Stanek, A.; Cholewka, A. Comparison of the Thermal Reaction of Patients after Conserving Procedures and after Mastectomy to the Radiation Dose Obtained during Radiotherapy. Int. J. Environ. Res. Public Health 2022, 19, 16085. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Perez-Raya, I.; Raghupathi, P.A.; Gonzalez-Hernandez, J.-L.; Dabydeen, D.; Medeiros, L.; Phatak, P. Infrared imaging technology for breast cancer detection—Current status, protocols and new directions. Int. J. Heat Mass Transf. 2017, 108, 2303–2320. [Google Scholar] [CrossRef]
- Owens, A.; Kandlikar, S.G.; Phatak, P. Potential of Infrared Imaging for Breast Cancer Detection: A Critical Evaluation. ASME J. Med. Diagn. 2021, 4, 041005. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, J.-L.; Recinella, A.N.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. Technology, application and potential of dynamic breast thermography for the detection of breast cancer. Int. J. Heat Mass Transf. 2019, 131, 558–573. [Google Scholar] [CrossRef]
- Tsietso, D.; Yahya, A.; Samikannu, R. A Review on Thermal Imaging-Based Breast Cancer Detection Using Deep Learning. Mob. Inf. Syst. 2022, 2022, 8952849. [Google Scholar] [CrossRef]
- Kolka, C.M.; Bergman, R.N. The barrier within: Endothelial transport of hormones. Physiology 2012, 27, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Saade, D.C.M.; Sequeiros, G.O.; Silva, A.C.; Paiva, A.C.; Bravo, R.S.; Conci, A. A New Database for Breast Research with Infrared Image. J. Med. Imaging Health Inform. 2014, 4, 92–100. [Google Scholar] [CrossRef]
- Pérez-Martín, J.; Sánchez-Cauce, R. Quality analysis of a breast thermal images database. Health Inform. J. 2023, 29, 14604582231153779. [Google Scholar] [CrossRef]
- Gershenson, M. Simple interpretation of time-domain electromagnetic sounding using similarities between wave and diffusion propagation. Geophysics 1997, 62, E763–E774. [Google Scholar] [CrossRef]
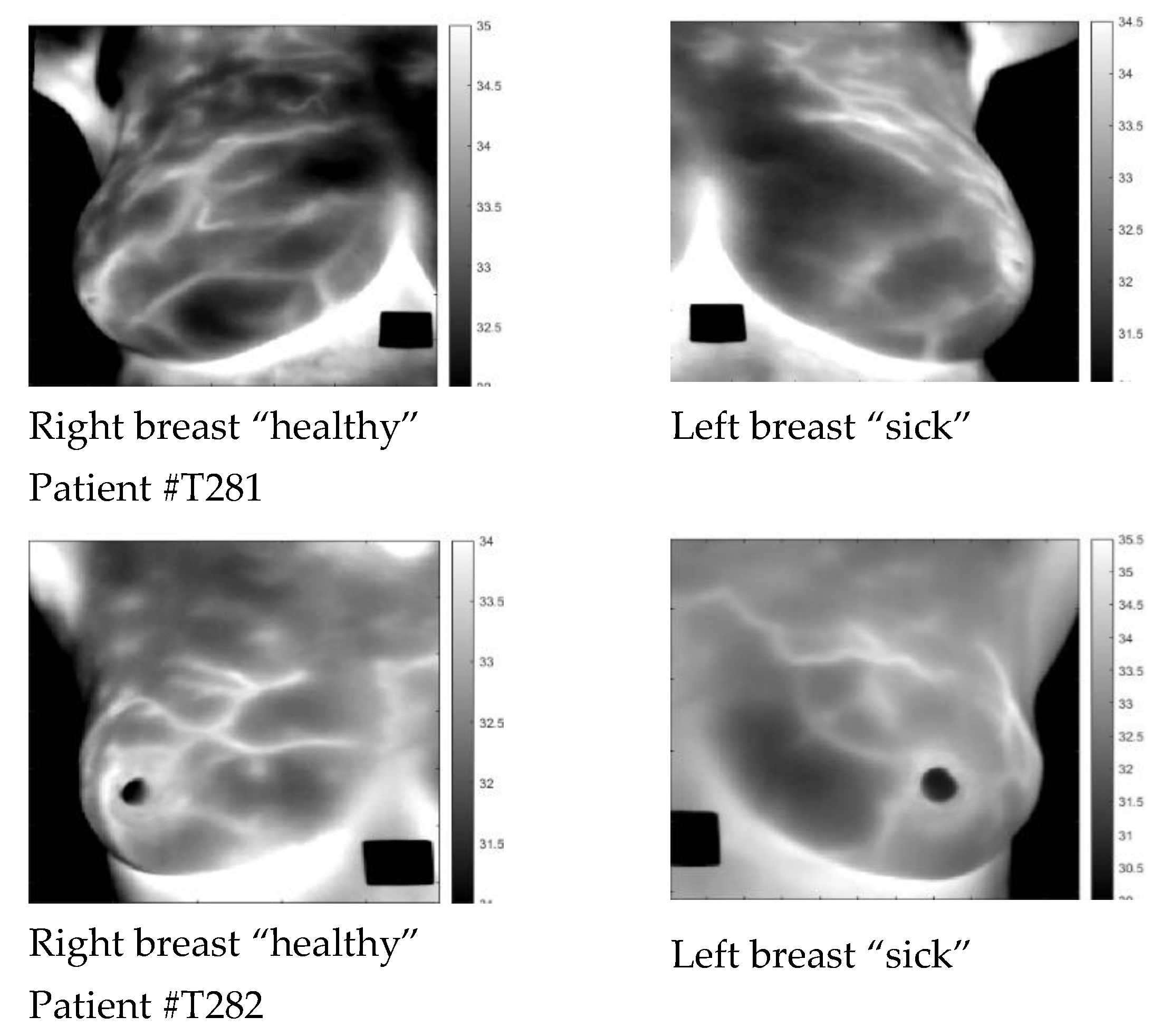



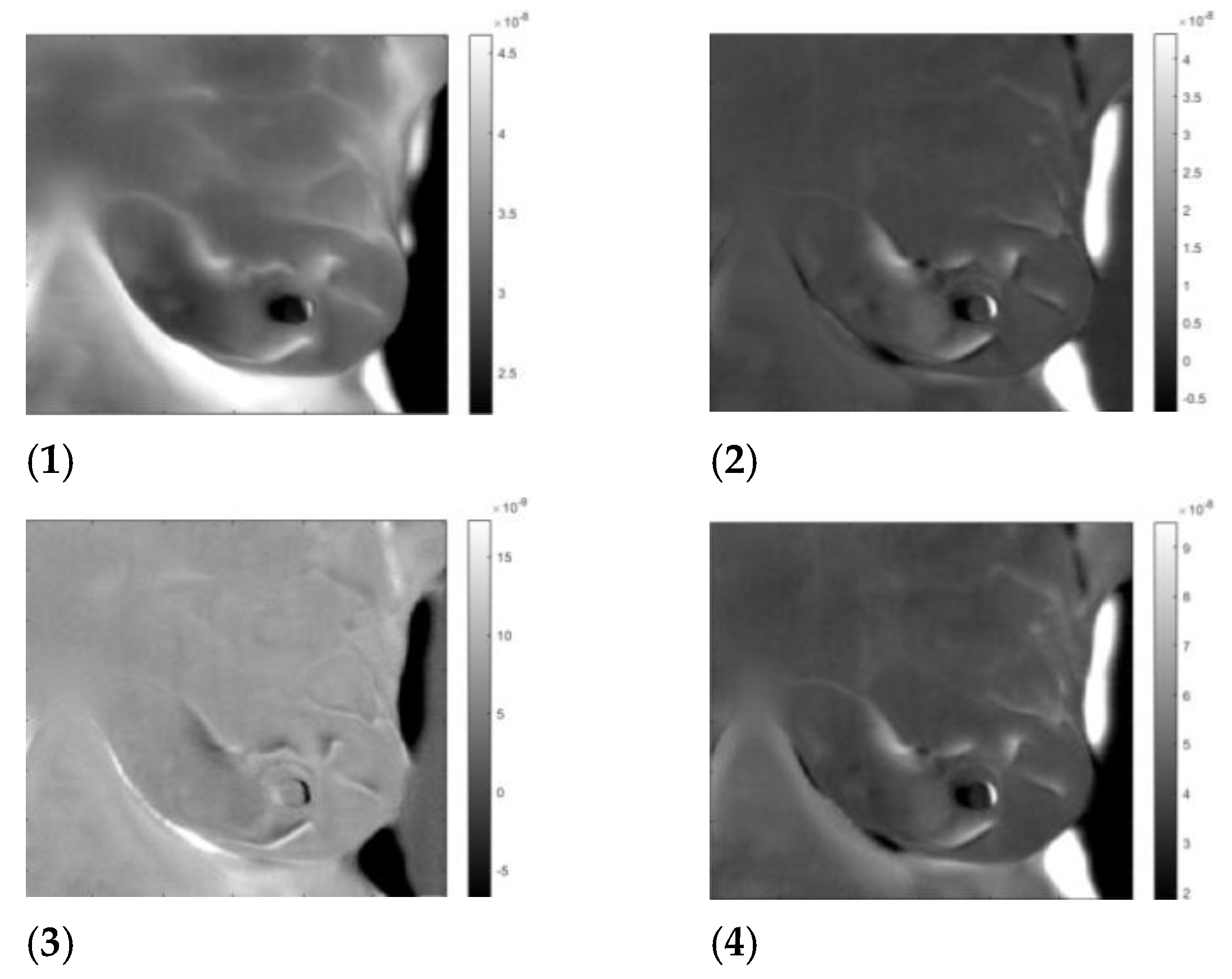
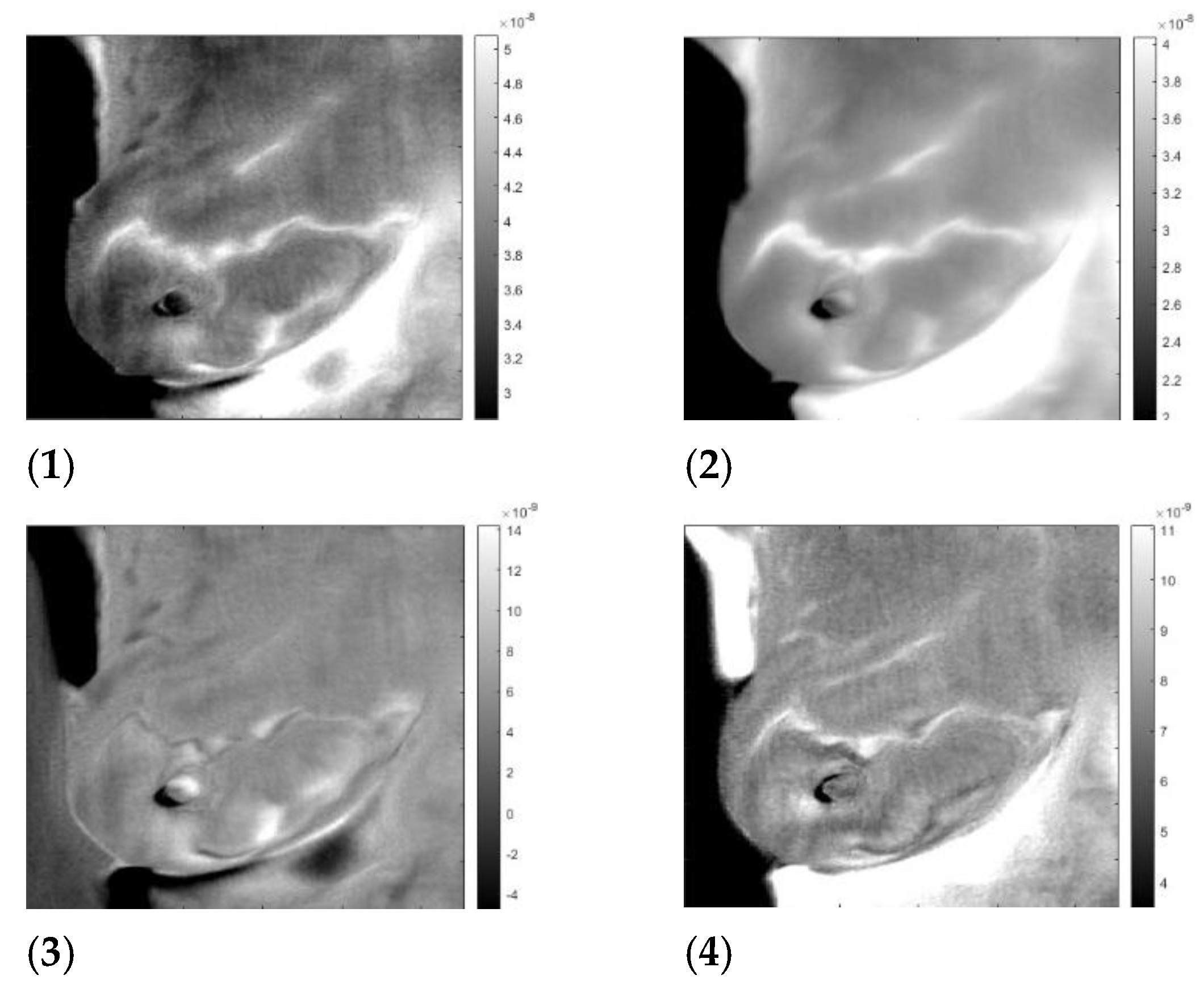
| Patient | Age | Exam Details | Diagnosis | Quadrant | Biopsy | Complaint |
|---|---|---|---|---|---|---|
| T281 | 43 | Left | Sick | QSL | left | Pain in both breasts with burning |
| T282 | 46 | Left | Sick | QIM | left | Pain in both breasts |
| T285 | 57 | Right | Sick | QIL | right | Pain in the right breast |
| T286 | 50 | Left | Infiltrating Ductal Carcinoma | QSL | left | Itching in both breasts, secretion in the left breast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gershenson, M.; Gershenson, J. Dynamic Vascular Imaging Using Active Breast Thermography. Sensors 2023, 23, 3012. https://doi.org/10.3390/s23063012
Gershenson M, Gershenson J. Dynamic Vascular Imaging Using Active Breast Thermography. Sensors. 2023; 23(6):3012. https://doi.org/10.3390/s23063012
Chicago/Turabian StyleGershenson, Meir, and Jonathan Gershenson. 2023. "Dynamic Vascular Imaging Using Active Breast Thermography" Sensors 23, no. 6: 3012. https://doi.org/10.3390/s23063012
APA StyleGershenson, M., & Gershenson, J. (2023). Dynamic Vascular Imaging Using Active Breast Thermography. Sensors, 23(6), 3012. https://doi.org/10.3390/s23063012









