Nanoflowers on Microporous Graphene Electrodes as a Highly Sensitive and Low-Cost As(III) Electrochemical Sensor for Water Quality Monitoring
Abstract
:1. Introduction
2. Materials and Methods
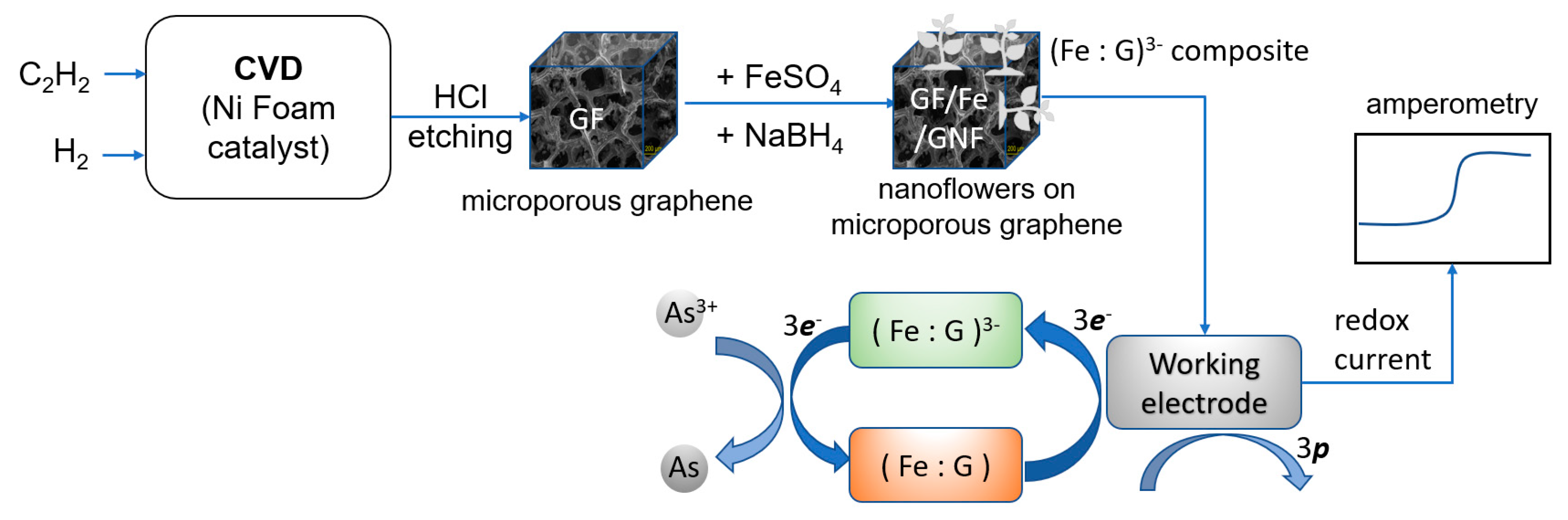
2.1. Fabrication of Microporous Graphene
2.2. Chemicals and Reagents
2.3. Nanoflower Decoration
2.4. Material Characterization
2.5. Overall Process
3. Results and Discussion
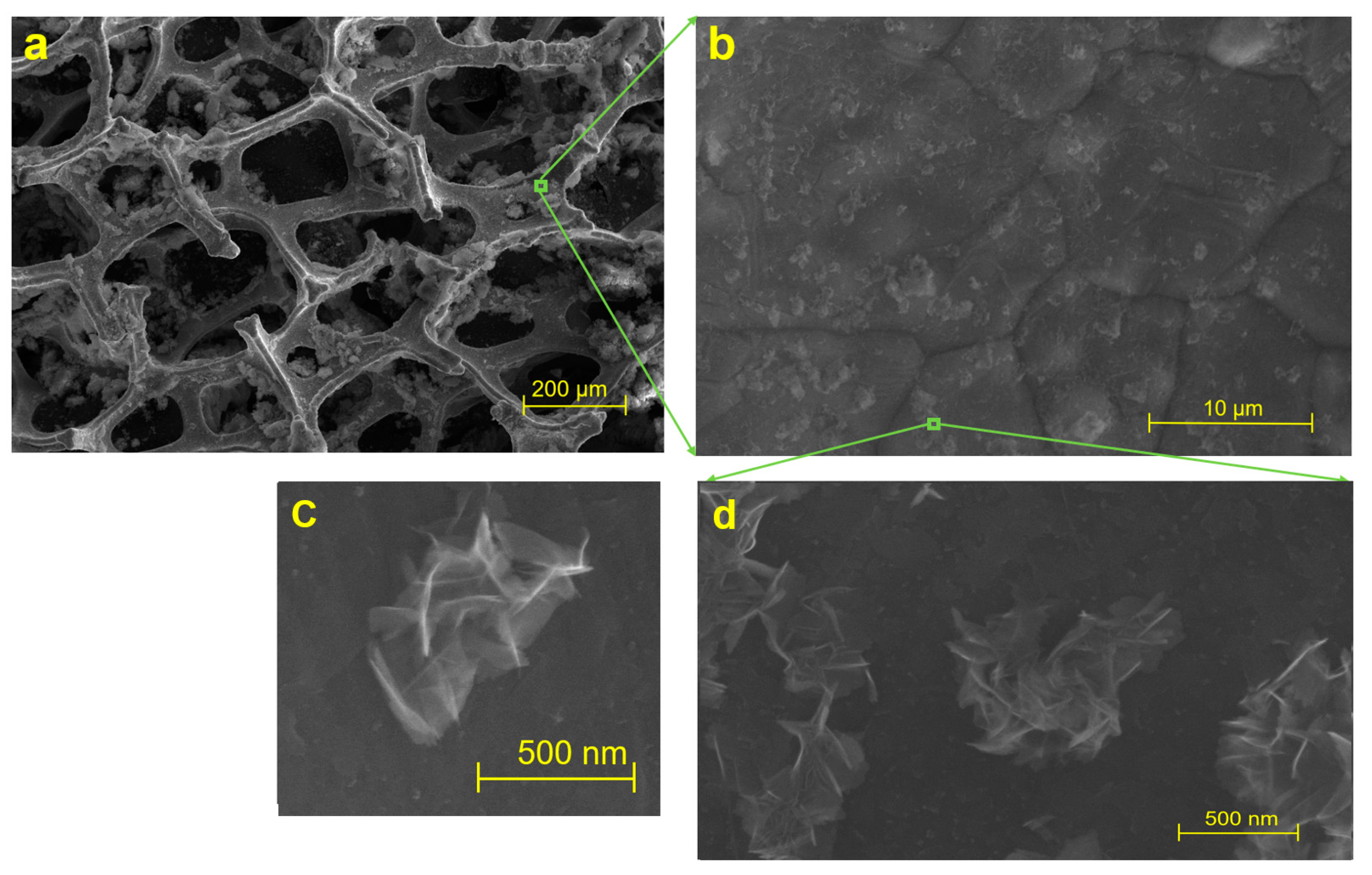
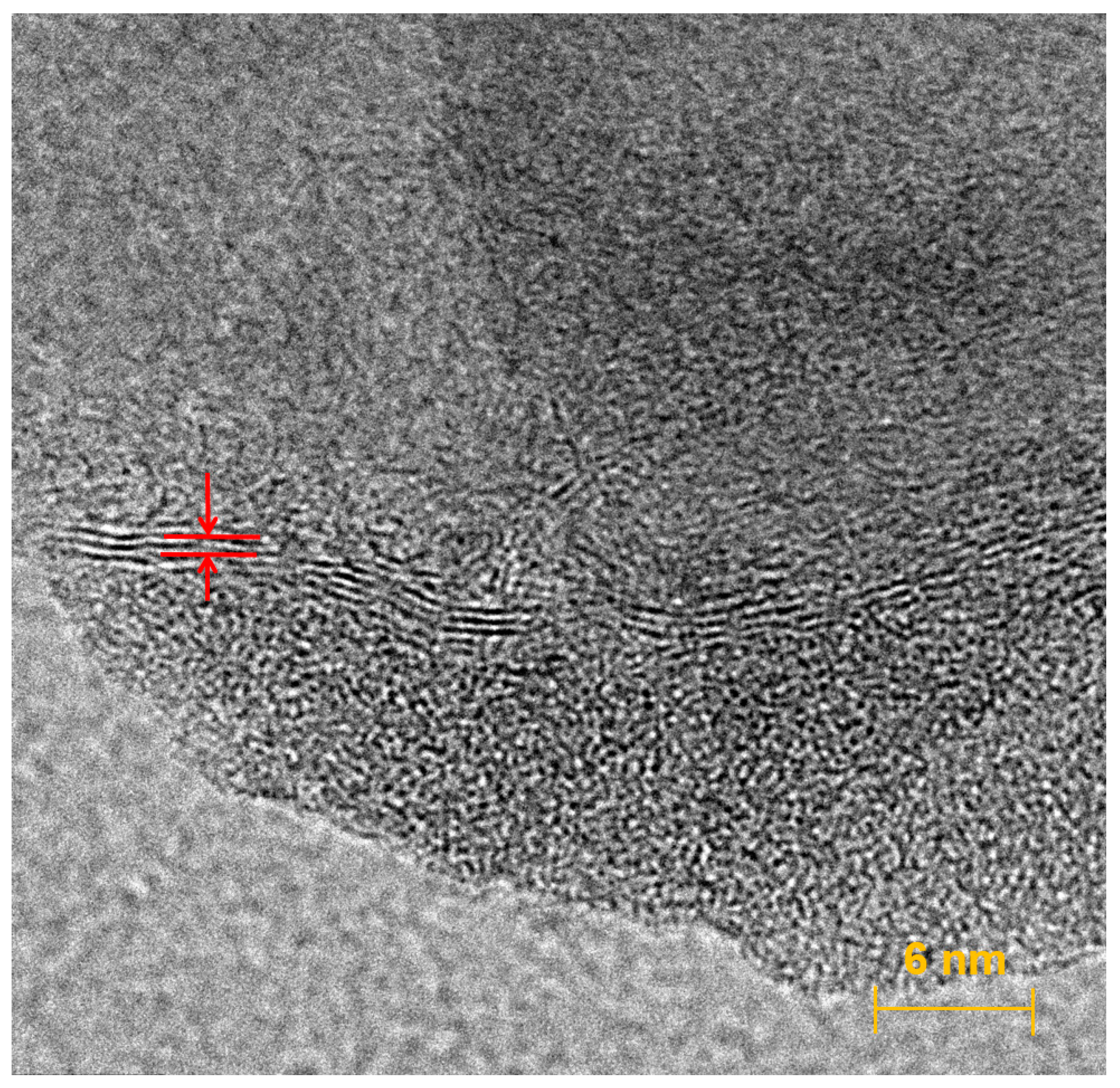
3.1. Structure of Graphene Nanoflowers or Fractal Micro-Nanoporous Graphene
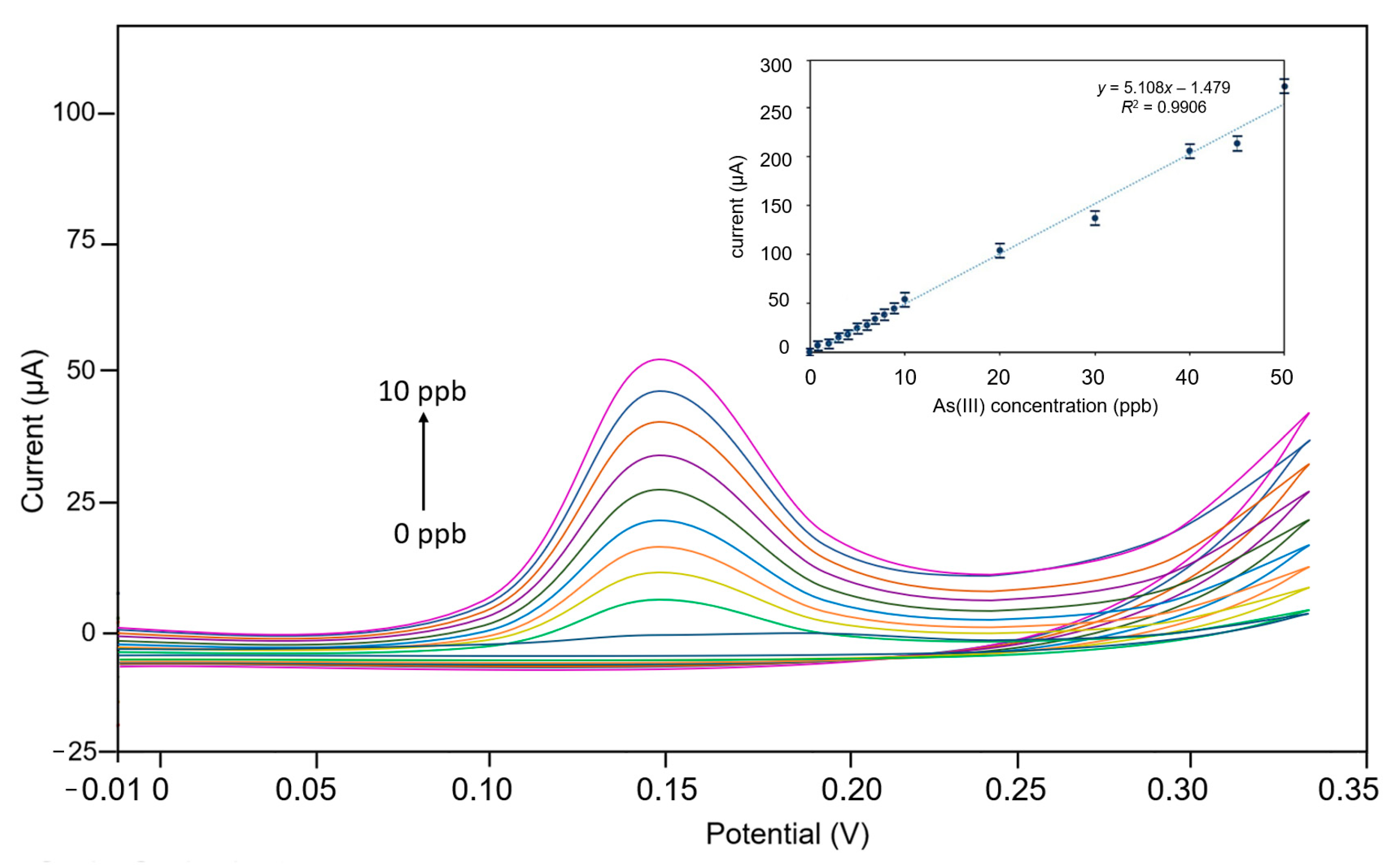
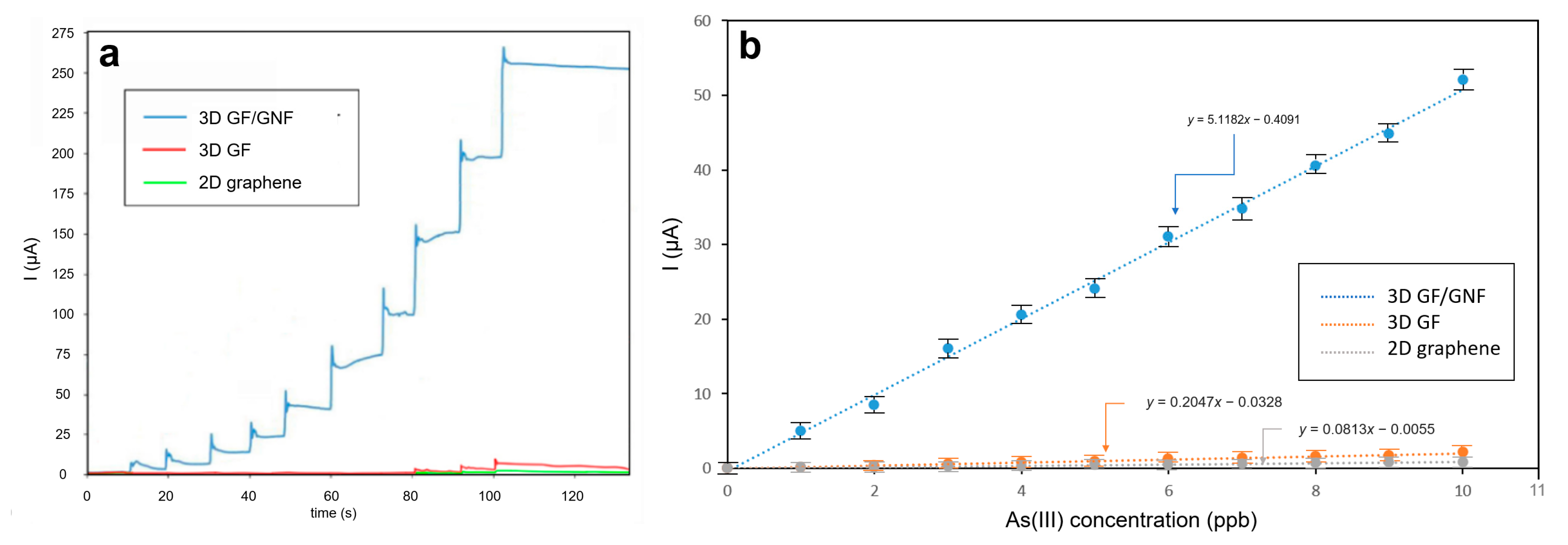
3.2. Electrochemical Measurements
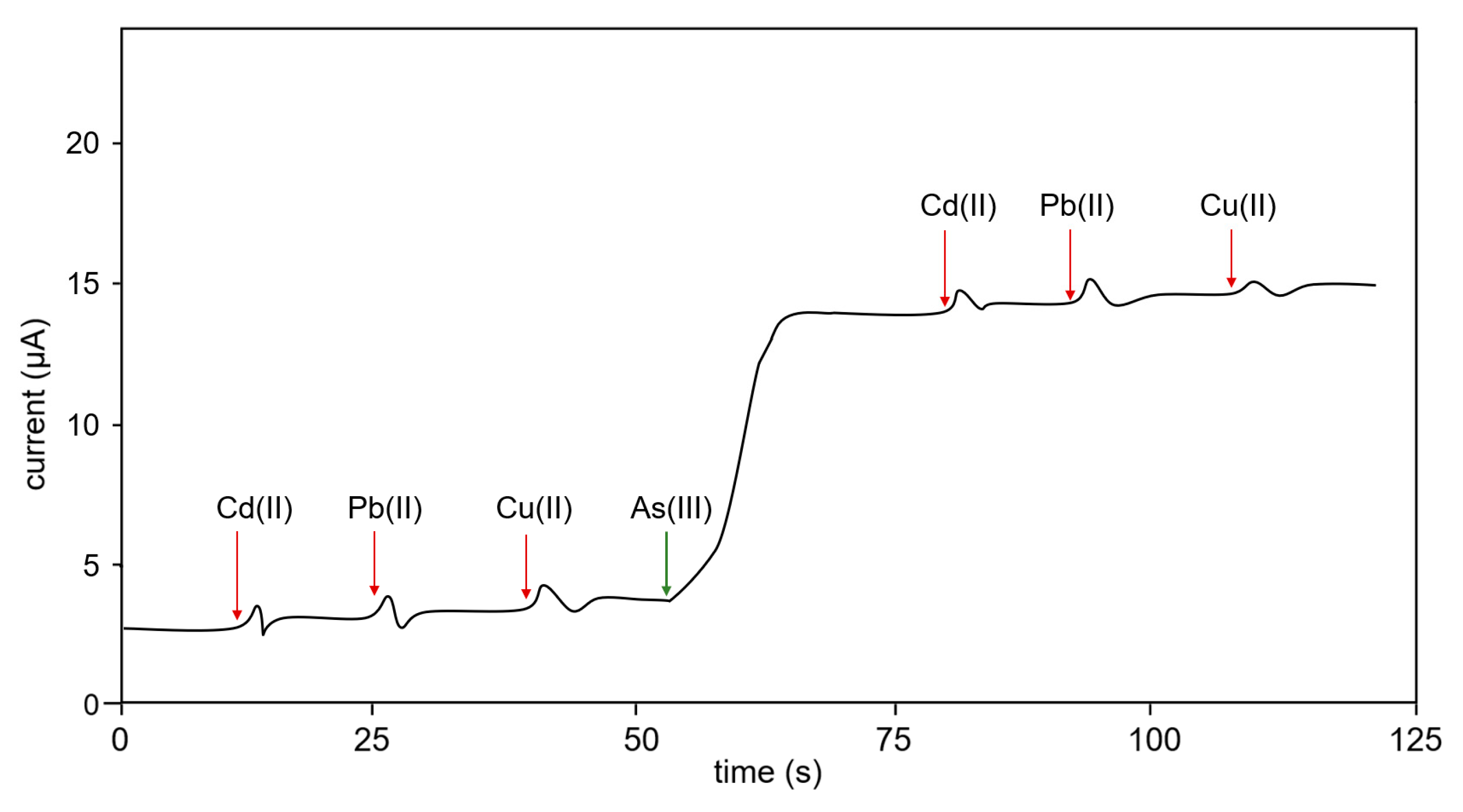
3.3. Interference Test
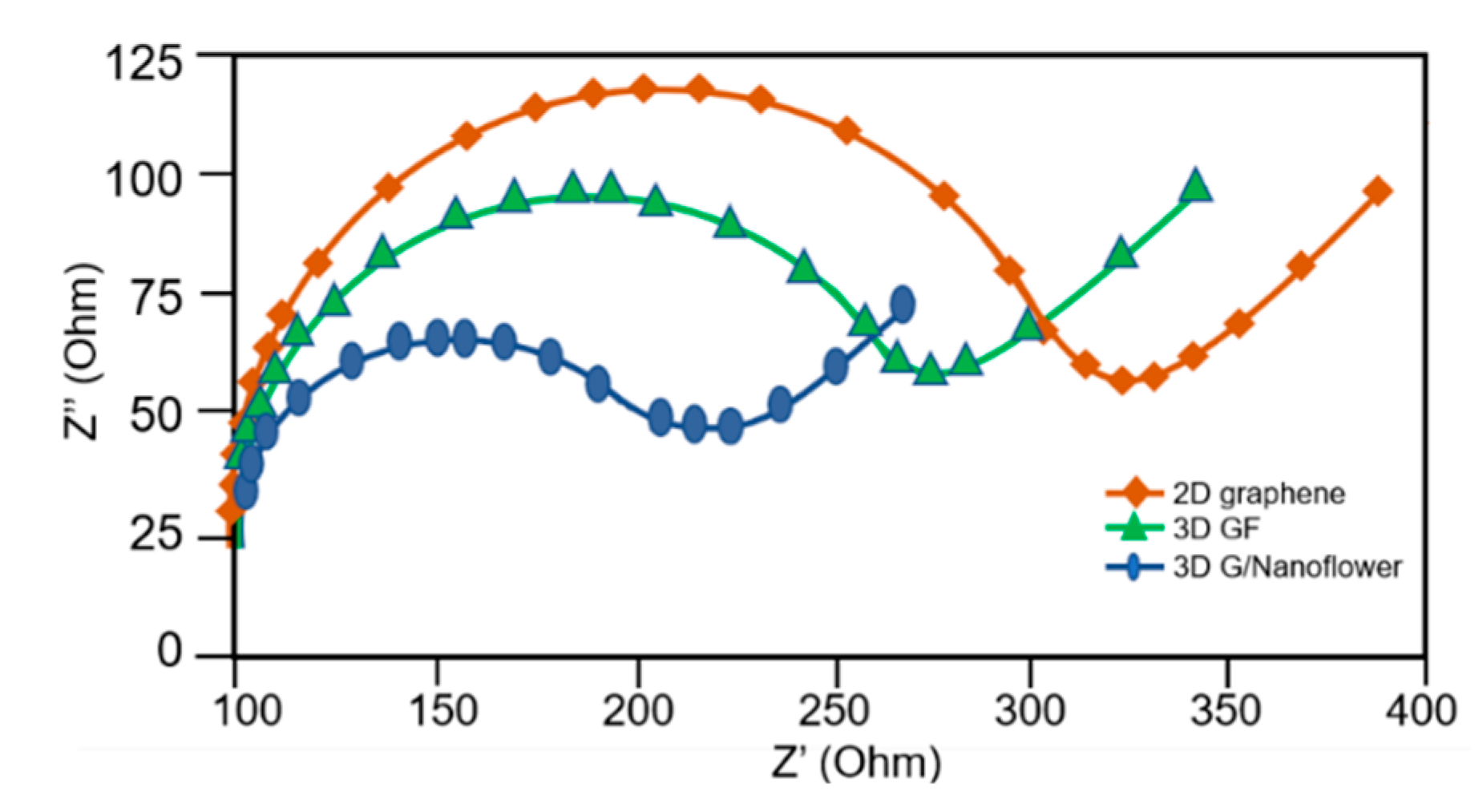
3.4. Electrochemical Impedance Spectroscopy
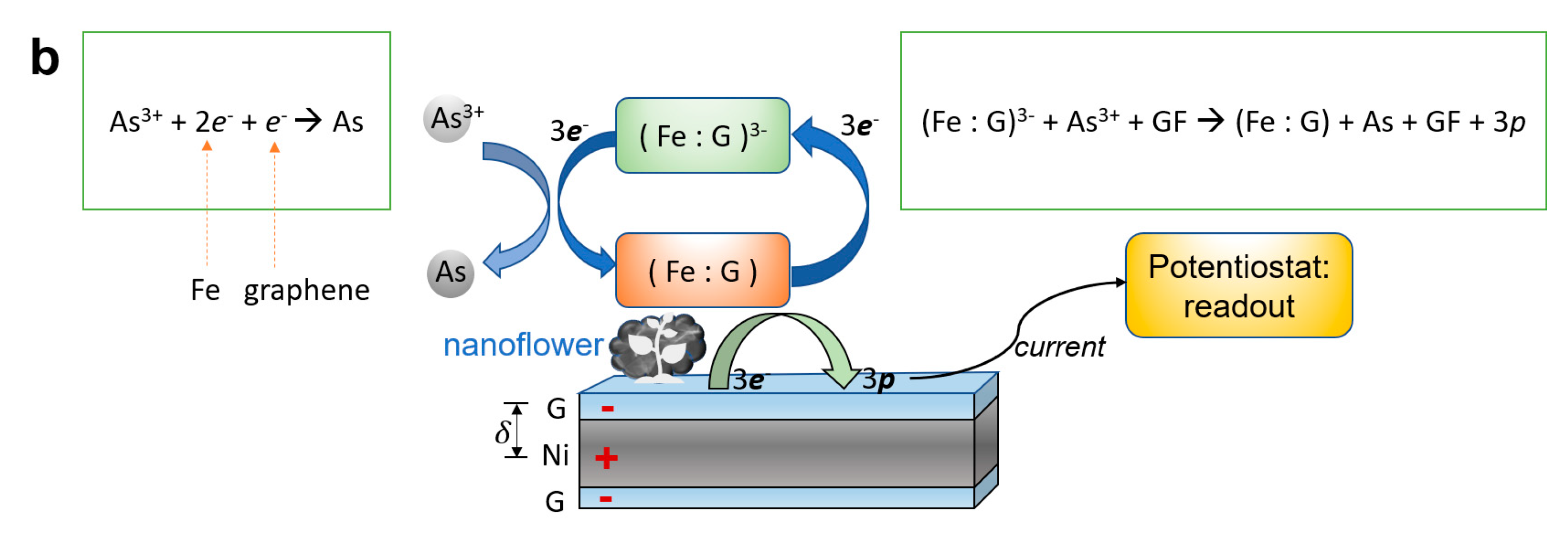
3.5. Sensing Mechanism
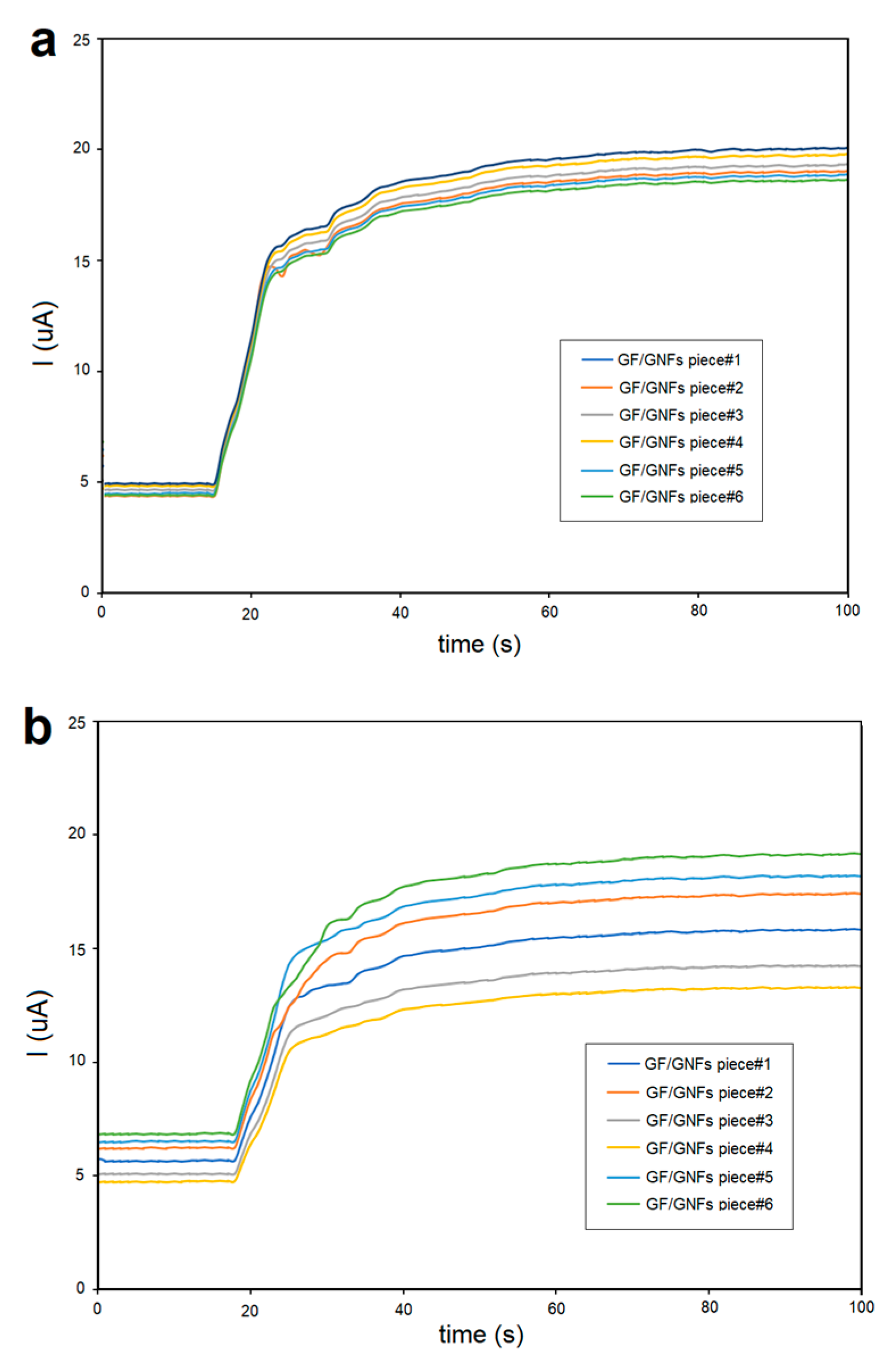
3.6. Reproducibility
3.7. Actual Tests on Environmental Water Sources
3.8. Discussion
- Novel nanoflowers grown on a microporous graphene foam surface formed a gigantic surface area enrichment and a highly sensitive electrode.
- LOD achieved was 1 ppb, comparable to lab-scale methods, such as ICP-MS, HPLC, and AAS.
- Sensitivity was measured as 5000 μA/mgL−1 (response current/As(III) concentration), which was 50–60 times greater than that of bare microporous graphene and flat graphene.
- It was highly specific to As(III) under Cd(II), Pb(II), and Cu(II) interference.
- It had a low cost compared to the AuNPs-based method and other large laboratory-scale methods.
- Portable devices and on-site investigation of As(III) contamination are possible for the offensive prevention of As(III) contamination in agricultural water and drinking water.
- It required a smaller sample size in comparison with other spectroscopic-based approaches, such as AAS, ICP MS, and AFS.
- Amperometric detection supports rapid readout and is suitable for field experiments for actual As(III) screening.
- Reproducibility was tested, and RSD% gradually increased from 6% to 10% within 1 week.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrıguez, V.M.; Jiménez-Capdeville, M.E.; Giordano, M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003, 145, 1–18. [Google Scholar] [CrossRef]
- de Mora, K.; Joshi, N.; Balint, B.L.; Ward, F.B.; Elfick, A.; French, C.E. A pH-based biosensor for detection of arsenic in drinking water. Anal Bioanal. Chem. 2011, 400, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Umar, A. Fabrication and characterization of highly sensitive and selective arsenic sensor based on ultra-thin graphene oxide nanosheets. Sens. Actuators B Chem. 2016, 227, 29–34. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, C.-C.; Ho, S.-C.; Chiu, H.-F. Is colon cancer mortality related to arsenic exposure? J. Toxicol. Environ. Health A 2008, 71, 533–538. [Google Scholar] [CrossRef]
- Kuivenhoven, M.; Mason, K. “Arsenic Toxicity” NIH National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541125/ (accessed on 19 January 2023).
- Montoro-Leal, P.; Garcia-Mesa, J.C.; Morales-Benitez, I.; Garcia de Torres, A.; Vereda Alonso, E. Semiautomatic method for the ultra-trace arsenic speciation in environmental and biological samples via magnetic solid phase extraction prior to HPLC-ICP-MS determination. Talanta 2021, 235, 122769. [Google Scholar] [CrossRef]
- Qi, Y.; Mao, X.; Liu, J.; Na, X.; Chen, G.; Liu, M.; Zheng, C.; Qian, Y. In Situ Dielectric Barrier Discharge Trap for Ultrasensitive Arsenic Determination by Atomic Fluorescence Spectrometry. Anal. Chem. 2018, 90, 6332–6338. [Google Scholar] [CrossRef]
- Shrivas, K.; Shankar, R.; Dewangan, K. Gold nanoparticles as a localized surface plasmon resonance based chemical sensor for on-site colorimetric detection of arsenic in water samples. Sens. Actuators B Chem. 2015, 220, 1376–1383. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, S.; Sinha, D.; Thakur, S.S.; Patle, T.K.; Kant, T.; Dewangan, K.; Satnami, M.L.; Nirmalkar, J.; Kumar, S. Colorimetric and smartphone-integrated paper device for on-site determination of arsenic (III) using sucrose modified gold nanoparticles as a nanoprobe. Mikrochim. Acta 2020, 187, 173. [Google Scholar] [CrossRef]
- Ge, H.; Yin, R.; Su, P.; Yu, L.; Lei, M.; Sun, M.; Sun, Z.; Wang, S. On-site detection of As(III) based on silver nanoparticles aggregation mediated by phosphates using surface-enhanced Raman scattering (SERS). Microchim. Acta 2022, 189, 44. [Google Scholar] [CrossRef]
- Melinte, G.; Hosu, O.; Lettieri, M.; Cristea, C.; Marrazza, G. Electrochemical Fingerprint of Arsenic (III) by Using Hybrid Nanocomposite-Based Platforms. Sensors 2019, 19, 2279. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Lu, W.; Liu, X.; Meng, F.; Zhu, J. A High-Response Electrochemical As(III) Sensor Using Fe3O4–rGO Nanocomposite Materials. Chemosensors 2021, 9, 150. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Jia, X.; Han, Y.; Wang, E. Electrochemical determination of arsenic(III) on mercaptoethylamine modified Au electrode in neutral media. Anal. Chim. Acta 2012, 733, 23–27. [Google Scholar] [CrossRef]
- Hu, H.; Xie, B.; Lu, Y.; Zhu, J. Advances in Electrochemical Detection Electrodes for As(III). Nanomaterials 2022, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Danvirutai, P.; Ekpanyapong, M.; Tuantranont, A.; Bohez, E.; Anutrakulchai, S.; Wisitsoraat, A.; Srichan, C. Ultra-sensitive and label-free neutrophil gelatinase-associated lipocalin electrochemical sensor using gold nanoparticles decorated 3D Graphene foam towards acute kidney injury detection. Sens. Bio-Sens. Res. 2020, 30, 100380. [Google Scholar] [CrossRef]
- Zeng, G.; Li, W.; Ci, S.; Jia, J.; Wen, Z. Highly Dispersed NiO Nanoparticles Decorating graphene Nanosheets for Non-enzymatic Glucose Sensor and Biofuel Cell. Sci. Rep. 2016, 6, 36454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srichan, C.; Ekpanyapong, M.; Horprathum, M.; Eiamchai, P.; Nuntawong, N.; Phokharatkul, D.; Danvirutai, P.; Bohez, E.; Wisitsoraat, A.; Tuantranont, A. Highly-Sensitive Surface-Enhanced Raman Spectroscopy (SERS)-based Chemical Sensor using 3D Graphene Foam Decorated with Silver Nanoparticles as SERS substrate. Sci. Rep. 2016, 6, 23733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcharbin, D.; Halets-Bui, I.; Abashkin, V.; Dzmitruk, V.; Loznikova, S.; Odabaşı, M.; Acet, Ö.; Önal, B.; Özdemir, N.; Shcharbina, N.; et al. Hybrid metal-organic nanoflowers and their application in biotechnology and medicine. Colloids Surf. B 2019, 182, 110354. [Google Scholar] [CrossRef]
- Babar, N.-U.; Joya, K.S.; Tayyab, M.A.; Ashiq, M.N.; Sohail, M. Highly Sensitive and Selective Detection of Arsenic Using Electrogenerated Nanotextured Gold Assemblage. ACS Omega 2019, 4, 13645–13657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lee, B.-J.; Li, H.; Samdani, J.; Kang, T.-H.; Yu, J.-S. Catalytic mechanism of graphene-nickel interface dipole layer for binder free electrochemical sensor applications. Commun. Chem. 2018, 1, 94. [Google Scholar] [CrossRef] [Green Version]
- Gubler, R.; ThomasArrigo, L.K. Ferrous iron enhances arsenic sorption and oxidation by non-stoichiometric magnetite and maghemite. J. Hazard. Mater. 2021, 402, 123425. [Google Scholar] [CrossRef]
- Çubik, S.; Taskan, M.C.; KÖk Yetimoglu, E.; Kahraman, M.V. A New Fluorescent Sensor for Arsenic(III) Determination in Aqueous Media. Anal. Sci. 2020, 36, 807–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ariza, J.L.; Sánchez-Rodas, D.; Giráldez, I.; Morales, E. A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples. Talanta 2000, 51, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kempegowda, R.; Antony, D.; Malingappa, P. Graphene–platinum nanocomposite as a sensitive and selective voltametric sensor for trace level arsenic quantification. Int. J. Smart Nano Mater. 2014, 5, 17–32. [Google Scholar] [CrossRef] [Green Version]

| Natural Water Resource Label | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| our method | 5.0 (ppb) | 3.0 (ppb) | 4.0 (ppb) | 5 (ppb) | 7 (ppb) |
| reference method | 5.21 (ppb) | 2.78 (ppb) | 4.15 (ppb) | 4.86 (ppb) | 7.20 (ppb) |
| Materials/ Methods | Detected Species | LOD (μg/L) | LR (μg/L) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| GO nanosheet | As(III) | 500 | not reported | Carbon-based: low toxicity and low cost | low sensitivity | [3] |
| HPLC-ICP-MS | Total arsenic and arsenic speciation | 0.6–6 | 0.005–10 | US EPA approved | expense | [6] |
| MSPE with HPLC-ICP-MS | As(III) | 0.0011 | 1–10 | ultra-high sensitivity | Expensive and not real-time available on site | [6] |
| AuNPs + colorimetry | As(III) | 2.0 | 5–500 | On-site detection | more expensive than carbon-based method | [8] |
| Pt/Au nanoparticles + PANI | As(III) | 1.48 | 2.47–14.98 | ease of use | Expenses of Au and Pt precursor | [11] |
| Fe3O4–rGO/SWV | As(III) | 1.19 | 1–20 | Carbon-based: low toxicity and low cost | moderate sensitivity | [12] |
| porous gold electrode | As(III) | 0.1 | 0.1–70 | high sensitivity | expense in Au nanostructure fabrication | [13] |
| Fluorescent | As(III) | 0.18 | 0.5–2.99 | moderate sensitivity | expensive, dilution required before measurement | [22] |
| ICP-MS | Total arsenic | ~0.1 | Tunable | US EPA approved | Expensive and Spectral interference | [23] |
| Graphene-Pt | As(III) | 0.008 | 0.75–7.5 | ultra-high sensitivity | cost and time to synthesize graphene-platinum composites and required dilution before measurement | [24] |
| GF + graphene nanoflowers | As(III) | 1.0 | 1–50 | lower cost, rapid readout and on-site availability | not reported | This work |
| Measurement Method | Value | SD |
|---|---|---|
| CV | I (μA) | 1.2 μA |
| Chronoamperometry | I (μA) | 1.51 μA |
| EIS | Rct (Ohm) | 4.1 Ohm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosuvun, M.; Danvirutai, P.; Hormdee, D.; Chaosakul, A.; Tanboonchuy, V.; Siritaratiwat, A.; Anutrakulchai, S.; Sharma, A.; Tuantranont, A.; Srichan, C. Nanoflowers on Microporous Graphene Electrodes as a Highly Sensitive and Low-Cost As(III) Electrochemical Sensor for Water Quality Monitoring. Sensors 2023, 23, 3099. https://doi.org/10.3390/s23063099
Kosuvun M, Danvirutai P, Hormdee D, Chaosakul A, Tanboonchuy V, Siritaratiwat A, Anutrakulchai S, Sharma A, Tuantranont A, Srichan C. Nanoflowers on Microporous Graphene Electrodes as a Highly Sensitive and Low-Cost As(III) Electrochemical Sensor for Water Quality Monitoring. Sensors. 2023; 23(6):3099. https://doi.org/10.3390/s23063099
Chicago/Turabian StyleKosuvun, Mahatthanah, Pobporn Danvirutai, Daranee Hormdee, Arnut Chaosakul, Visanu Tanboonchuy, Apirat Siritaratiwat, Sirirat Anutrakulchai, Amod Sharma, Adisorn Tuantranont, and Chavis Srichan. 2023. "Nanoflowers on Microporous Graphene Electrodes as a Highly Sensitive and Low-Cost As(III) Electrochemical Sensor for Water Quality Monitoring" Sensors 23, no. 6: 3099. https://doi.org/10.3390/s23063099






