Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensory Test
2.2. Reagents
2.3. Lipid/Polymer Membrane
- Prepare a 45 mm petri dish and a 20 mL vial snap;
- Dissolve TFPB, NPOE, and PVC with 10 mL THF in the vial snap;
- Add Na+ solution ionophore accordingly into the vial snap.
- Stir the vial snap for 1 h and pour the content into the petri dish.
- Dry the petri dish for three days in a draft chamber with a temperature of 25 °C to let the THF be fully volatilized.
2.4. Samples
2.5. Sensor Preparation
2.6. Measurement Procedures
3. Results
3.1. Saltiness Enhancement Effect of Quinine
3.2. Response to NaCl
3.3. Response to Saltiness Enhancement Samples
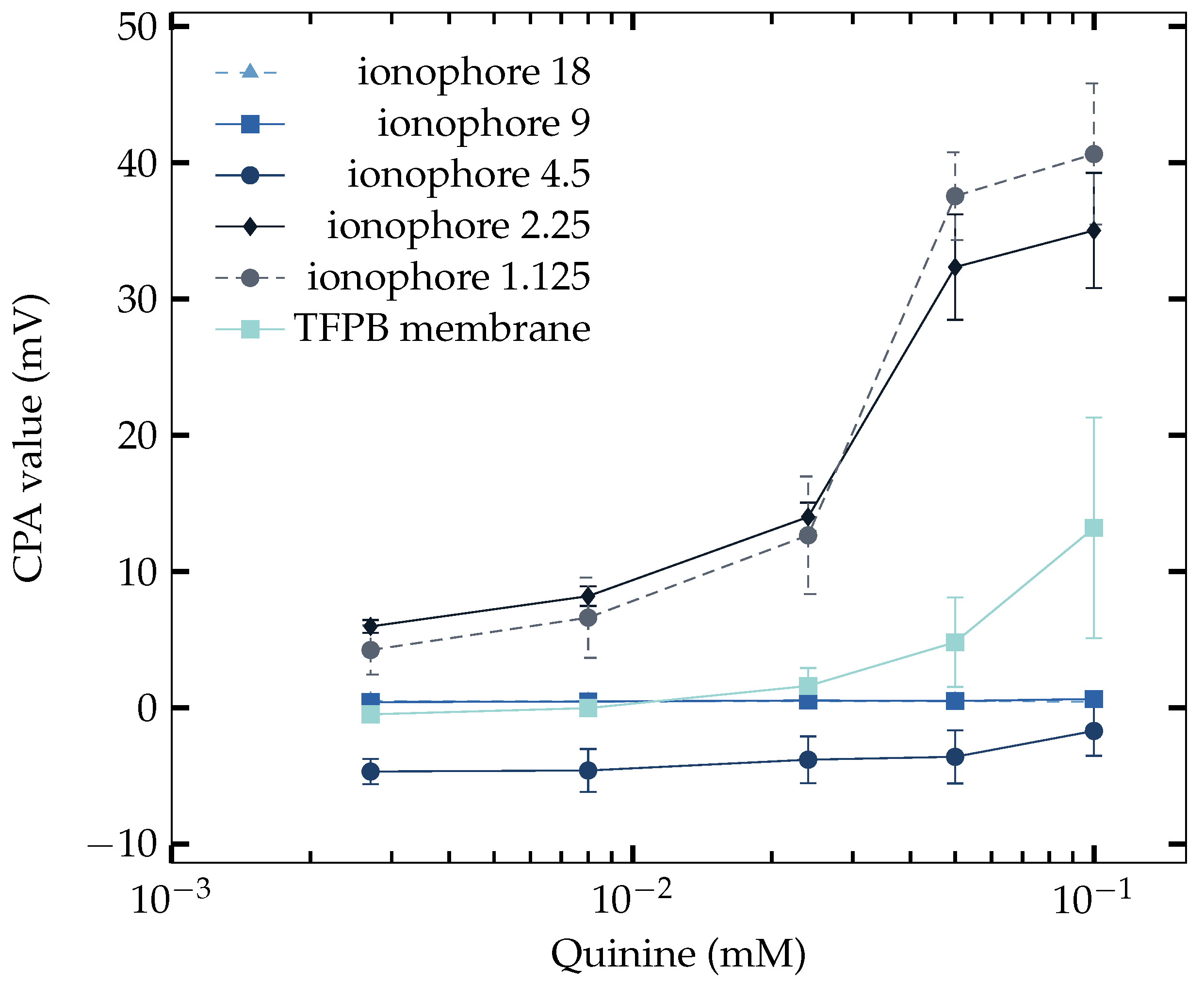
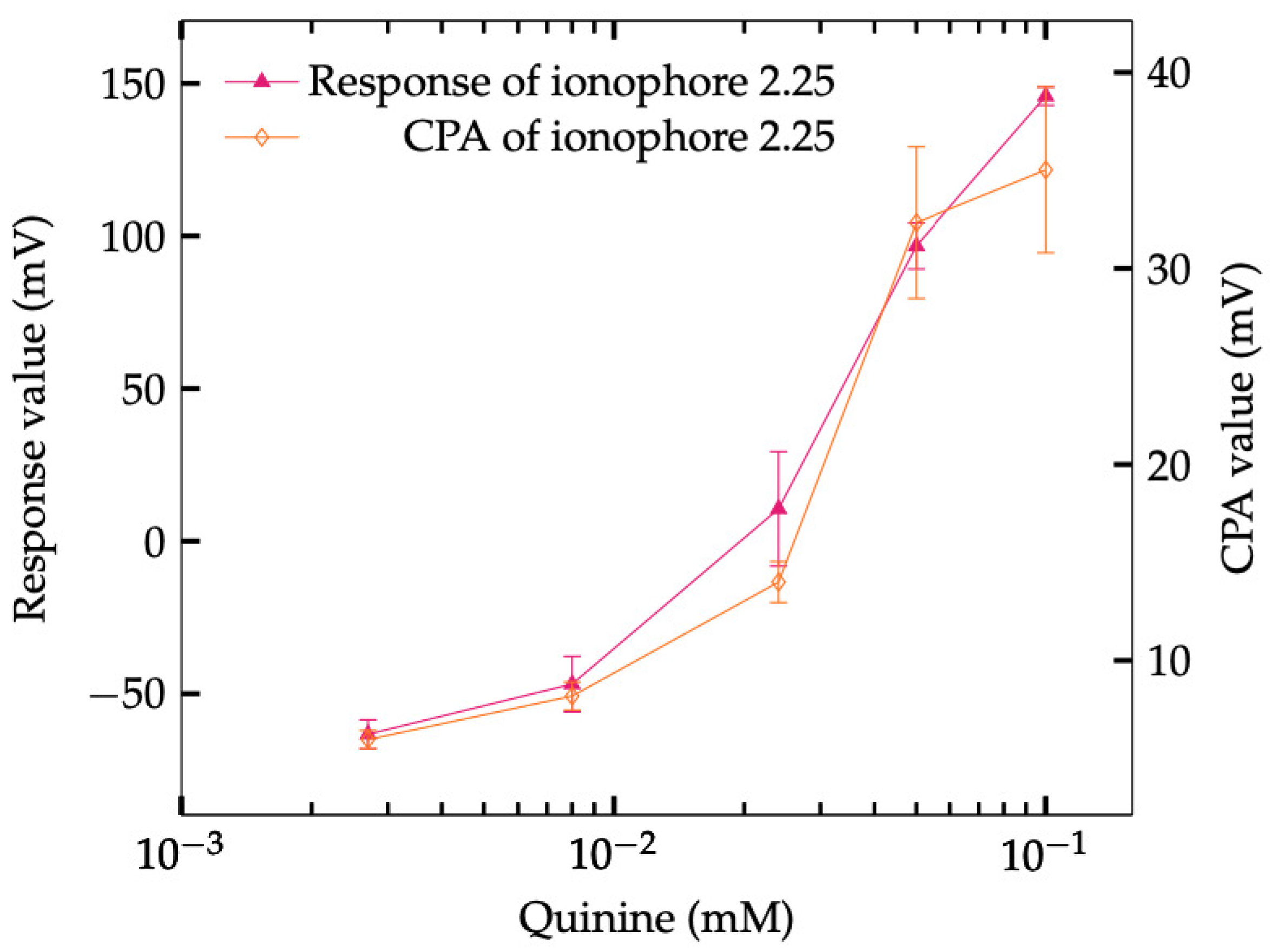
3.4. Saltiness Enhancement Effect Measurement
3.5. Adsorption Property
3.6. Proposal Composition of NaISE
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PADE | phosphoric acid di-n-decyl ester |
| TFPB | Bis[(12-crown-4)methyl] 2-dodecyl-2-methylmalonate |
| NaISE | Na+ ion-selective electrode |
| BCAAs | branched-chain amino acids |
| PVC | polyvinyl chloride |
References
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Kirsanov, D.; Ciosek, P.; del Valle, M.; Yaroshenko, I.; Wesoły, M.; Zabadaj, M.; Gonzalez-Calabuig, A.; Wróblewski, W.; Legin, A. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2015, 114, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, P.; Du, L.; Wu, C. Advances in gustatory biomimetic biosensing technologies: In vitro and in vivo bioelectronic tongue. Trends Anal. Chem. 2022, 157, 116778. [Google Scholar] [CrossRef]
- Banerjee, R.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. A review on combined odor and taste sensor systems. J. Food Eng. 2016, 190, 10–21. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in recent applications of electronic tongues in food analysis. Anal. Chim. Acta 2010, 665, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing – electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F. Voltammetric electronic tongues – basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Natale, C.D.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues–a review. IEEE Sensors J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Wu, X.; Toko, K. Taste sensor with multiarray lipid/polymer membranes. Trends Anal. Chem. 2023, 158, 116874. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kondo, R.; Mabuchi, R.; Watanabe, E.; Nobayashi, K.; Fujita, Y. Antioxidant activity and taste-active component distribution in the bran layer of rice grain. Food Sci. Technol. Res. 2020, 26, 855–862. [Google Scholar] [CrossRef]
- Ujihara, T.; Hayashi, N.; Ikezaki, H. Objective evaluation of astringent and umami taste intensities of matcha using a taste sensor system. Food Sci. Technol. Res. 2013, 19, 1099–1105. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ismail, I.; Joo, S.T. The relationship between muscle fiber composition and pork taste-traits assessed by electronic tongue system. Korean J. Food Sci. Anim. Resour. 2018, 38, 1305–1314. [Google Scholar] [CrossRef]
- Toko, K. Biochemical Sensors: Mimicking Gustatory and Olfactory Senses; Pan Stanford Publishing Pte. Ltd.: Singapore, 2013. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hamada, H.; Yamaguchi, Y.; Ikezaki, H.; Toko, K. Development of an artificial lipid-based membrane sensor with high selectivity and sensitivity to the bitterness of drugs and with high correlation with sensory score. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 710–719. [Google Scholar] [CrossRef]
- Harada, T.; Uchida, T.; Yoshida, M.; Kobayashi, Y.; Narazaki, R.; Ohwaki, T. A new method for evaluating the bitterness of medicines in development using a taste sensor and a disintegration testing apparatus. Chem. Pharm. Bull. 2010, 58, 1009–1014. [Google Scholar] [CrossRef]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Prescott, J.; Johnstone, V.; Francis, J. Odor–taste interactions: Effects of attentional strategies during exposure. Chem. Senses 2004, 29, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Zatorre, R.; Jones-Gotman, M. Effects of perceived and imagined odors on taste detection. Chem. Senses 2004, 29, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Onitake, H.; Huang, Z.; Shiino, T.; Tahara, Y.; Yatabe, R.; Ikezaki, H.; Toko, K. Improved Durability and Sensitivity of Bitterness-Sensing Membrane for Medicines. Sensors 2017, 17, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y. Qualitative discrimination among “umami” and the four basic taste substances in mice. In Umami: A Basic Taste; Kawamura, Y., Kare, M.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1987; pp. 365–385. [Google Scholar]
- Dahl, L.K.; Love, R.A. Evidence for relationship between sodium (chloride) intake and human essential hypertension. A.M.A. Arch. Intern. Med. 1954, 94, 525–531. [Google Scholar] [CrossRef]
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013; Technical Report; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Tamura, M.; Seki, T.; Kawasaki, Y.; Tada, M.; Kikuchi, E.; Okai, H. An enhancing effect on the saltiness of sodium chloride of added amino acids and their esters. Agric. Biol. Chem. 1989, 53, 1625–1633. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kohori, J.; Koike, S.; Katsuragi, Y.; Shoji, T. Sodium aspartate as a specific enhancer of salty taste perception—Sodium aspartate is a possible candidate to decrease excessive intake of dietary salt. Chem. Senses 2014, 39, 781–786. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Takahashi, C. Interactions of monosodium glutamate and sodium chloride on saltiness and palatability of a clear soup. J. Food Sci. 1984, 49, 82–85. [Google Scholar] [CrossRef]
- Rocha, R.A.R.; Ribeiro, M.N.; Silva, G.A.; Rocha, L.C.R.; Pinheiro, A.C.M.; Nunes, C.A.; Carneiro, J.d.D.S. Temporal profile of flavor enhancers MAG, MSG, GMP, and IMP, and their ability to enhance salty taste, in different reductions of sodium chloride. J. Food Sci. 2020, 85, 1565–1575. [Google Scholar] [CrossRef]
- Nakatani, F.; Ienaga, T.; Wu, X.; Tahara, Y.; Ikezaki, H.; Sano, H.; Muto, Y.; Kaneda, Y.; Toko, K. Development of a sensor with a lipid/polymer membrane comprising Na+ ionophores to evaluate the saltiness enhancement effect. Sensors 2019, 19, 5251. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. general characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yoshinaga, M.; Funaki, K.; Shibutani, Y.; Yakabe, K.; Shono, T.; Kasai, M.; Mizufune, H.; Tanaka, M. Effects of substituents on ion selectivity of bis(12-crown-4-methyl) malonates as neutral carriers for sodium ion-selective electrodes. Anal. Sci. 1996, 12, 67–70. [Google Scholar] [CrossRef]
- Haraguchi, T.; Uchida, T.; Yoshida, M.; Kojima, H.; Habara, M.; Ikezaki, H. The utility of the artificial taste sensor in evaluating the bitterness of drugs: Correlation with responses of human taste2 receptors (hTAS2Rs). Chem. Pharm. Bull. 2018, 66, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Jing, Y.; Ikezaki, H.; Toko, K. Electrical properties of two types of membrane component used in taste sensors. Sensors 2021, 21, 8343. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Katsuragi, Y.; Matsuoka, I.; Kashiwayanagi, M.; Kumazawa, T.; Shoji, T. Receptor mechanisms of bitter substances. Physiol. Behav. 1994, 56, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Herman, K. Taste of food especially more bitter or sweeter, and its relationship to the chemical structure of taste giving substances. Ernahrungs-Umschau 1972, 19, 251–256. [Google Scholar] [CrossRef]
- Yamaguchi, S. Fundamental properties of umami in human taste sensation. In Umami: A Basic Taste; Kawamura, Y., Kare, M.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1987; pp. 41–73. [Google Scholar]
- Indow, T. A general equi-distance scale of the four qualities of taste. Jpn. Psychol. Res. 1966, 8, 136–150. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, Y.; Tahara, Y.; Habara, M.; Ikezaki, H.; Toko, K. Reusability enhancement of taste sensor using lipid polymer membranes by surfactant cleaning treatment. IEEE Sensors J. 2020, 20, 4579–4586. [Google Scholar] [CrossRef]
- Yatabe, R.; Noda, J.; Tahara, Y.; Naito, Y.; Ikezaki, H.; Toko, K. Analysis of a lipid/polymer membrane for bitterness sensing with a preconditioning process. Sensors 2015, 15, 22439–22450. [Google Scholar] [CrossRef]
- Harada, Y.; Noda, J.; Yatabe, R.; Ikezaki, H.; Toko, K. Research on the changes to the lipid/polymer membrane used in the acidic bitterness sensor caused by preconditioning. Sensors 2016, 16, 230. [Google Scholar] [CrossRef]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the amount of bitter substances adsorbed onto lipid/polymer membrane and the electric response of taste sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, S.; Azuma, Y.; Nagaishi, M.; Toko, K. Change in electric characteristics of membranes in response to taste stimuli with increasing amount of lipids in membrane matrix of PVC and plasticizer. Biophys. Chem. 1996, 61, 23–27. [Google Scholar] [CrossRef] [PubMed]
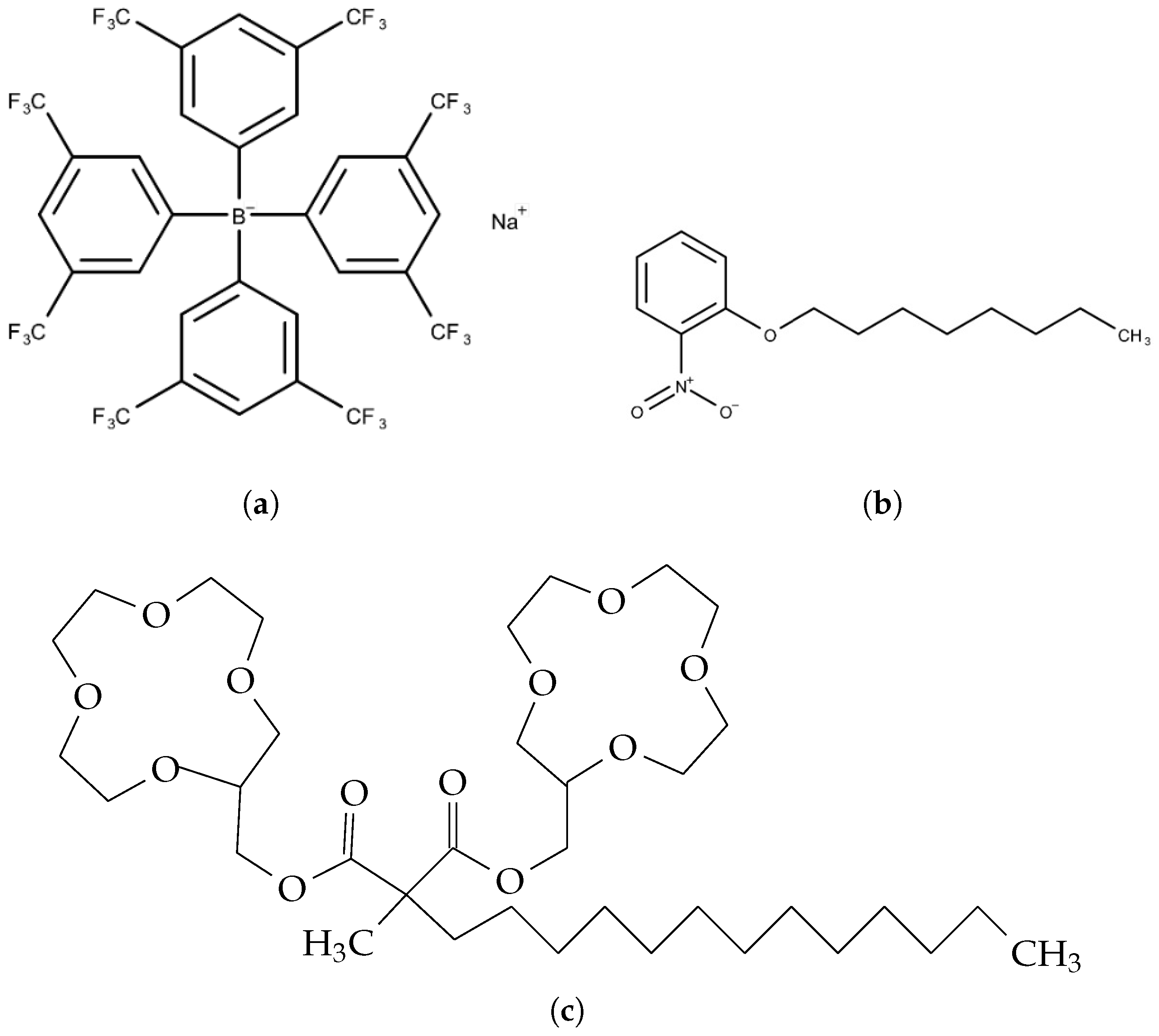
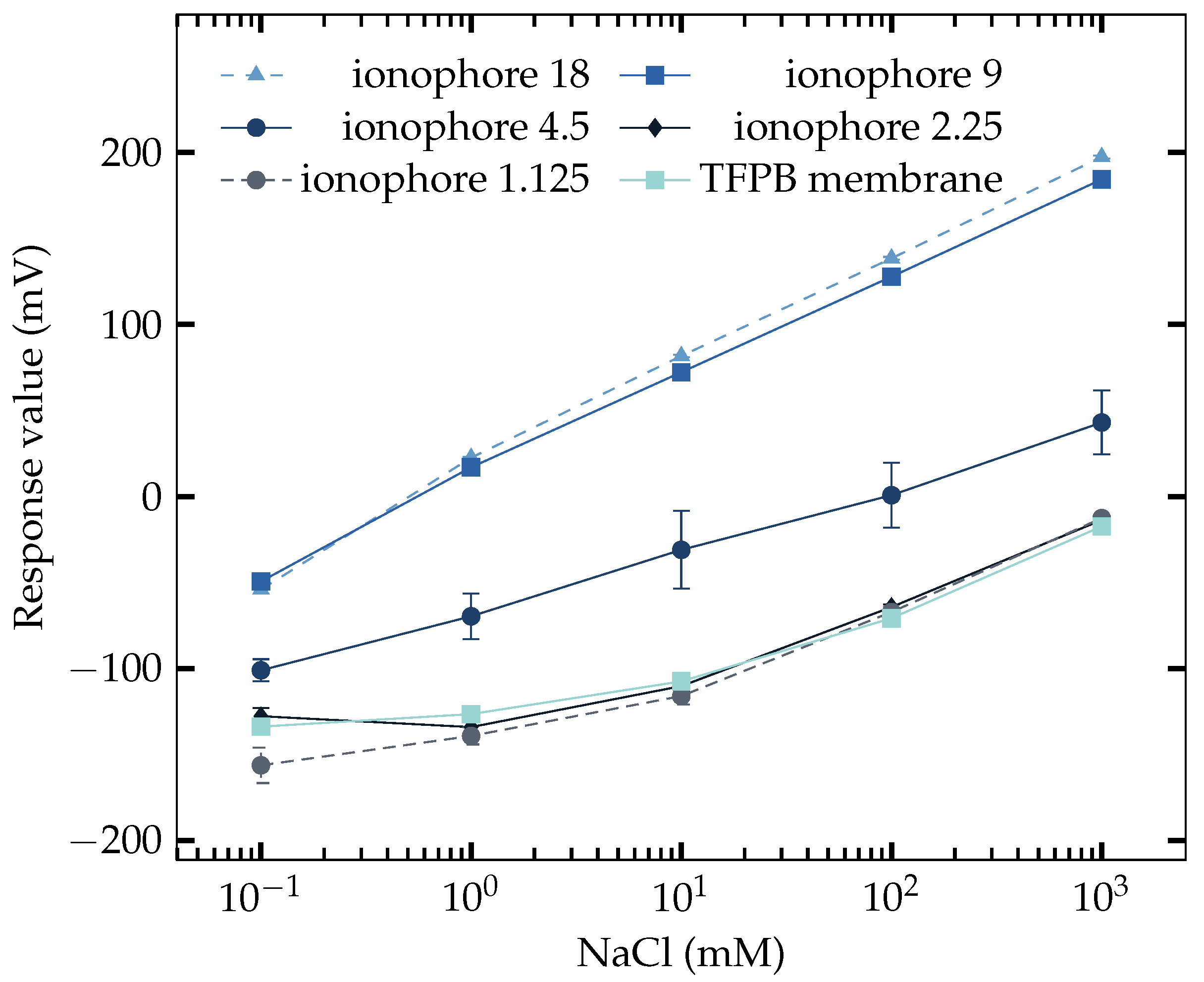
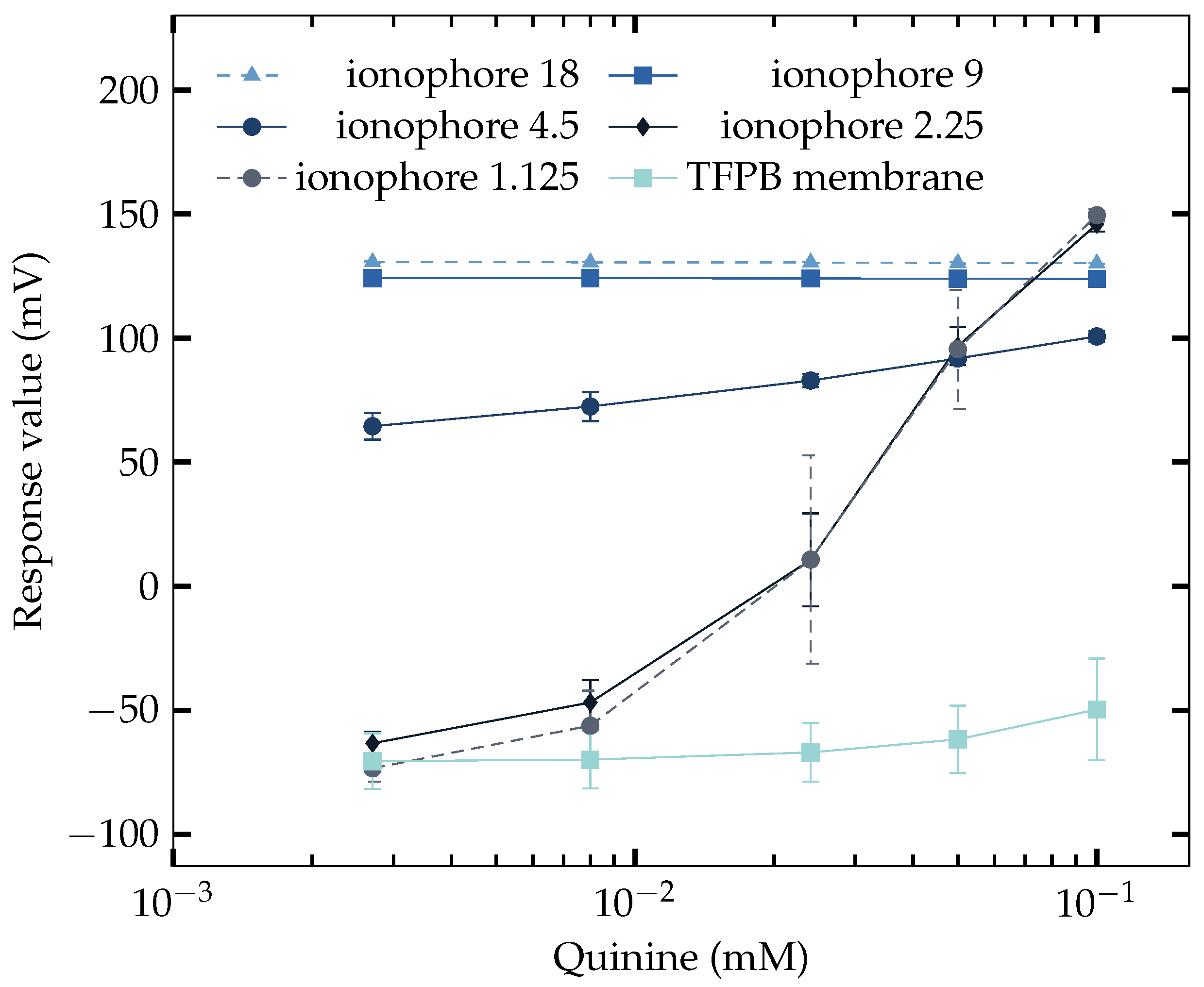
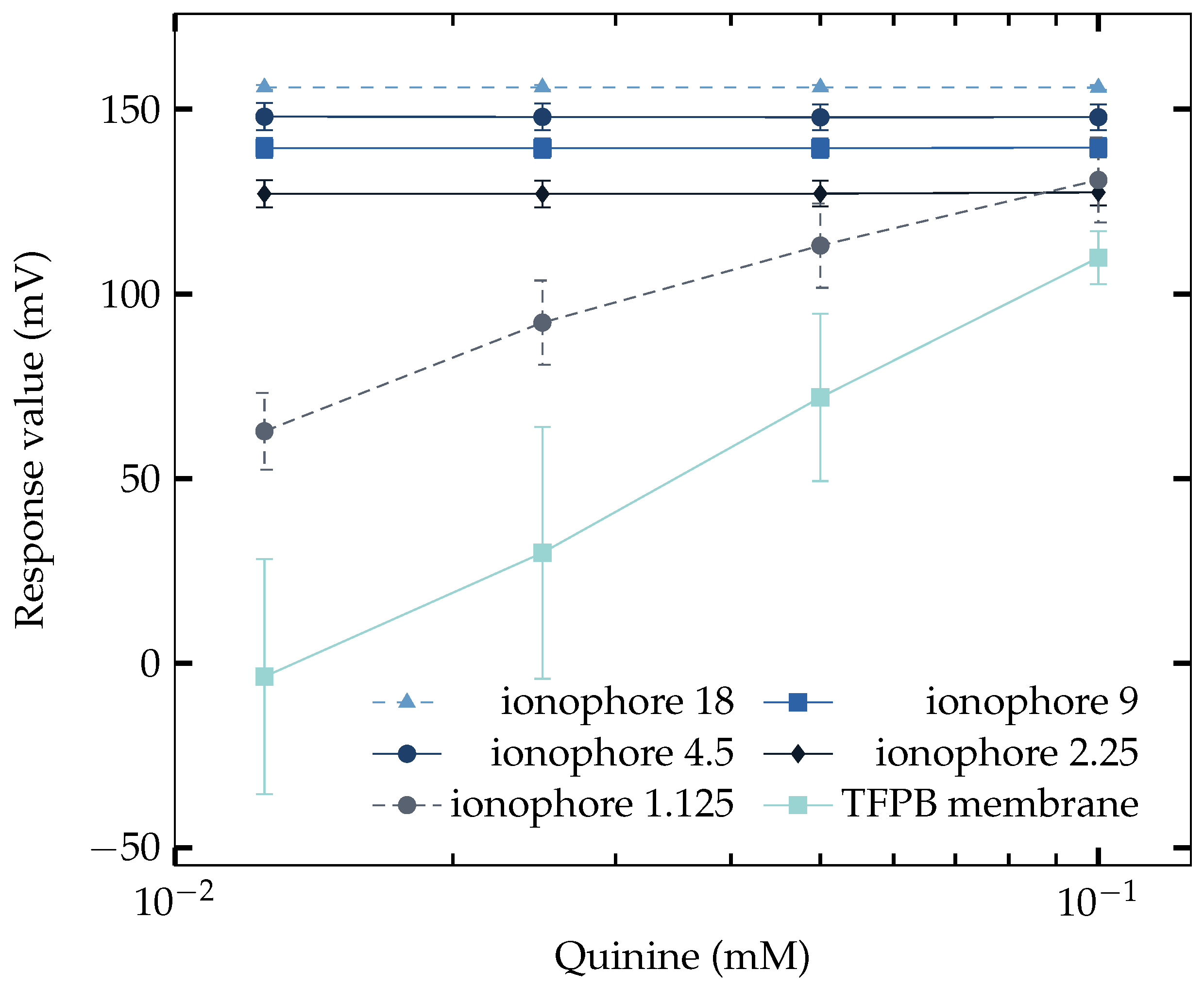
| Component | Amount |
|---|---|
| Na+ ionophore | 18, 9, 4.5, 2.25, 1.125 mg |
| TFPB | 1.5 mg |
| NPOE | 400 uL |
| PVC | 200 mg |
| Sample | Concentration |
|---|---|
| NaCl | 0.1, 1, 10, 100, 1000 mM |
| NaCl + quinine | NaCl (70, 200 mM) + quinine hydrochloride dihydrate (0.0027, 0.008, 0.024, 0.05, 0.1 mM) |
| Comparaing Samples | NaCl + Quinine | NaCl | Same | |
|---|---|---|---|---|
| NaCl (0.375%) | + quinine (0.0027 mM) | 10 | 0 | 0 |
| + quinine (0.0080 mM) | 10 | 0 | 0 | |
| + quinine (0.024 mM) | 10 | 0 | 0 | |
| NaCl (1%) | + quinine (0.0027 mM) | 0 | 10 | 0 |
| + quinine (0.0080 mM) | 0 | 10 | 0 | |
| + quinine (0.024 mM) | 1 | 9 | 0 | |
| Membrane | Plasticizer & Polymer Support | Additional Compositions |
|---|---|---|
| TFPB membrane | NPOE 400 uL; PVC 200 mg | TFPB 1.5 mg |
| NaISE | TFPB 1.5 mg; Na+ ionophore |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Watanabe, K.; Watanabe, T.; Kimura, S.; Toko, K. Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect. Sensors 2023, 23, 3178. https://doi.org/10.3390/s23063178
Jing Y, Watanabe K, Watanabe T, Kimura S, Toko K. Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect. Sensors. 2023; 23(6):3178. https://doi.org/10.3390/s23063178
Chicago/Turabian StyleJing, Yifei, Kentaro Watanabe, Tatsukichi Watanabe, Shunsuke Kimura, and Kiyoshi Toko. 2023. "Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect" Sensors 23, no. 6: 3178. https://doi.org/10.3390/s23063178
APA StyleJing, Y., Watanabe, K., Watanabe, T., Kimura, S., & Toko, K. (2023). Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect. Sensors, 23(6), 3178. https://doi.org/10.3390/s23063178







