Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells
Abstract
:1. Introduction
2. Metal Affinity Based H2S Detection
2.1. Accuracy and Reliability
2.2. Sensitivity
2.3. Biocompatibility
2.4. Detection Medium
2.5. Repeatability
3. Reducibility Based H2S Detection
3.1. Accuracy and Reliability
3.2. Sensitivity
3.3. Response Time
3.4. Water Solubility
4. Nucleophilicity Based H2S Detection
4.1. Accuracy and Reliability
4.2. Nucleophilic Reaction Time
4.3. Test Material Preparation
5. Summary and Outlook
- Biocompatibility: Firstly, the detection materials with good biocompatibility and low toxicity should be applied. In addition, it can be combined with secondary substances with good biocompatibility, such as peptide-based substances, among others. Especially when detecting H2S via its metal affinity, metal ions are most likely to reduce cell activity or even cause toxicity to organisms. For this, precious metals, especially precious metal nanostructures such as gold nanoparticles/clusters, can be used, which have good biocompatibility. At present, a green and simple synthesis method has been proposed.
- Sensitivity: The detection technology with high sensitivity, such as ECL technology, or the combination of several detection technologies, such as PEC technology and EC technology, can be used to improve the sensitivity of the electrochemical sensor. Secondly, the detection materials could be optimized, such as nanomaterials with multi-dimensional structure, which have excellent chemical and optical properties and can provide a larger specific surface area to play the sensitization effect. Alternatively, to employ materials with higher conductivity is instrumental for the improvement of the sensitivity of electrochemical sensors.
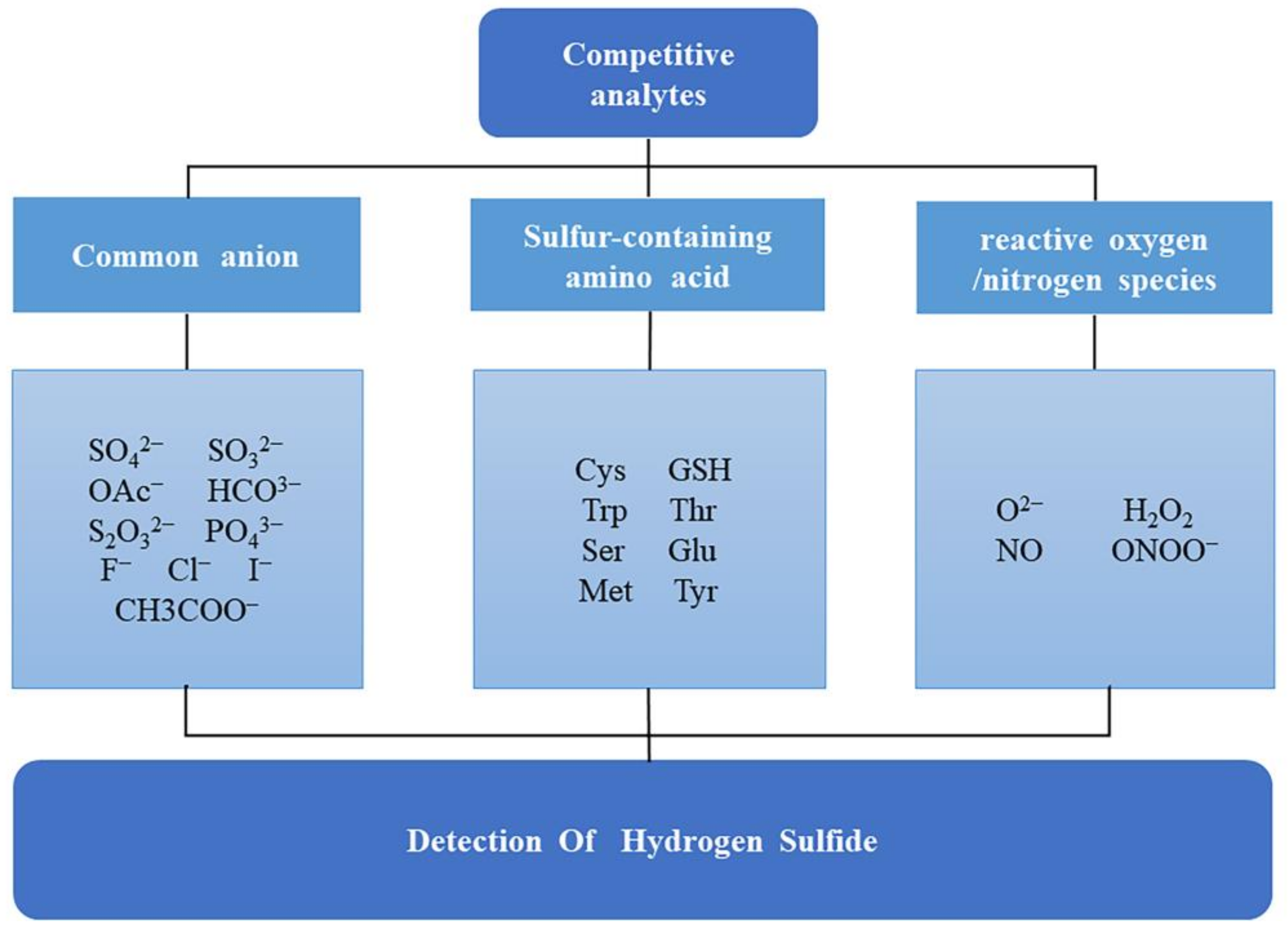
- Reliability: Ratio detection, multi-channel detection, and multi-signal detection improve the reliability of sensors and ensure the accuracy of detection results by mutual evidence. With respect to the fluorescence chemical sensor, in order to solve the problem of false positives/false negatives, apart from the above methods, low background fluorescence interference and non-biological sample automatic fluorescence detection materials are preferable, such as UCNPs, PTMCs, etc.
- Response time: Detection methods based on the reducibility and nucleophilicity of H2S ultimately lie in the gain and loss of electrons. As a reducing agent and nucleophilic reagent for the loss of electrons, H2S can be introduced into the detection materials to reduce the response time of the sensor by introducing electron-absorbing groups, parent nuclear molecules, or multiple catalytic systems.
- Repeatability: H2S chemical sensors are mostly disposable, especially in fluorescence and colorimetric sensors, which cannot be reused. At present, almost all the repeatable chemical sensors are based on the affinity of H2S toward metal, which can be reused by repeated displacement or reversible reaction. However, the detection can only be repeated several times in a short period of time, which cannot guarantee the long-term stability, and the detection outcome becomes worse after multiple repetitions.
- Detection medium: At present, the detection of H2S is mostly in the liquid phase because of less interference under this condition. In order to realize the wearable H2S-detection device, it is urgent to transfer the detection medium to the solid phase. In this way, sensors can be combined with materials such as thin films and fibers. At the same time, it is also necessary to improve the detection selectivity to prevent interference of other gases except common anions and biological mercaptans in the solid phase.
- Detection limits: The amount of H2S in the human body is usually at the micromolar level, but in some systems, it is as low as the nanomolar level. Therefore, the detection limit of H2S sensor should be at least micromolar level, which can meet the detection in most cases. However, it is more necessary to develop sensors with detection limits of nanomolar and below so as to further realize the detection of very low content.
- Dye preparation: The problem of complex dye preparation often exists when dyes are used to detect H2S. The one-step synthesis method reduces the synthesis steps to a certain extent, but at the cost of long synthesis period wherein poor long-term stability of materials after synthesis always exists. More research should be carried out to this end.
- Real-time monitoring: Some H2S fluorescence and colorimetric chemical sensors possess long reaction time, which largely hinders the real-time monitoring. Therefore, it is very necessary to further improve the sensor sensitivity and shorten the response time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of Mitochondrial Bioenergetic Function by Hydrogen Sulfide. Part I. Biochemical and Physiological Mechanisms: Biochemistry of H2S and Mitochondrial Function. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [Green Version]
- Gadalla, M.M.; Snyder, S.H. Hydrogen Sulfide as a Gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, C. Hydrogen Sulphide and Its Therapeutic Potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gasotransmitters in Cancer: From Pathophysiology to Experimental Therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Dalapati, R.; Balaji, S.N.; Trivedi, V.; Khamari, L.; Biswas, S. A Dinitro-Functionalized Zr(IV)-Based Metal-Organic Framework as Colorimetric and Fluorogenic Probe for Highly Selective Detection of Hydrogen Sulphide. Sens. Actuators B Chem. 2017, 245, 1039–1049. [Google Scholar] [CrossRef]
- Oguz, A.; Oguz, M.; Kursunlu, A.N.; Yilmaz, M. A Fully Water-Soluble Calix[4]Arene Probe for Fluorometric and Colorimetric Detection of Toxic Hydrosulfide and Cyanide Ions: Practicability in Living Cells and Food Samples. Food Chem. 2023, 401, 134132. [Google Scholar] [CrossRef]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. Int. Ed. 2011, 50, 9672–9675. [Google Scholar] [CrossRef] [Green Version]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [Green Version]
- Khattak, S.; Zhang, Q.-Q.; Sarfraz, M.; Muhammad, P.; Ngowi, E.E.; Khan, N.H.; Rauf, S.; Wang, Y.-Z.; Qi, H.-W.; Wang, D.; et al. The Role of Hydrogen Sulfide in Respiratory Diseases. Biomolecules 2021, 11, 682. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Yuan, Z.; Lu, C. Colorimetric Detection of Biological Hydrogen Sulfide Using Fluorosurfactant Functionalized Gold Nanorods. Analyst 2015, 140, 7443–7450. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, F.; Peng, M.; Wang, C.-W.; Tseng, Y.-T.; Du, Y.; Cai, N.; Lien, C.-W.; Chang, H.-T.; He, Y.; et al. Selective Colorimetric Detection of Hydrogen Sulfide Based on Primary Amine-Active Ester Cross-Linking of Gold Nanoparticles. Anal. Chem. 2015, 87, 7267–7273. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-B.; Liu, Y.-L.; Zhang, H.-W.; Xiao, C.; Qin, Y.; Duo, H.-H.; Xu, J.-Q.; Guo, S.; Pang, D.-W.; Huang, W.-H. Electrochemical Monitoring of Hydrogen Sulfide Release from Single Cells. ChemElectroChem 2016, 3, 1998–2002. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Saha, T.; Desai, A.V.; Talukdar, P.; Ghosh, S.K. Metal-Organic Framework Based Highly Selective Fluorescence Turn-on Probe for Hydrogen Sulphide. Sci. Rep. 2015, 4, 7053. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Lu, Y.; Liu, B.; Chen, Y.; Zhang, J.; Zhou, Y. A H2S-Triggered Two-Photon Ratiometric Fluorescent Theranostic Prodrug for Bio-Imaging. Chin. Chem. Lett. 2021, 32, 2380–2384. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, W.; Yin, C.; Zhang, Y.; Huo, F. A Tracer-Type Fluorescent Probe for Imaging Adenosine Triphosphate under the Stresses of Hydrogen Sulfide and Hydrogen Peroxide in Living Cells. Analyst 2022, 147, 4222–4227. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Recent Developments of Fluorescent Probes for the Detection of Gasotransmitters (NO, CO and H2S). Coord. Chem. Rev. 2013, 257, 2335–2347. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Belal, F.; El-Shabrawy, Y.; El-Maghrabey, M. Nanostructures-Based Sensing Strategies for Hydrogen Sulfide. Trends Environ. Anal. Chem. 2021, 31, e00133. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, Y.; Wang, C.; Jia, H.; Gao, X.; Zhang, R.; Wang, Y.; Zhang, Z. NBD-Based Fluorescent Chemosensor for the Selective Quantification of Copper and Sulfide in an Aqueous Solution and Living Cells. Org. Biomol. Chem. 2015, 13, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gong, Y.; Qi, L.; Gao, Y.; Yu, L. A Polyoxometalate-Based Supramolecular Chemosensor for Rapid Detection of Hydrogen Sulfide with Dual Signals. J. Colloid Interf. Sci. 2017, 485, 280–287. [Google Scholar] [CrossRef]
- Wang, S.; Ding, H.; Wang, Y.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. An “off–on–off” Sensor for Sequential Detection of Cu2+ and Hydrogen Sulfide Based on a Naphthalimide–Rhodamine B Derivative and Its Application in Dual-Channel Cell Imaging. RSC Adv. 2018, 8, 33121–33128. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, Y.; Cui, L.; Zheng, F.; Song, Q. Electroactive Au@Ag Nanoparticles Driven Electrochemical Sensor for Endogenous H2S Detection. Biosens. Bioelectron. 2018, 117, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ornet-Martínez, N.; Hakobyan, L.; Argente-García, A.I.; Molins-Legua, C.; Campíns-Falcó, P. Nylon-Supported Plasmonic Assay Based on the Aggregation of Silver Nanoparticles: In Situ Determination of Hydrogen Sulfide-like Compounds in Breath Samples as a Proof of Concept. ACS Sens. 2019, 4, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Kim, D.-H.; Choi, S.-J.; Koo, W.-T.; Kim, I.-D. Sub-Parts-per-Million Hydrogen Sulfide Colorimetric Sensor: Lead Acetate Anchored Nanofibers toward Halitosis Diagnosis. Anal. Chem. 2018, 90, 8769–8775. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, J.; Wang, S.; Zhu, A.; Hou, X. Sensing during In Situ Growth of Mn-Doped ZnS QDs: A Phosphorescent Sensor for Detection of H2S in Biological Samples. Chem. Eur. J. 2014, 20, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Wang, Y.; Wu, N.; Feng, W.; Wei, D.; Li, Z.; Yu, M. A Mitochondrial-Targeted Ratiometric Probe for Detecting Intracellular H2S with High Photostability. Chin. Chem. Lett. 2021, 32, 1799–1802. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Y.; Zhang, X.; Zhang, S. A Ratiometric Strategy to Detect Hydrogen Sulfide with a Gold Nanoclusters Based Fluorescent Probe. Talanta 2016, 154, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, C.; Zhang, W.; Ta, H.T.; Yuan, J.; Zhang, R.; Xu, Z.P. Responsive Nanosensor for Ratiometric Luminescence Detection of Hydrogen Sulfide in Inflammatory Cancer Cells. Anal. Chim. Acta 2020, 1103, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Xu, H.; Wang, C.; Chen, C.; Wang, C.; Jin, L.; Du, Y. A New Ratiometric Electrochemical Sensor Using Electroactive GO/MB/Ag Nanocomposites for H2S Detection in Biological Samples. J. Nanopart. Res. 2020, 22, 75. [Google Scholar] [CrossRef]
- Jarosz, A.P.; Yep, T.; Mutus, B. Microplate-Based Colorimetric Detection of Free Hydrogen Sulfide. Anal. Chem. 2013, 85, 3638–3643. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Liu, Q.; Wang, C.; Di, J.; Chen, C.; Jin, L.; Du, Y. Dual Mode Electrochemical-Photoelectrochemical Sensing Platform for Hydrogen Sulfide Detection Based on the Inhibition Effect of Titanium Dioxide/Bismuth Tungstate/Silver Heterojunction. J. Colloid Interf. Sci. 2021, 581, 323–333. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Lin, W.; Li, J.; Fang, L.; Wang, X.; Zhang, Y.; Chen, Y.; Lin, Z. Signal-On and Highly Sensitive Electrochemiluminescence Biosensor for Hydrogen Sulfide in Joint Fluid Based on Silver-Ion-Mediated Base Pairs and Hybridization Chain Reaction. Chemosensors 2022, 10, 250. [Google Scholar] [CrossRef]
- Gao, Z.; Tang, D.; Tang, D.; Niessner, R.; Knopp, D. Target-Induced Nanocatalyst Deactivation Facilitated by Core@Shell Nanostructures for Signal-Amplified Headspace-Colorimetric Assay of Dissolved Hydrogen Sulfide. Anal. Chem. 2015, 87, 10153–10160. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Wang, Y.; Zhang, R.; Zou, C.; Zhou, Y. Mesoporous WS2-Decorated Cellulose Nanofiber-Templated CuO Heterostructures for High-Performance Chemiresistive Hydrogen Sulfide Sensors. Anal. Chem. 2022, 94, 16160–16170. [Google Scholar] [CrossRef]
- Viles, J.H. Metal Ions and Amyloid Fiber Formation in Neurodegenerative Diseases. Copper, Zinc and Iron in Alzheimer’s, Parkinson’s and Prion Diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. [Google Scholar] [CrossRef]
- Ding, L.; Ma, C.; Li, L.; Zhang, L.; Yu, J. A Photoelectrochemical Sensor for Hydrogen Sulfide in Cancer Cells Based on the Covalently and in Situ Grafting of CdS Nanoparticles onto TiO2 Nanotubes. J. Electroanal. Chem. 2016, 783, 176–181. [Google Scholar] [CrossRef]
- Hao, C.; Li, Y.; Fan, B.; Zeng, G.; Zhang, D.; Bian, Z.; Wu, J. A New Peptide-Based Chemosensor for Selective Imaging of Copper Ion and Hydrogen Sulfide in Living Cells. Microchem. J. 2020, 154, 104658. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Y.; Zhang, H.; Niu, X.; Xu, L.; Ren, C.; Chen, X. Fluorescence Resonance Energy Transfer-Based Ratiometric Fluorescent Assay for Highly Sensitive and Selective Determination of Sulfide Anions. Analyst 2015, 140, 6711–6719. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Niu, Q.; Gao, P.; Zhang, G.; Dong, C.; Shuang, S. Gold Nanoclusters as Fluorescent Sensors for Selective and Sensitive Hydrogen Sulfide Detection. Talanta 2017, 171, 143–151. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Carpenter, T.S.; Xue, Z.-L. Bismuth-Based, Disposable Sensor for the Detection of Hydrogen Sulfide Gas. Anal. Chem. 2016, 88, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Li, Y.-C.; Ma, J.-Y.; Huang, J.-Y.; Chen, C.-F.; Chang, H.-T. Size-Tunable Copper Nanocluster Aggregates and Their Application in Hydrogen Sulfide Sensing on Paper-Based Devices. Sci. Rep. 2016, 6, 24882. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, J.; Rosqvist, E.; Ihalainen, P.; Peltonen, J. Electro-Optical Gas Sensor Consisting of Nanostructured Paper Coating and an Ultrathin Sensing Element. Chemosensors 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Carrero-Ferrer, I.; Molins-Legua, C.; Campíns-Falcó, P. Plasmonic Sensor for Hydrogen Sulphide in Saliva: Multisensor Platform and Bag Format. Talanta 2022, 245, 123449. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-Temperature High-Performance H2S Sensor Based on Porous CuO Nanosheets Prepared by Hydrothermal Method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gu, Z.; Xing, G. Design and Synthesis of a Cu(II)-Complex-Based Carbazole-Hemicyanine Hybrid for Fluorescent Sensing of H2S in SDS Micellar Solution. Tetrahedron 2017, 73, 2123–2130. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Chemically Reversible Binding of H2S to a Zinc Porphyrin Complex: Towards Implementation of a Reversible Sensor via a “Coordinative-Based Approach”. Dalton Trans. 2017, 46, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Dulac, M.; Melet, A.; Galardon, E. Reversible Detection and Quantification of Hydrogen Sulfide by Fluorescence Using the Hemoglobin I from Lucina Pectinata. ACS Sens. 2018, 3, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, H.; Ma, Y.; Chen, X. An NBD Fluorophore-Based Colorimetric and Fluorescent Chemosensor for Hydrogen Sulfide and Its Application for Bioimaging. Tetrahedron 2013, 69, 867–870. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, W.; Tan, L. A Phenanthroimidazole-Based Fluorescent Chemosensor for Imaging Hydrogen Sulfide in Living Cells. Org. Biomol. Chem. 2012, 10, 9683–9688. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Hu, C.; Cao, J.; Zhang, H.; Xiong, X.; Wang, J.; Cui, S.; Sun, S.; Peng, X. A Two-Photon Fluorescent Probe with near-Infrared Emission for Hydrogen Sulfide Imaging in Biosystems. Chem. Commun. 2013, 49, 3890–3892. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Ren, W.; Ai, H. Reaction-Based Genetically Encoded Fluorescent Hydrogen Sulfide Sensors. J. Am. Chem. Soc. 2012, 134, 9589–9592. [Google Scholar] [CrossRef] [PubMed]
- Manibalan, K.; Mani, V.; Chang, P.-C.; Huang, C.-H.; Huang, S.-T.; Marchlewicz, K.; Neethirajan, S. Electrochemical Latent Redox Ratiometric Probes for Real-Time Tracking and Quantification of Endogenous Hydrogen Sulfide Production in Living Cells. Biosens. Bioelectron. 2017, 96, 233–238. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Zhang, M. Reduction of Ammineruthenium(III) by Sulfide Enables In Vivo Electrochemical Monitoring of Free Endogenous Hydrogen Sulfide. Anal. Chem. 2017, 89, 5382–5388. [Google Scholar] [CrossRef] [PubMed]
- Gatty, H.; Stemme, G.; Roxhed, N. A Miniaturized Amperometric Hydrogen Sulfide Sensor Applicable for Bad Breath Monitoring. Micromachines 2018, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Liu, X.; Wang, Y.; Wu, Z. A Polymer-Based Turn-on Fluorescent Sensor for Specific Detection of Hydrogen Sulfide. RSC Adv. 2013, 3, 14543–14548. [Google Scholar] [CrossRef]
- Kim, G.; Jang, E.; Page, A.M.; Ding, T.; Carlson, K.A.; Cao, H. Investigation of a Sensing Approach Based on a Rapid Reduction of Azide to Selectively Measure Bioavailability of H2S. RSC Adv. 2016, 6, 95920–95924. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, F.; Yin, C. Photocontrolled Single-/Dual-Site Alternative Fluorescence Probes Distinguishing Detection of H2S/SO2 In Vivo. Org. Lett. 2019, 21, 5277–5280. [Google Scholar] [CrossRef] [PubMed]
- Jothi, D.; Iyer, S.K. A Highly Sensitive Naphthalimide Based Fluorescent “Turn-on” Sensor for H2S and Its Bio-Imaging Applications. J. Photochem. Photobiol. A Chem. 2022, 427, 113802. [Google Scholar] [CrossRef]
- Hall, J.R.; Schoenfisch, M.H. Direct Electrochemical Sensing of Hydrogen Sulfide without Sulfur Poisoning. Anal. Chem. 2018, 90, 5194–5200. [Google Scholar] [CrossRef]
- Kim, S.G.; Tran, T.V.; Lee, J.S. Iron Oxide-Immobilized Porous Carbon Nanofiber-Based Radio Frequency Identification (RFID) Tag Sensor for Detecting Hydrogen Sulfide. J. Ind. Eng. Chem. 2022, 112, 423–429. [Google Scholar] [CrossRef]
- Li, D.-W.; Qu, L.-L.; Hu, K.; Long, Y.-T.; Tian, H. Monitoring of Endogenous Hydrogen Sulfide in Living Cells Using Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2015, 54, 12758–12761. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-Dot-Based Ratiometric Fluorescent Sensor for Detecting Hydrogen Sulfide in Aqueous Media and inside Live Cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Zhang, S.; Ai, H. A Genetically Encoded, Ratiometric Fluorescent Biosensor for Hydrogen Sulfide. ACS Sens. 2019, 4, 1626–1632. [Google Scholar] [CrossRef]
- Yao, Y.; Kong, C.; Yin, L.; Jain, A.D.; Ratia, K.; Thatcher, G.R.J.; Moore, T.W.; Driver, T.G.; Miller, L.W. Time-Gated Detection of Cystathionine γ-Lyase Activity and Inhibition with a Selective, Luminogenic Hydrogen Sulfide Sensor. Chem. Eur. J. 2017, 23, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Yeh, H.-W.; Karmach, O.; Ji, A.; Carter, D.; Martins-Green, M.M.; Ai, H. Red-Shifted Luciferase–Luciferin Pairs for Enhanced Bioluminescence Imaging. Nat. Methods 2017, 14, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Sozmen, F.; Mamur, S.; Tekinay, T.; Akkaya, E.U. Fast Responding and Selective Near-IR Bodipy Dye for Hydrogen Sulfide Sensing. Chem. Commun. 2014, 50, 5455–5457. [Google Scholar] [CrossRef] [Green Version]
- Ji, A.; Fan, Y.; Ren, W.; Zhang, S.; Ai, H. A Sensitive Near-Infrared Fluorescent Sensor for Mitochondrial Hydrogen Sulfide. ACS Sens. 2018, 3, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Silindir-Gunay, M.; Sarcan, E.T.; Ozer, A.Y. Near-infrared Imaging of Diseases: A Nanocarrier Approach. Drug Dev. Res. 2019, 80, 521–534. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, Z.; Ashraf, G.; Wang, J.; Luo, H.; Chen, X.; Xiao, F.; Liu, H. Hierarchical CNTs@CuMn Layered Double Hydroxide Nanohybrid with Enhanced Electrochemical Performance in H2S Detection from Live Cells. Anal. Chem. 2019, 91, 3912–3920. [Google Scholar] [CrossRef]
- Jha, R.K.; D’Costa, J.V.; Sakhuja, N.; Bhat, N. MoSe2 Nanoflakes Based Chemiresistive Sensors for Ppb-Level Hydrogen Sulfide Gas Detection. Sens. Actuators B Chem. 2019, 297, 126687. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; He, L.; Li, P.; Li, X.; Zhang, P. A Direct Electrochemical H2S Sensor Based on Ti3C2Tx MXene. ChemElectroChem 2021, 8, 3658–3665. [Google Scholar] [CrossRef]
- Choi, S.-A.; Park, C.S.; Kwon, O.S.; Giong, H.-K.; Lee, J.-S.; Ha, T.H.; Lee, C.-S. Structural Effects of Naphthalimide-Based Fluorescent Sensor for Hydrogen Sulfide and Imaging in Live Zebrafish. Sci. Rep. 2016, 6, 26203. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Jang, J.-S.; Koo, W.-T.; Choi, S.-J.; Cho, H.-J.; Kim, M.-H.; Kim, S.-J.; Kim, I.-D. Bioinspired Cocatalysts Decorated WO3 Nanotube Toward Unparalleled Hydrogen Sulfide Chemiresistor. ACS Sens. 2018, 3, 1164–1173. [Google Scholar] [CrossRef]
- Xu, Q.; He, L.; Wei, H.; Lin, W. An ICT-Based Hydrogen Sulfide Sensor with Good Water Solubility for Fluorescence Imaging in Living Cells. J. Fluoresc. 2018, 28, 5–11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yue, D.; Zhang, J.; Wang, J.; Li, B.; Yang, Y.; Cui, Y.; Qian, G. Flexible Metal-Organic Framework-Based Mixed-Matrix Membranes: A New Platform for H2S Sensors. Small 2018, 14, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huo, F.; Wen, Y.; Glass, T.E.; Yin, C. A Novel Water-Soluble Fluorescence Probe Based on ICT Lighten for Detecting Hydrogen Sulfide and Its Application in Bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 355–359. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. A Near-Infrared Ratiometric Fluorescent Probe for Rapid and Highly Sensitive Imaging of Endogenous Hydrogen Sulfide in Living Cells. Chem. Sci. 2013, 4, 2551–2556. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, T.; Liu, J.-T.; Miao, J.-Y.; Zhao, B.-X. A New Ratiometric Fluorescent Probe for Rapid, Sensitive and Selective Detection of Endogenous Hydrogen Sulfide in Mitochondria. Chem. Commun. 2016, 52, 3131–3134. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, B.; Li, S.; Park, C.-M.; Whorton, A.R.; Xian, M. Reaction Based Fluorescent Probes for Hydrogen Sulfide. Org. Lett. 2012, 14, 2184–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yang, D.; Tan, R.; Zhou, Z.J.; Xu, R.; Zhang, J.F.; Zhou, Y. A Cyanine-Based Colorimetric and Fluorescence Probe for Detection of Hydrogen Sulfide in Vivo. Sens. Actuators B Chem. 2017, 247, 883–888. [Google Scholar] [CrossRef]
- Du, Z.; Song, B.; Zhang, W.; Duan, C.; Wang, Y.-L.; Liu, C.; Zhang, R.; Yuan, J. Quantitative Monitoring and Visualization of Hydrogen Sulfide In Vivo Using a Luminescent Probe Based on a Ruthenium(II) Complex. Angew. Chem. Int. Ed. 2018, 57, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, C.; Huo, F.; Zhang, Y.; Chao, J. A Ratiometric Colorimetric and Fluorescent Chemosensor for Rapid Detection Hydrogen Sulfide and Its Bioimaging. Sens. Actuators B Chem. 2014, 203, 596–601. [Google Scholar] [CrossRef]
- Karakuş, E.; Üçüncü, M.; Emrullahoğlu, M. Electrophilic Cyanate as a Recognition Motif for Reactive Sulfur Species: Selective Fluorescence Detection of H2S. Anal. Chem. 2016, 88, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Shang, X.; Li, C.; Xue, Z.; Chen, H.; Wu, H.; Wang, T. The Synthesis, Crystal, Hydrogen Sulfide Detection and Cell Assement of Novel Chemsensors Based on Coumarin Derivatives. Sci. Rep. 2018, 8, 16159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yang, W.; Song, Y.; Hu, Y. Novel Turn-on Fluorescence Sensor for Detection and Imaging of Endogenous H2S Induced by Sodium Nitroprusside. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118775. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huo, F.; Yin, C. A ‘Naked-Eye’ Ratiometric and NIR Fluorescent Detection for Hydrogen Sulphide with Quick Response and High Selectivity for and Its Bioimaging. Dyes Pigm. 2019, 165, 31–37. [Google Scholar] [CrossRef]
- Fan, J.; Wu, E.; Dong, J.; Zhu, R.; Li, M.; Gao, J.; Han, H.; Ding, L. A Minimalist Ratiometric Fluorescent Sensor Based on Non-Covalent Ternary Platform for Sensing H2S in Aqueous Solution and Serum. Colloid Surf. A Physicochem. Eng. Aspects 2021, 616, 126299. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Li, W. Three-Channel Fluorescent Sensing via Organic White Light-Emitting Dyes for Detection of Hydrogen Sulfide in Living Cells. Biomaterials 2013, 34, 7429–7436. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, J.; Li, P.; Wang, H.; Tang, B. Dual Signaling Molecule Sensor for Rapid Detection of Hydrogen Sulfide Based on Modified Tetraphenylethylene. Anal. Chem. 2015, 87, 8964–8969. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, W.; Peng, Y.; Wang, M.-X.; Chen, G.; Chen, Z.; Zhu, J.-J. A Spectral Shift-Based Electrochemiluminescence Sensor for Hydrogen Sulfide. Anal. Chem. 2018, 90, 1334–1339. [Google Scholar] [CrossRef]
- Peng, J.; Teoh, C.L.; Zeng, X.; Samanta, A.; Wang, L.; Xu, W.; Su, D.; Yuan, L.; Liu, X.; Chang, Y.-T. Development of a Highly Selective, Sensitive, and Fast Response Upconversion Luminescent Platform for Hydrogen Sulfide Detection. Adv. Funct. Mater. 2016, 26, 191–199. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, K.Y.; Liang, H.; Zhao, Q.; Yang, T.; Liu, S.; Zhang, C.; Shi, Z.; Xu, W.; Huang, W. Dual-Emissive Nanohybrid for Ratiometric Luminescence and Lifetime Imaging of Intracellular Hydrogen Sulfide. ACS Appl. Mater. Interfaces 2015, 7, 5462–5470. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qu, W.; Zhu, J.; Liu, H.; Wen, W.; Zhang, X.; Wang, S. Electrochemiluminescent Sensor Based on Ru(Bpy)32+-Doped Silica Nanoprobe by Incorporating a New Co-Reactant NBD-Amine for Selective Detection of Hydrogen Sulfide. Sens. Actuators B Chem. 2019, 284, 451–455. [Google Scholar] [CrossRef]
- Reja, S.I.; Sharma, N.; Gupta, M.; Bajaj, P.; Bhalla, V.; Parihar, R.D.; Ohri, P.; Kaur, G.; Kumar, M. A Highly Selective Fluorescent Probe for Detection of Hydrogen Sulfide in Living Systems: In Vitro and In Vivo Applications. Chem. Eur. J. 2017, 23, 9872–9878. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, G.; Periasamy, V. Hydrogen Sulfide Detection by ESIPT Based Fluorescent Sensor: Potential in Living Cells Imaging. J. Photochem. Photobiol. A Chem. 2019, 369, 97–105. [Google Scholar] [CrossRef]
- Zheng, J.; Noh, H.L.; Chun, H.W.; Oh, B.M.; Lee, J.; Choi, S.-K.; Kim, E.; Jung, D.; Lee, W.S.; Kim, J.H. Highly Sensitive, Selective, and Rapid Response Colorimetric Chemosensor for Naked Eye Detection of Hydrogen Sulfide Gas under Versatile Conditions: Solution, Thin-Film, and Wearable Fabric. Sens. Actuators B Chem. 2021, 341, 130013. [Google Scholar] [CrossRef]
- Noh, H.L.; Oh, B.M.; Park, Y.K.; Chun, H.W.; Lee, J.; Kim, J.K.; Zheng, J.; Jung, D.; Lee, W.; Kim, J.H. Chromogenic Detection of Hydrogen Sulfide Using Squarylium-Based Chemosensors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118457. [Google Scholar] [CrossRef] [PubMed]














| Precipitation | Test Material | Linear Range | Limit of Detection | Response Time | Ref |
|---|---|---|---|---|---|
| CuS | NBD/Cu2+ | 0–20 μM | 0.17 μM | — | [19] |
| CuS | [C16-2-C16im]Br2/Eu-POM/Cu2+ | 1.25–175 μM | 1.25 μM | Seconds | [20] |
| CuS | naphthalimide–rhodamine B/Cu2+ | 0–40 mM/0–80 mM | 0.23 mM/0.4 mM | <30 s | [21] |
| Ag2S | Au@Ag NPs | 0.1–500 nM | 0.04 nM | — | [22] |
| Ag2S | AgNPs | 150–1000 ppbv | 45 ppbv | 10 min | [23] |
| PbS | Pb(Ac)2 | — | 400 ppb | 1 min | [24] |
| ZnS | Mn-doped Zn2+ QDs | 2–100 μM | 0.3 μM | — | [25] |
| Reaction Type | Sensor Type | Test Material | Linear Range | Limit of Detection | Response Time | Ref |
|---|---|---|---|---|---|---|
| azide to amino | Fluorescent | AISA/poly | - | 2.5 mM | - | [55] |
| azide to amino | Fluorescent | 2,3-naphthalimide | 0–80 μM | - | - | [56] |
| azide to amino | Fluorescent | 4-azide-1,8-naphthalic anhydride/spiropyran | - | 0.101 μM | 25 min | [57] |
| azide to amino | Fluorescent | NPN-N3 | - | 1.2 μM | 10 s | [58] |
| REDOX | Electrochemical | glassy carbon electrode | 15 nM–15 μM | <100 nM | <10 s | [59] |
| REDOX | Electrochemical | Fe2O3-MPCNF | 0.2–100 ppm | 0.2 ppm | - | [60] |
| Reaction Type | Test Material | Linear Range | Limit of Detection | Response Time | Ref |
|---|---|---|---|---|---|
| Nucleophilic addition | diethylaminocoumarin–hemicyanine | - | 14 nM | - | [82] |
| Nucleophilic addition | 3-hydroxyflavone | - | 0.25 μM | <60 s | [83] |
| Thiolysis | coumarin derivatives/fluorobenzene | 0–8 × 10−6 M/L | 4 × 10−6 mol/L | - | [84] |
| Nucleophilic addition | BOTD | 0–50 μM/L | 27.3 nM/L | 30 s | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhou, Y.; Wang, K.; Luo, C.; Xie, M.; Shi, X.; Lin, X. Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells. Sensors 2023, 23, 3316. https://doi.org/10.3390/s23063316
Yang M, Zhou Y, Wang K, Luo C, Xie M, Shi X, Lin X. Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells. Sensors. 2023; 23(6):3316. https://doi.org/10.3390/s23063316
Chicago/Turabian StyleYang, Mengjie, Yong Zhou, Ke Wang, Chunfeng Luo, Mingna Xie, Xiang Shi, and Xiaogang Lin. 2023. "Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells" Sensors 23, no. 6: 3316. https://doi.org/10.3390/s23063316
APA StyleYang, M., Zhou, Y., Wang, K., Luo, C., Xie, M., Shi, X., & Lin, X. (2023). Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells. Sensors, 23(6), 3316. https://doi.org/10.3390/s23063316








