Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns
Abstract
:1. Introduction
2. Materials and Methods
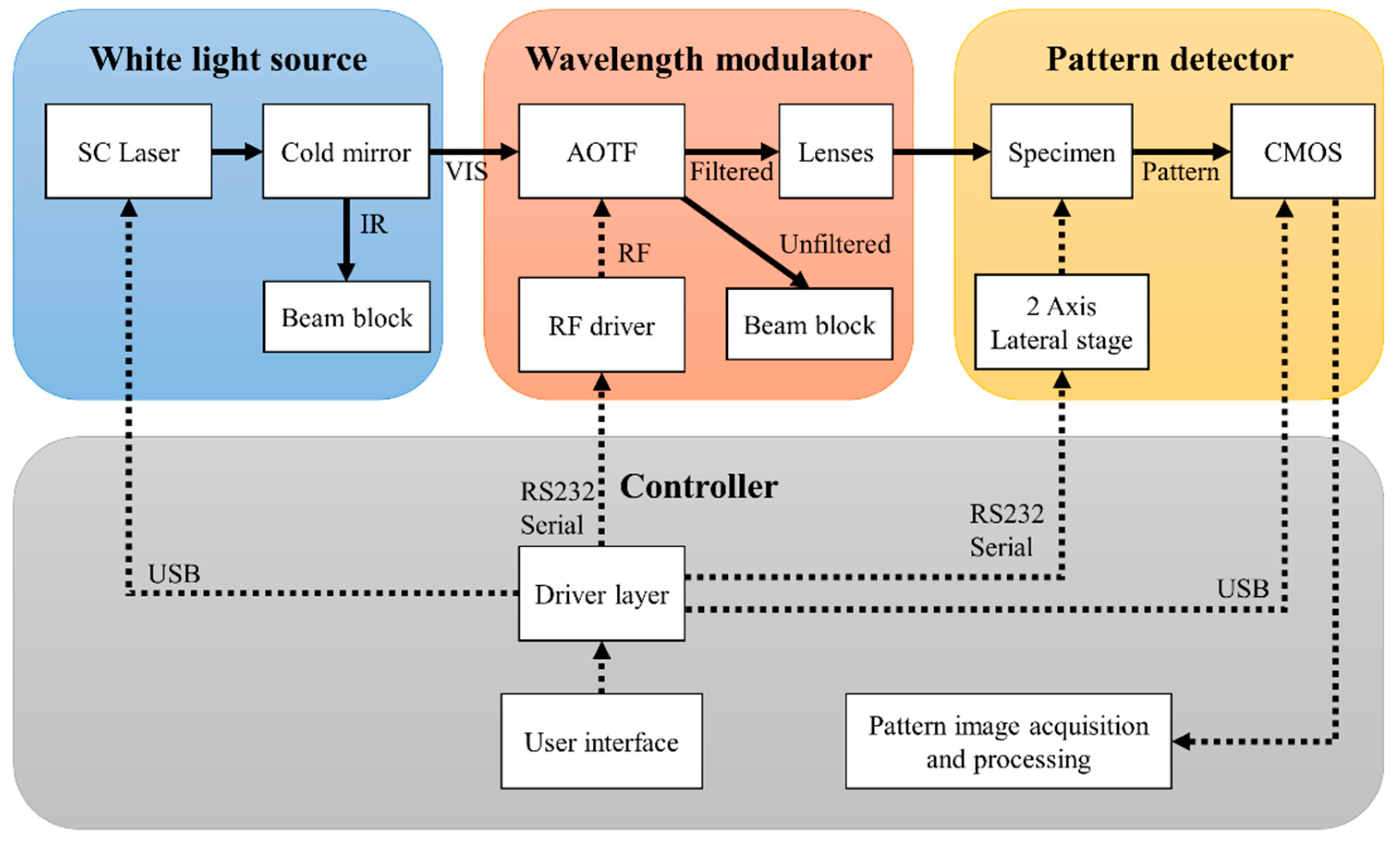
2.1. Instrumentation Design
2.2. Sample Preparation
2.3. Pattern Image Preprocessing
2.4. Feature Extraction
2.5. Classification and Feature Interpretation
3. Results
3.1. Hyperspectral ELS Patterns
3.2. Hyperspectral ELS Pattern Classification
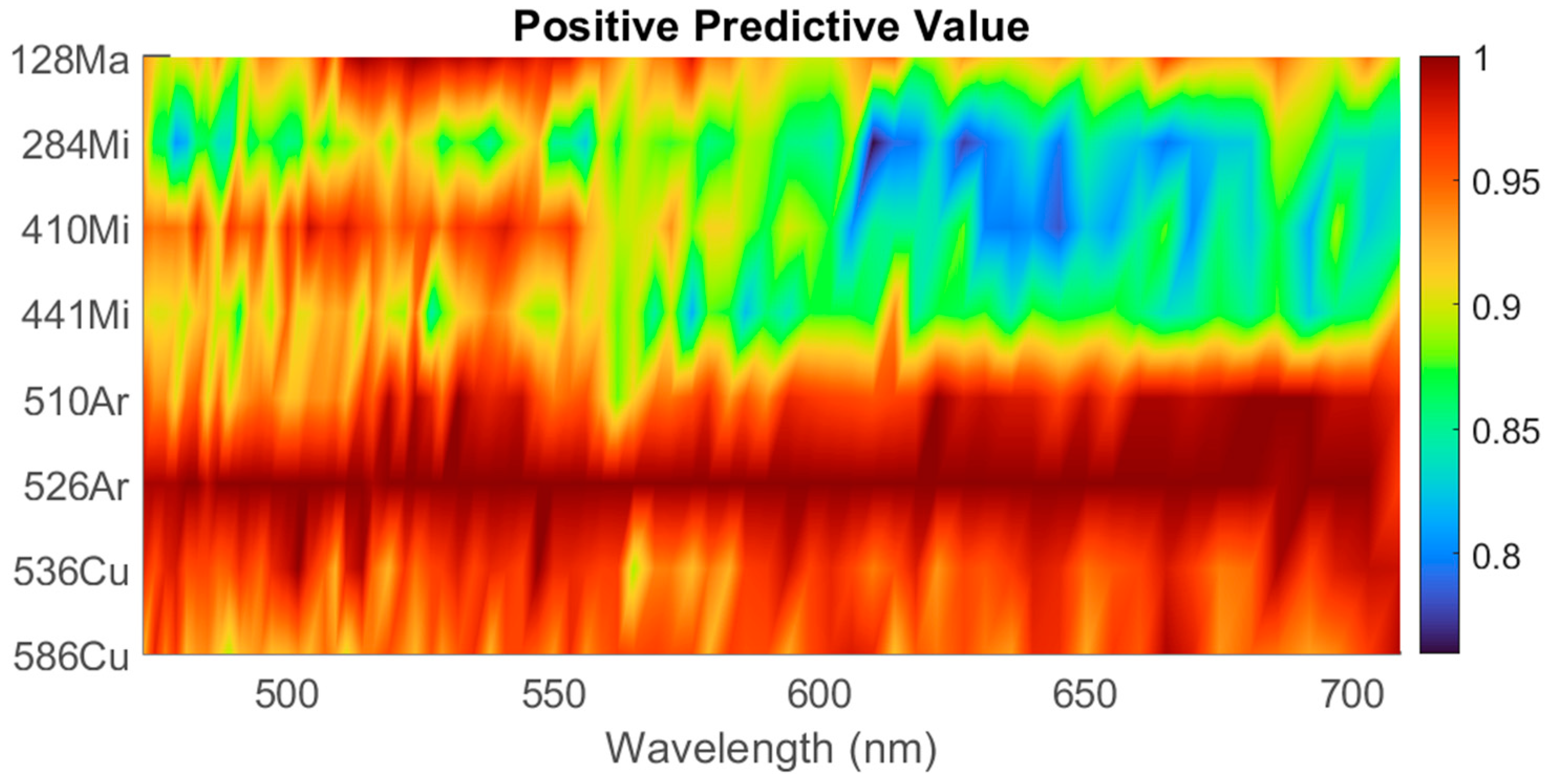
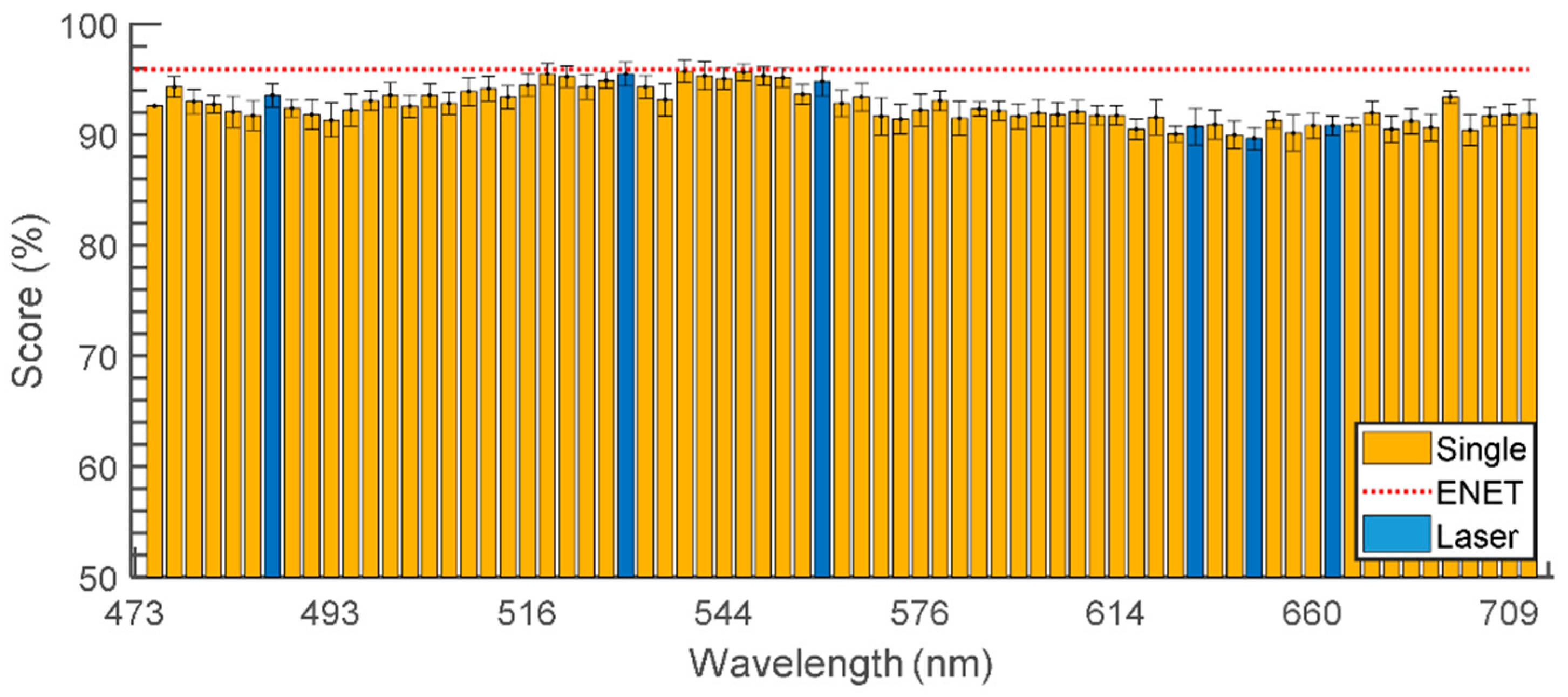
3.3. Feature Selection and Classification
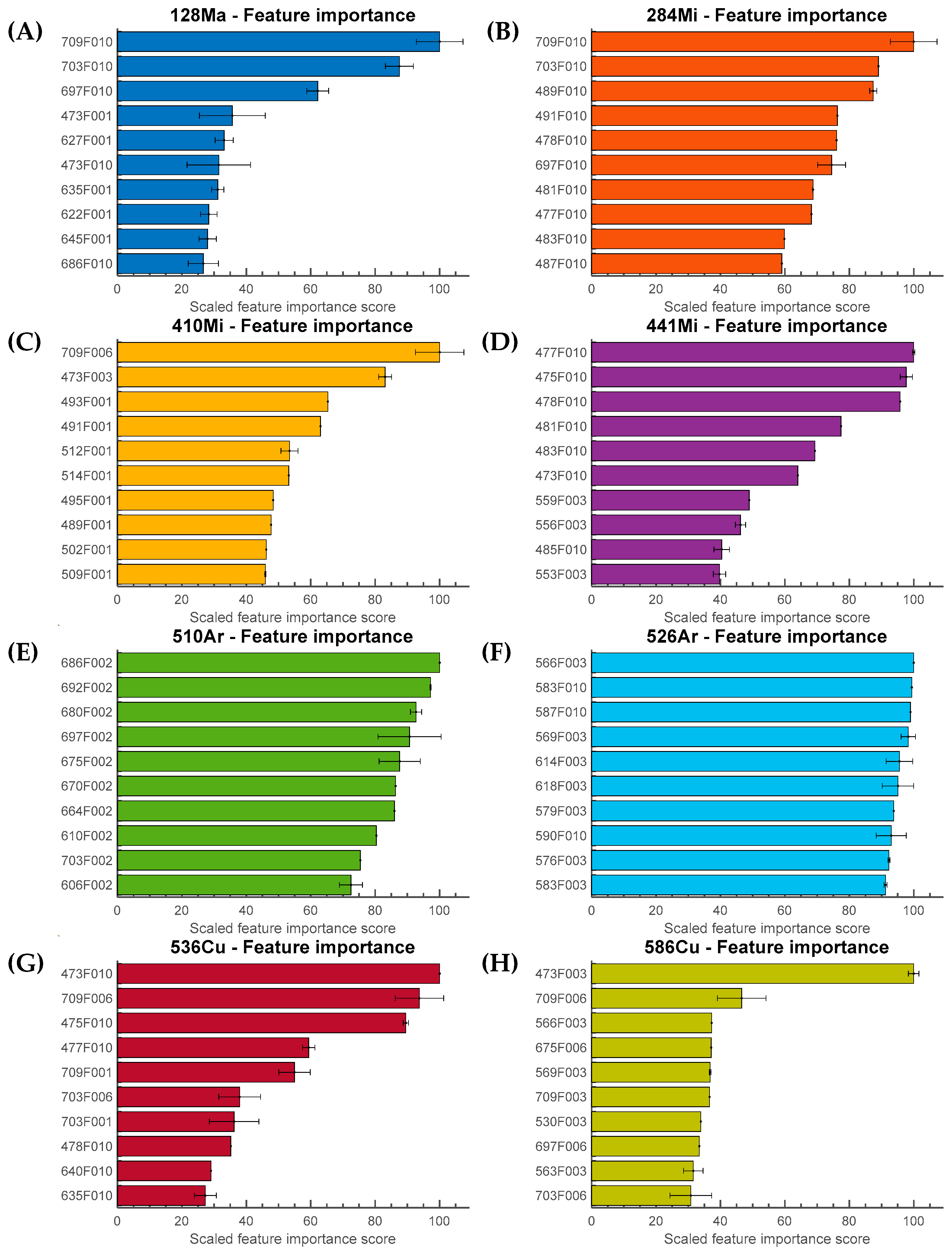
3.4. Feature Interpretation
4. Discussion
4.1. Motivation for Developing the Hyperspectral ELS System
4.2. Prototype Design
4.3. Classification Result
4.4. Feature Selection and Importance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.; Fitzgerald, S.; Mahadevan-Jansen, A. Advances in Optical Detection of Human-Associated Pathogenic Bacteria. Molecules 2020, 25, 5256. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Kim, H.; Rajwa, B.; Thomas, J.G.; Robinson, J.P. Current Status and Future Prospects of Using Advanced Computer-Based Methods to Study Bacterial Colonial Morphology. Expert Rev. Anti Infect. Ther. 2016, 14, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Minoni, U.; Signoroni, A.; Nassini, G. On the Application of Optical Forward-Scattering to Bacterial Identification in an Automated Clinical Analysis Perspective. Biosens. Bioelectron. 2015, 68, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Marcoux, P.R.; Dupoy, M.; Cuer, A.; Kodja, J.-L.; Lefebvre, A.; Licari, F.; Louvet, R.; Narassiguin, A.; Mallard, F. Optical Forward-Scattering for Identification of Bacteria within Microcolonies. Appl. Microbiol. Biotechnol. 2014, 98, 2243–2254. [Google Scholar] [CrossRef]
- Buzalewicz, I.; Suchwałko, A.; Korzekwa, K. The Label-Free Optical Biosensor for an Automated, Ultra-Sensitive and Highly Accurate Microorganisms Identification. Measurement 2021, 178, 109408. [Google Scholar] [CrossRef]
- Doh, I.; Sturgis, J.; Sarria Zuniga, D.V.; Pruitt, R.E.; Robinson, J.P.; Bae, E. Generalized Spectral Light Scatter Models of Diverse Bacterial Colony Morphologies. J. Biophotonics 2019, 12, e201900149. [Google Scholar] [CrossRef]
- Suchwalko, A.; Buzalewicz, I.; Wieliczko, A.; Podbielska, H. Bacteria Species Identification by the Statistical Analysis of Bacterial Colonies Fresnel Patterns. Opt. Express 2013, 21, 11322. [Google Scholar] [CrossRef]
- Kim, H.; Rajwa, B.; Bhunia, A.K.; Robinson, J.P.; Bae, E. Development of a Multispectral Light-Scatter Sensor for Bacterial Colonies. J. Biophotonics 2017, 10, 634–644. [Google Scholar] [CrossRef]
- Doh, I.-J.; Kim, H.; Sturgis, J.; Rajwa, B.; Robinson, J.P.; Bae, E. Optical Multi-Channel Interrogation Instrument for Bacterial Colony Characterization. PLoS ONE 2021, 16, e0247721. [Google Scholar] [CrossRef]
- Buzalewicz, I.; Karwańska, M.; Wieliczko, A.; Podbielska, H. On the Application of Multi-Parametric Optical Phenotyping of Bacterial Colonies for Multipurpose Microbiological Diagnostics. Biosens. Bioelectron. 2021, 172, 112761. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.; Chetty, K.; Bulcock, H. A Review of Hyperspectral Remote Sensing and Its Application in Vegetation and Water Resource Studies. Water SA 2009, 33, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Pampoukis, G.; Lytou, A.E.; Argyri, A.A.; Panagou, E.Z.; Nychas, G.-J.E. Recent Advances and Applications of Rapid Microbial Assessment from a Food Safety Perspective. Sensors 2022, 22, 2800. [Google Scholar] [CrossRef]
- ElMasry, G.; Sun, D.-W. Principles of Hyperspectral Imaging Technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–43. ISBN 978-0-12-374753-2. [Google Scholar]
- Yoon, S.-C.; Shin, T.-S.; Heitschmidt, G.W.; Lawrence, K.C.; Park, B.; Gamble, G. Hyperspectral Image Recovery Using a Color Camera for Detecting Colonies of Foodborne Pathogens on Agar Plate. J. Biosyst. Eng. 2019, 44, 169–185. [Google Scholar] [CrossRef]
- Gowen, A.A.; Feng, Y.; Gaston, E.; Valdramidis, V. Recent Applications of Hyperspectral Imaging in Microbiology. Talanta 2015, 137, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.M.; Genty, G.; Coen, S. Supercontinuum Generation in Photonic Crystal Fiber. Rev. Mod. Phys. 2006, 78, 1135–1184. [Google Scholar] [CrossRef]
- Batshev, V.; Machikhin, A.; Gorevoy, A.; Martynov, G.; Khokhlov, D.; Boritko, S.; Pozhar, V.; Lomonov, V. Spectral Imaging Experiments with Various Optical Schemes Based on the Same AOTF. Materials 2021, 14, 2984. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of Spectral Imaging Technology in Biomedical Engineering: Achievements and Challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef] [Green Version]
- Kasili, P.M.; Vo-Dinh, T. Hyperspectral Imaging System Using Acousto-Optic Tunable Filter for Flow Cytometry Applications. Cytometry A 2006, 69A, 835–841. [Google Scholar] [CrossRef]
- Annamdevula, N.S.; Sweat, B.; Favreau, P.; Lindsey, A.S.; Alvarez, D.F.; Rich, T.C.; Leavesley, S.J. An Approach for Characterizing and Comparing Hyperspectral Microscopy Systems. Sensors 2013, 13, 9267–9293. [Google Scholar] [CrossRef] [Green Version]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, C.F.; Watt, R.S.; Elder, A.D.; Frank, J.H.; Hult, J. Supercontinuum Radiation for Applications in Chemical Sensing and Microscopy. Appl. Phys. B 2008, 92, 367. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Hyyppä, J.; Wang, N.; Jiang, C.; Meng, F.; Tang, L.; Puttonen, E.; Li, C. A 10-Nm Spectral Resolution Hyperspectral LiDAR System Based on an Acousto-Optic Tunable Filter. Sensors 2019, 19, 1620. [Google Scholar] [CrossRef] [Green Version]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, A.; Clarke, K.; Lewis, M. Hyperspectral Classification of Plants: A Review of Waveband Selection Generalisability. Remote Sens. 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.; Xia, Z.; Qiao, J.; Lin, W. Deep Dual-Channel Neural Network for Image-Based Smoke Detection. IEEE Trans. Multimed. 2020, 22, 311–323. [Google Scholar] [CrossRef]
- Gu, K.; Liu, H.; Xia, Z.; Qiao, J.; Lin, W.; Thalmann, D. PM₂.₅ Monitoring: Use Information Abundance Measurement and Wide and Deep Learning. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4278–4290. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, Y.; Qiao, J. Ensemble Meta-Learning for Few-Shot Soot Density Recognition. IEEE Trans. Ind. Inform. 2021, 17, 2261–2270. [Google Scholar] [CrossRef]
- Bayraktar, B.; Banada, P.P.; Hirleman, E.D.; Bhunia, A.K.; Robinson, J.P.; Rajwa, B. Feature Extraction from Light-Scatter Patterns of Listeria Colonies for Identification and Classification. J. Biomed. Opt. 2006, 11, 034006. [Google Scholar] [CrossRef] [Green Version]
- Rajwa, B.; Dundar, M.M.; Akova, F.; Bettasso, A.; Patsekin, V.; Dan Hirleman, E.; Bhunia, A.K.; Robinson, J.P. Discovering the Unknown: Detection of Emerging Pathogens Using a Label-Free Light-Scattering System. Cytometry A 2010, 77A, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Pappu, V.; Pardalos, P.M. High-Dimensional Data Classification. In Clusters, Orders, and Trees: Methods and Applications: In Honor of Boris Mirkin’s 70th Birthday; Aleskerov, F., Goldengorin, B., Pardalos, P.M., Eds.; Springer Optimization and Its Applications; Springer: New York, NY, USA, 2014; pp. 119–150. ISBN 978-1-4939-0742-7. [Google Scholar]
- Jovic, A.; Brkic, K.; Bogunovic, N. A Review of Feature Selection Methods with Applications. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 25–29 May 2015; pp. 1200–1205. [Google Scholar]
- Richardson, J.T.E. Eta Squared and Partial Eta Squared as Measures of Effect Size in Educational Research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Sun, D.-W.; Zeng, X.-A. Recent Advances in Wavelength Selection Techniques for Hyperspectral Image Processing in the Food Industry. Food Bioprocess Technol. 2014, 7, 307–323. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why Should I Trust You?”: Explaining the Predictions of Any Classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]


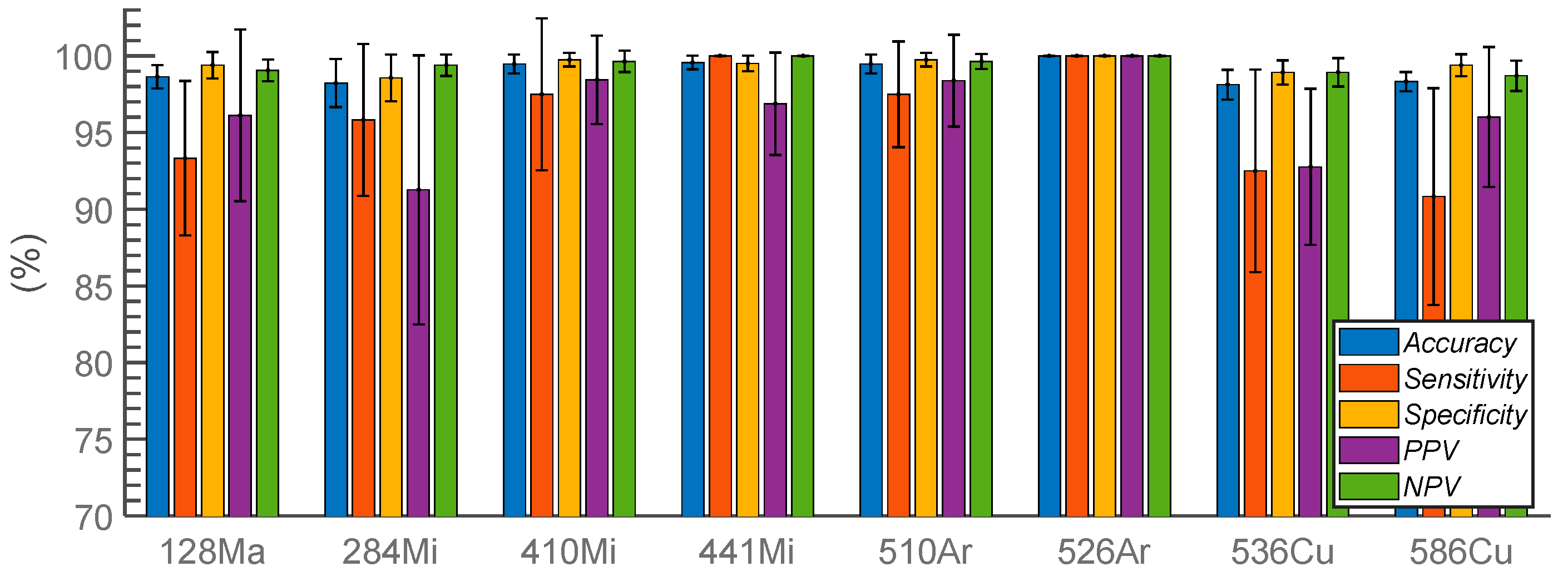


| 128Ma | 284Mi | 410Mi | 441Mi | 510Ar | 526Ar | 536Cu | 586Cu | |
|---|---|---|---|---|---|---|---|---|
| SVM | 93.73 | 85.64 | 90.46 | 88.63 | 95.80 | 99.87 | 96.15 | 94.62 |
| Single wavelength | (3.4) | (3.7) | (5.8) | (3.2) | (2.7) | (0.5) | (2.0) | (2.0) |
| SVM | 93.76 | 95.71 | 94.74 | 94.94 | 100 | 100 | 97.22 | 94.81 |
| Entire feature set | (0) | (3.9) | (3.0) | (6.4) | (0) | (0) | (3.5) | (2.9) |
| ENET | 96.13 | 91.27 | 98.44 | 96.88 | 98.39 | 100 | 92.77 | 96.02 |
| Entire feature set | (5.6) | (8.8) | (3.0) | (3.3) | (3.0) | (0) | (5.1) | (4.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doh, I.-J.; Zuniga, D.V.S.; Shin, S.; Pruitt, R.E.; Rajwa, B.; Robinson, J.P.; Bae, E. Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns. Sensors 2023, 23, 3485. https://doi.org/10.3390/s23073485
Doh I-J, Zuniga DVS, Shin S, Pruitt RE, Rajwa B, Robinson JP, Bae E. Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns. Sensors. 2023; 23(7):3485. https://doi.org/10.3390/s23073485
Chicago/Turabian StyleDoh, Iyll-Joon, Diana Vanessa Sarria Zuniga, Sungho Shin, Robert E. Pruitt, Bartek Rajwa, J. Paul Robinson, and Euiwon Bae. 2023. "Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns" Sensors 23, no. 7: 3485. https://doi.org/10.3390/s23073485
APA StyleDoh, I. -J., Zuniga, D. V. S., Shin, S., Pruitt, R. E., Rajwa, B., Robinson, J. P., & Bae, E. (2023). Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns. Sensors, 23(7), 3485. https://doi.org/10.3390/s23073485









