The MotoNet: A 3 Tesla MRI-Conditional EEG Net with Embedded Motion Sensors
Abstract
1. Introduction
2. Materials and Methods
- -
- Fabrication: High-resistance polymer thick film (PTF) technology (Figure 1) was used to fabricate electrode pads and leads. Conductive inks (Engineered Conductive Materials, Delaware, OH, USA) were screen-printed onto Melinex (DuPont Teijin Films U.S. Limited Partnership, Chester, VA, USA) substrate, with EEG electrodes on one side and a loop on the opposite side. A dielectric ink (ECM) was used to coat the leads and loops for electrical and environmental insulation. The MotoNet circuits were fit to a commercial elastomer structure (Figure 1 and Figure S6). An adapter (Figure 2 and Figure S1) was built to interface the MotoNet to two EEG amplifiers (Brain Products GmbH, Gilching, Germany), with the EEG channels and the motion sensors routed to different amplifiers in order to have separate grounds and references.
- -
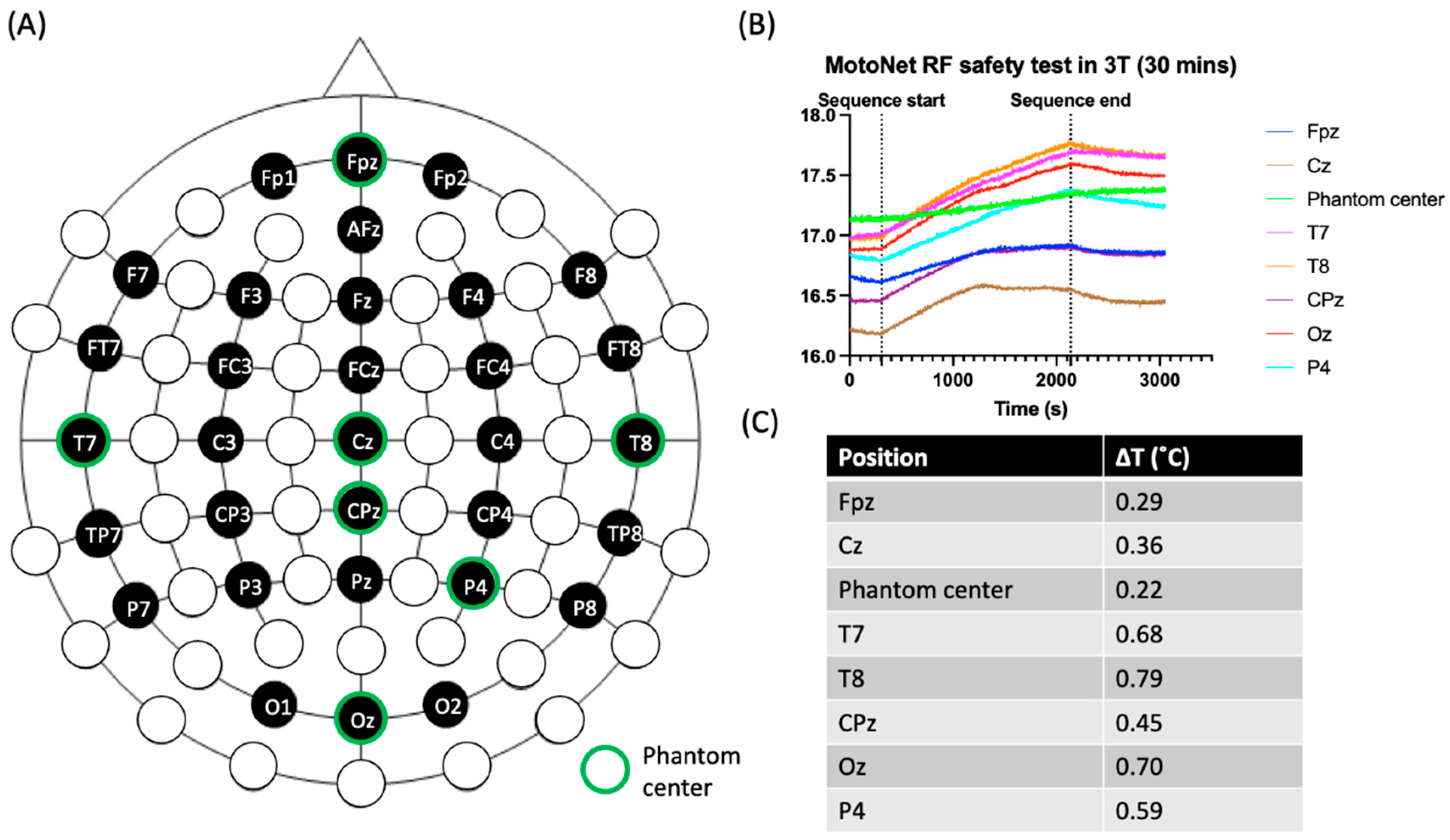
- Safety: The Radiofrequency (RF) safety of the MotoNet was tested in a 3 T Prisma (Siemens Healthineers, Erlangen, Germany) MRI using an adult head-sized agar phantom. The dielectric properties of the phantom were selected to be similar to the adult brain properties at 128 MHz [28,29], i.e., σ = 0.52 S/m, εr = 65.4. For temperature measurements, 8-channel fiber optic probes (OSENSA Innovations Corp., Coquitlam, BC, Canada) were positioned at distributed locations across the MotoNet, including three hot spots estimated from the thermal simulation [30]. Thermal paste was used to keep the fiber optic probes in contact with the surface of the agar phantom and EEG electrodes, to assess RF-induced heating. A high-power turbo spin-echo sequence (21 slices, 0.9 × 0.9 × 5.0 mm voxels, TR/TE = 7600/86 ms, FA = 120°, 20 averages) was used to deliver 100% SAR for 30 min (SARhead: 3.07 W/kg, 10 g SARtorso local: 9.60 W/kg) to reach the maximum power deposition within the allowable RF safety limit in a clinical scan.
- -
- Movement Estimation: We assume that the subject’s head wearing the MotoNet is an ideal sphere centered at the origin with surface points P(x,y,z) satisfying the equation:
- -
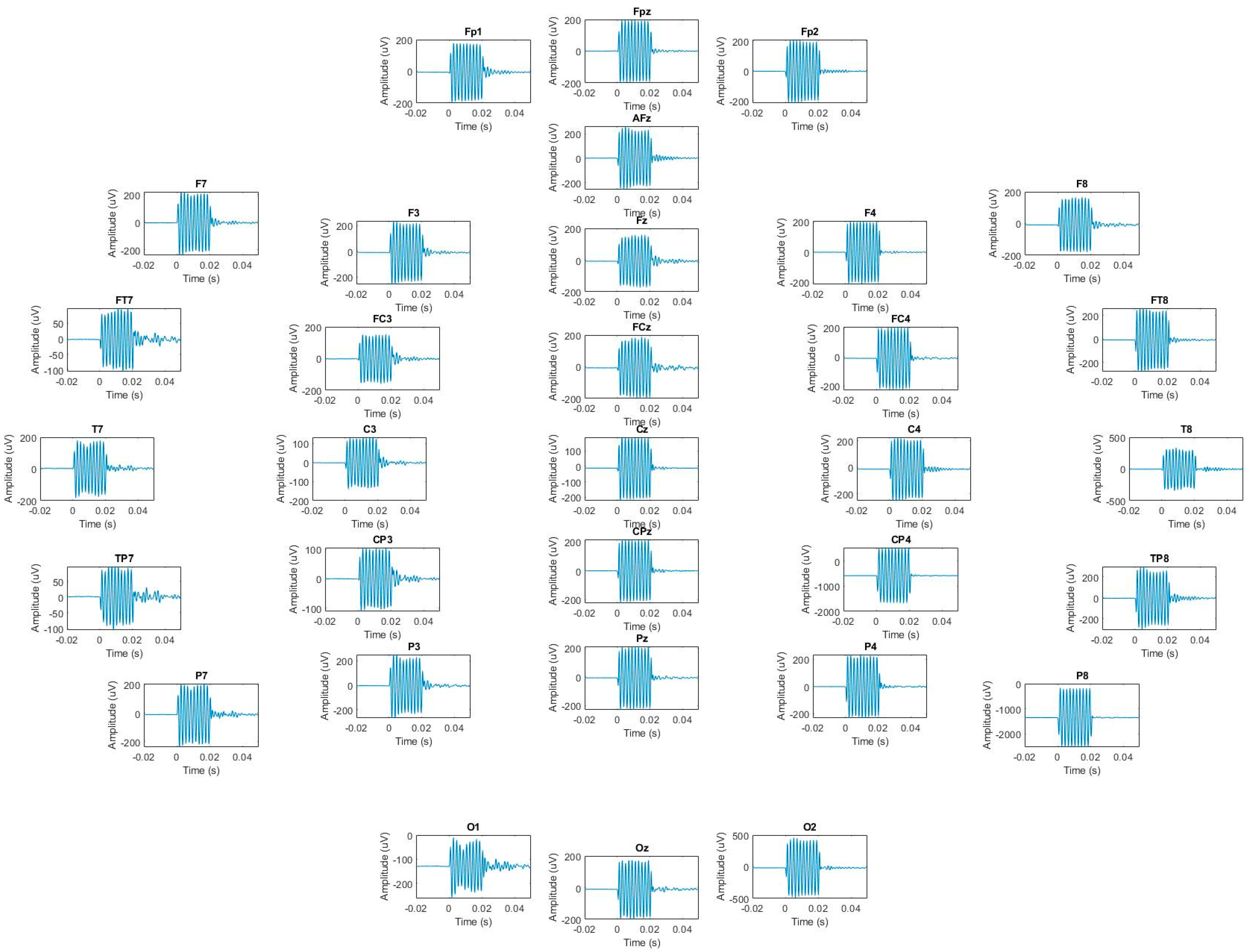
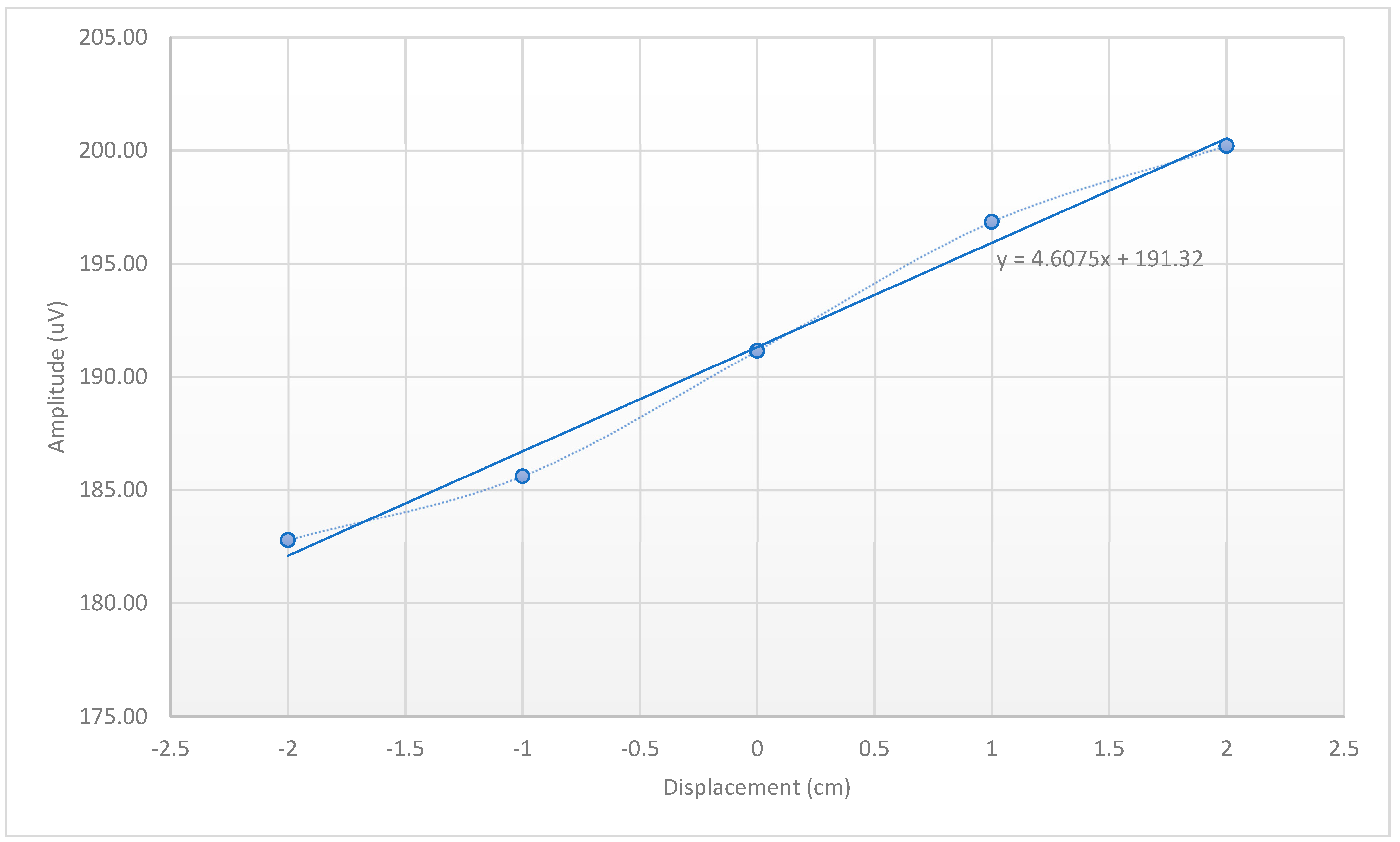
- Phantom Motion Tracking Scans: We placed the head-shaped agar phantom in a 3 T Siemens Prisma scanner and imaged it in the initial position, plus three other positions. The x-, y- and z-gradient responses were measured and ensemble-averaged across epochs (128 repetitions). We translated the phantom, approximately guided by a measured template. The EEG system was set up to record EEG signals at the highest sampling rate available (5000 S/s) with no filter settings beyond those present in the hardware. The sinusoidal amplitude estimation was performed as follows: prior to finding the amplitude of each sinusoidal response, each was normalized by subtracting out its mean to remove DC drift. The amplitude of each normalized sinusoidal response was then calculated as the amplitude of the 500 Hz sine wave that minimized the root mean square error (RMSE) between that sine wave and the normalized sinusoidal response.
- -
- EEG Recordings in a Human Subject. To test the Kalman noise cancellation capability of the MotoNet, we recorded EEG on a healthy adult subject wearing the MotoNet. The Massachusetts General Hospital Institutional Review Board approved the study procedures, and the subject provided informed consent. The MotoNet EEG signals were measured inside the 3 T scanner while the participant alternated between periods of eyes open and eyes closed states. The PTF leads were passed through the opening at the top of a Siemens 64-channel head/neck coil and connected to a 64-channel EEG amplifier system (BrainProducts GMBH) through a custom-made interface (see Figure 2 or Supplementary Material Figure S1).
- -
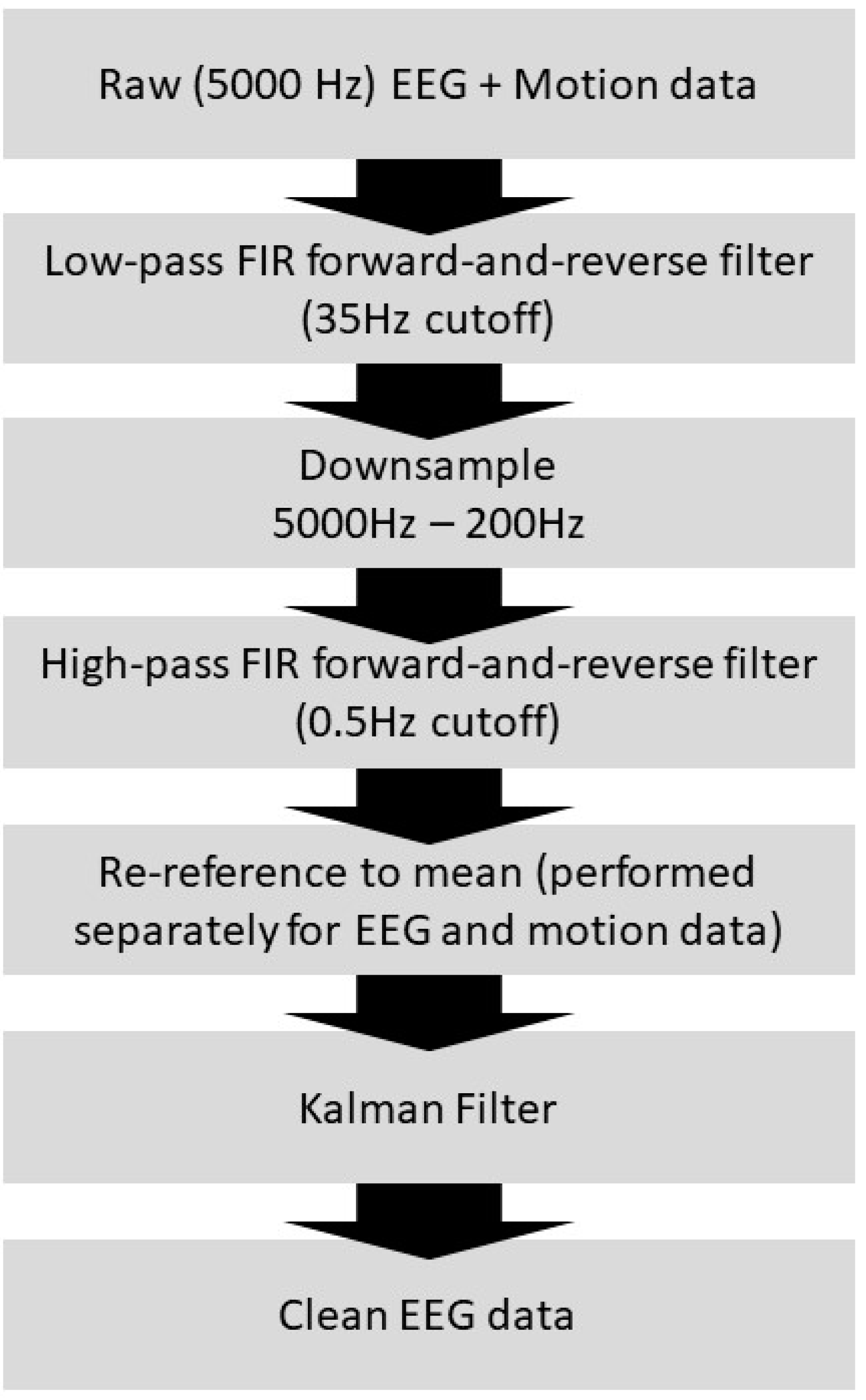
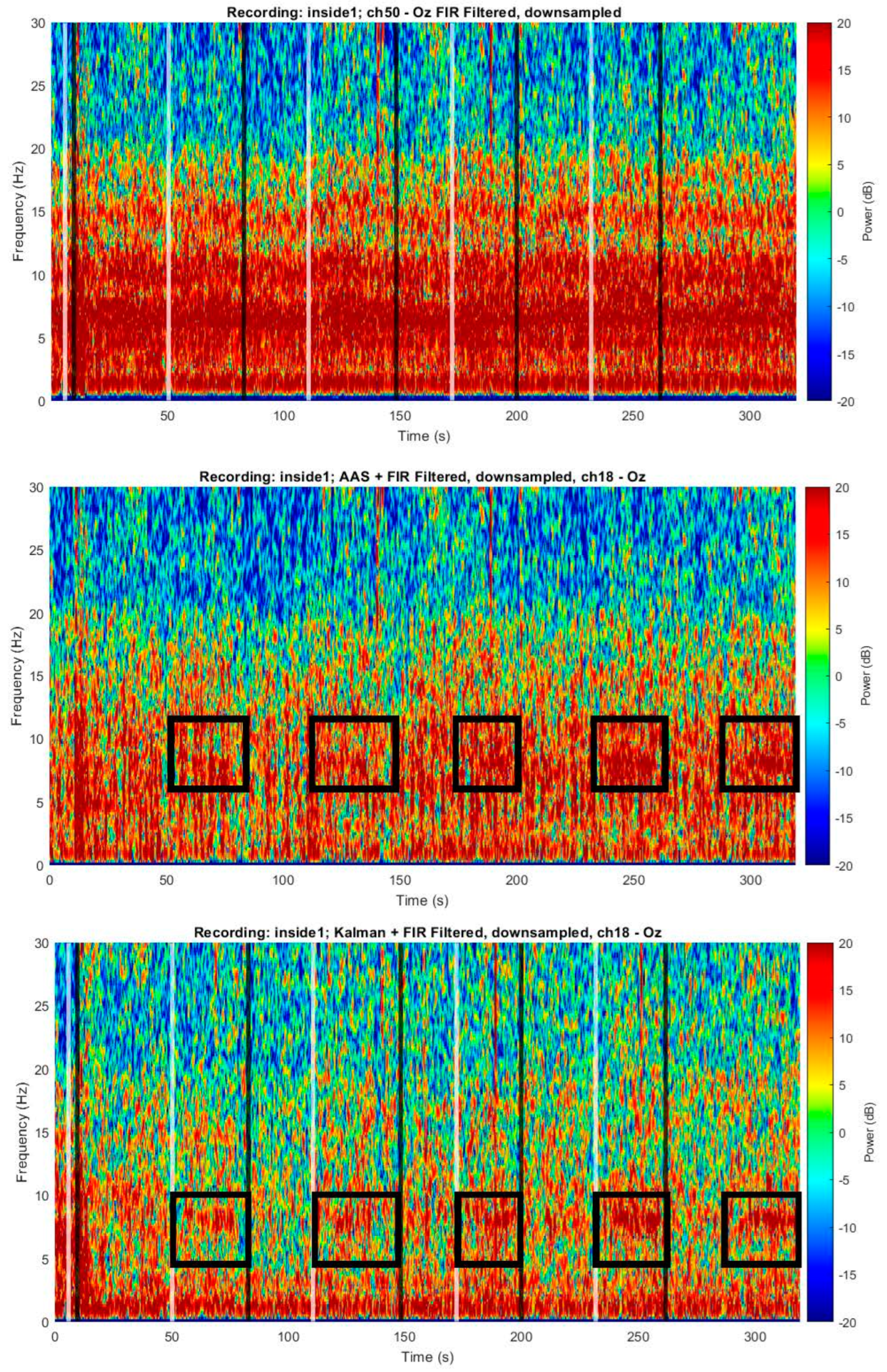
- EEG Kalman Noise Cancellation: To remove noise, EEG signals recorded inside a 3 T MRI on a human volunteer were Kalman filtered as follows. We modeled the n-channel recorded EEG scalar signal as the sum of a “true” underlying EEG (scalar) signal and , or the motion sensor signal vector with size 1 × (n + 1) (i.e., a motion sensor for each EEG channel) multiplied by or the weight vector with size (n + 1) × 1 and t = 1…T:where i and t are indices tracking the current EEG channel number (i.e., spatial index) and sample number (i.e., temporal index), respectively; n is the number of EEG or motion sensors channels (i.e., a motion sensor for each EEG electrode, plus one additional constant value result in n + 1 channels), and ‘’ is the vector or matrix product. The was initialized prior to beginning the filtering procedure to a vector of zeros. The state can be found (see Figure S3):where is the following Kalman gain vector with size (n + 1) × 1:where is the measurement noise covariance scalar, a constant model hyperparameter, in this case, estimated to be 100, and is the error covariance matrix at the current step of size (n + 1) × (n + 1):where Q is the noise covariance matrix, which is a model hyperparameter and is held constant and is assumed to be an identity matrix I of size (n + 1) × (n + 1) multiplied by a scalar (estimated to be 0.01 in this case), and where is the error covariance matrix (at the previous time step), which is initialized prior to each recording to be an identity matrix of size (n + 1) × (n + 1). Finally, and are also related as follows, limiting the unbounded increase in in Equation (21):
3. Results
3.1. MotoNet Fabrication
3.2. MRI Safety
3.3. Displacement Estimation in Phantoms
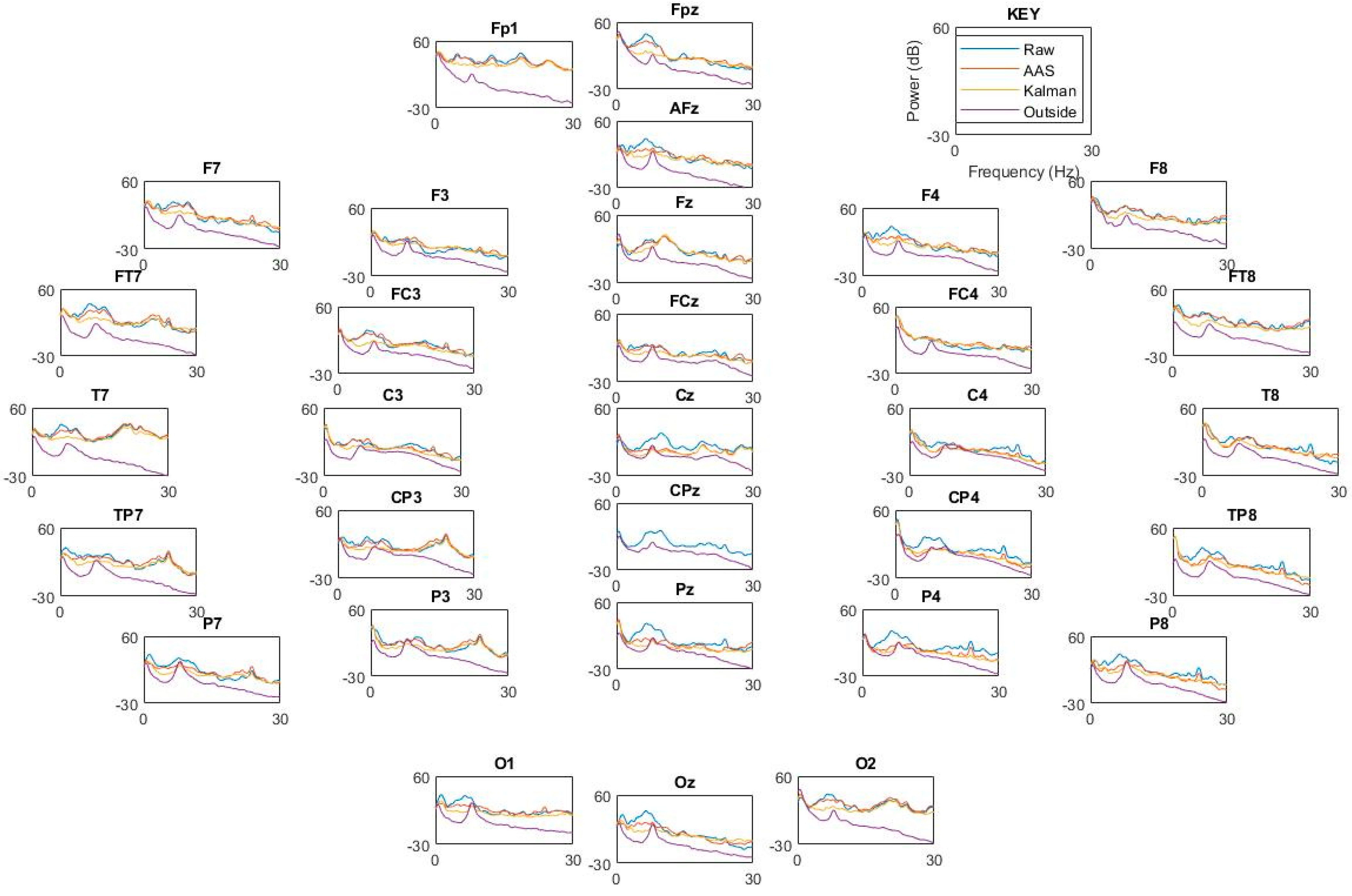
3.4. BCG Adaptive Noise Cancellation
4. Discussion
4.1. Safety
4.2. Displacement Estimation in Phantoms
4.3. MotoNet Design
4.4. BCG Adaptive Noise Cancellation
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jorge, J.; Grouiller, F.; Gruetter, R.; Van Der Zwaag, W.; Figueiredo, P. Towards high-quality simultaneous EEG-fMRI at 7 T: Detection and reduction of EEG artifacts due to head motion. Neuroimage 2015, 120, 143–153. [Google Scholar] [CrossRef]
- Schulte-Uentrop, L.; Goepfert, M.S. Anaesthesia or sedation for MRI in children. Curr. Opin. Anaesthesiol. 2010, 23, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Mulert, C.; Lemieux, L. EEG-fMRI: Physiological Basis, Technique, and Applications, 2nd ed.; Mulert, C., Lemieux, L., Eds.; Springer: Cham, Switzerland, 2023; Volume IX, p. 789. [Google Scholar]
- Kuperman, J.M.; Brown, T.T.; Ahmadi, M.E.; Erhart, M.J.; White, N.S.; Roddey, J.C.; Shankaranarayanan, A.; Han, E.T.; Rettmann, D.; Dale, A.M. Prospective motion correction improves diagnostic utility of pediatric MRI scans. Pediatr. Radiol. 2011, 41, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Placidi, G. MRI: Essentials for Innovative Technologies; CRC Press: Boca Raton, FL, USA, 2012; 192p. [Google Scholar]
- FDA. FDA Drug Safety Communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children. In Drug Safety and Availability; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Zaitsev, M.; Maclaren, J.; Herbst, M. Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging 2015, 42, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Reali, M.; Bertini, G.; Wiechmann, L.; Spagnolo, A.; Tangucci, M.; Rubaltelli, F. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. Eur. Respir. J. 1999, 14, 155–159. [Google Scholar] [CrossRef]
- Allen, P.J.; Josephs, O.; Turner, R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 2000, 12, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.; Hill, D.; Stoyle, P.; Summers, P.; Keevil, S. Automatic correction of motion artifacts in magnetic resonance images using an entropy focus criterion. IEEE Trans. Med. Imaging 1997, 16, 903–910. [Google Scholar] [CrossRef]
- Küstner, T.; Armanious, K.; Yang, J.; Yang, B.; Schick, F.; Gatidis, S. Retrospective correction of motion-affected MR images using deep learning frameworks. Magn. Reson. Med. 2019, 82, 1527–1540. [Google Scholar] [CrossRef]
- Maclaren, J.; Herbst, M.; Speck, O.; Zaitsev, M. Prospective motion correction in brain imaging: A review. Magn. Reson. Med. 2013, 69, 621–636. [Google Scholar] [CrossRef]
- Slipsager, J.M.; Glimberg, S.L.; Højgaard, L.; Paulsen, R.R.; Wighton, P.; Tisdall, M.D.; Jaimes, C.; Gagoski, B.A.; Grant, P.E.; van der Kouwe, A.; et al. Comparison of prospective and retrospective motion correction in 3D-encoded neuroanatomical MRI. Magn. Reson. Med. 2022, 87, 629–645. [Google Scholar] [CrossRef]
- Slipsager, J.M.; Ellegaard, A.H.; Glimberg, S.L.; Paulsen, R.R.; Tisdall, M.D.; Wighton, P.; Van Der Kouwe, A.; Marner, L.; Henriksen, O.M.; Law, I.; et al. Markerless motion tracking and correction for PET, MRI, and simultaneous PET/MRI. PLoS ONE 2019, 14, e0215524. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, M.; Dold, C.; Sakas, G.; Hennig, J.; Speck, O. Magnetic resonance imaging of freely moving objects: Prospective real-time motion correction using an external optical motion tracking system. Neuroimage 2006, 31, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Kober, T.; Marques, J.P.; Gruetter, R.; Krueger, G. Head motion detection using FID navigators. Magn. Reson. Med. 2011, 66, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gallichan, D.; Marques, J.P.; Gruetter, R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magn. Reson. Med. 2016, 75, 1030–1039. [Google Scholar] [CrossRef]
- Tisdall, M.D.; Hess, A.T.; Reuter, M.; Meintjes, E.M.; Fischl, B.; van der Kouwe, A.J.W. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn. Reson. Med. 2012, 68, 389–399. [Google Scholar] [CrossRef] [PubMed]
- White, N.; Roddey, C.; Shankaranarayanan, A.; Han, E.; Rettmann, D.; Santos, J.; Kuperman, J.; Dale, A. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn. Reson. Med. 2010, 63, 91–105. [Google Scholar] [CrossRef]
- Ooi, M.B.; Aksoy, M.; Maclaren, J.; Watkins, R.D.; Bammer, R. Prospective motion correction using inductively coupled wireless RF coils. Magn. Reson. Med. 2013, 70, 639–647. [Google Scholar] [CrossRef]
- Purdon, P.L.; Millan, H.; Fuller, P.L.; Bonmassar, G. An open-source hardware and software system for acquisition and real-time processing of electrophysiology during high field MRI. J. Neurosci. Methods 2008, 175, 165–186. [Google Scholar] [CrossRef]
- Laustsen, M.; Andersen, M.; Xue, R.; Madsen, K.H.; Hanson, L.G. Tracking of rigid head motion during MRI using an EEG system. Magn. Reson. Med. 2022, 88, 986–1001. [Google Scholar] [CrossRef]
- Krishnaswamy, P.; Bonmassar, G.; Poulsen, C.; Pierce, E.T.; Purdon, P.L.; Brown, E.N. Reference-free removal of EEG-fMRI ballistocardiogram artifacts with harmonic regression. Neuroimage 2016, 128, 398–412. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Glover, G.H. Ballistocardiogram artifact removal with a reference layer and standard EEG cap. J. Neurosci. Methods 2014, 233, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Bonmassar, G.; Purdon, P.L.; Jääskeläinen, I.P.; Chiappa, K.; Solo, V.; Brown, E.N.; Belliveau, J.W. Motion and Ballistocardiogram Artifact Removal for Interleaved Recording of EEG and EPs during MRI. Neuroimage 2002, 16, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, A.; Meintjes, E.; van der Kouwe, A. A Wireless Radio Frequency Triggered Acquisition Device (WRAD) for Self-Synchronised Measurements of the Rate of Change of the MRI Gradient Vector Field for Motion Tracking. IEEE Trans. Med. Imaging 2019, 38, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Leite, M.; Jorge, J.; Grouiller, F.; van der Zwaag, W.; Leal, A.; Figueiredo, P. Ballistocardiogram artifact correction taking into account physiological signal preservation in simultaneous EEG-fMRI. Neuroimage 2016, 135, 45–63. [Google Scholar] [CrossRef]
- Duan, Q.; Duyn, J.H.; Gudino, N.; de Zwart, J.A.; van Gelderen, P.; Sodickson, D.K.; Brown, R. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Med. Phys. 2014, 41, 102303. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. 2022. Available online: Itis.Swiss/Database (accessed on 11 November 2020).
- Atefi, S.R.; Serano, P.; Poulsen, C.; Angelone, L.M.; Bonmassar, G. Numerical and Experimental Analysis of Radiofrequency-Induced Heating Versus Lead Conductivity During EEG-MRI at 3 T. IEEE Trans. Electromagn. Compat. 2018, 61, 852–859. [Google Scholar] [CrossRef]
- Laustsen, M.; Andersen, M.; Lehmann, P.M.; Xue, R.; Madsen, K.H.; Hanson, L.G. Slice-wise motion tracking during simultaneous EEG-fMRI. In Proceedings of the Joint Annual Meeting ISMRM-ESMRMB, Paris, France, 16–21 June 2018. [Google Scholar]
- Gholipour, A.; Polak, M.; van der Kouwe, A.; Nevo, E.; Warfield, S.K. Motion-robust MRI through real-time motion tracking and retrospective super-resolution volume reconstruction. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- De Cusatis, C.; Optical Society of America. Handbook of Applied Photometry; AIP Press: Woodbury, NY, USA; Optical Society of America: Washington, DC, USA, 1997; 463p. [Google Scholar]
- Allen, P.J.; Polizzi, G.; Krakow, K.; Fish, D.R.; Lemieux, L. Identification of EEG events in the MR scanner: The problem of pulse artifact and a method for its subtraction. Neuroimage 1998, 8, 229–239. [Google Scholar] [CrossRef]
- FDA. Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment; Center for Devices and Radiological Health, Ed.; FDA: Silver Spring, MD, USA, 2021.
- Chou, C.K.; Bassen, H.; Osepchuk, J.; Balzano, Q.; Petersen, R.; Meltz, M.; Cleveland, R.; Lin, J.C.; Heynick, L. Radio frequency electromagnetic exposure: Tutorial review on experimental dosimetry. Bioelectromagnetics 1996, 17, 195–208. [Google Scholar] [CrossRef]
- NCRP. Radiofrequency Electromagnetic Fields: Properties, Quantities and Units, Biophysical Interaction, and Measurement; National Council Radiation Protection and Measurements: Bethesda, MD, USA, 1981.
- Ibrahim, T.S.; Kangarlu, A.; Chakeress, D.W. Design and performance issues of RF coils utilized in ultra high field MRI: Experimental and numerical evaluations. IEEE Trans. Biomed. Eng. 2005, 52, 1278–1284. [Google Scholar] [CrossRef]
- Collins, C.M.; Liu, W.; Wang, J.; Gruetter, R.; Vaughan, J.T.; Ugurbil, K.; Smith, M.B. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J. Magn. Reson. Imaging 2004, 19, 650–656. [Google Scholar] [CrossRef]
- Finelli, D.A.; Rezai, A.R.; Ruggieri, P.M.; Tkach, J.A.; Nyenhuis, J.A.; Hrdlicka, G.; Sharan, A.; Gonzalez-Martinez, J.; Stypulkowski, P.H.; Shellock, F.G. MR imaging-related heating of deep brain stimulation electrodes: In Vitro study. AJNR Am. J. Neuroradiol. 2002, 23, 1795–1802. [Google Scholar]
- Gajsek, P.; Walters, T.J.; Hurt, W.D.; Ziriax, J.M.; Nelson, D.A.; Mason, P.A. Empirical validation of SAR values predicted by FDTD modeling. Bioelectromagnetics 2002, 23, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Noth, U.; Laufs, H.; Stoermer, R.; Deichmann, R. Simultaneous electroencephalography-functional MRI at 3 T: An analysis of safety risks imposed by performing anatomical reference scans with the EEG equipment in place. J. Magn. Reson. Imaging 2012, 35, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G. Magnetic Resonance Procedures: Health Effects and Safety; Amirsys: Salt Lake City, UT, USA, 2003; 456p. [Google Scholar]
- Vasios, C.E.; Angelone, L.M.; Purdon, P.L.; Ahveninen, J.; Belliveau, J.W.; Bonmassar, G. EEG/(f)MRI measurements at 7 Tesla using a new EEG cap (“InkCap”). Neuroimage 2006, 33, 1082–1092. [Google Scholar] [CrossRef]
- Jeong, H.; Ntolkeras, G.; Grant, P.E.; Bonmassar, G. Numerical simulation of the radiofrequency safety of 128-channel hd-EEG nets on a 29-month-old whole-body model in a 3 Tesla MRI. IEEE Trans. Electromagn. Compat. 2021, 63, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, A.; van der Kouwe, A.; Meintjes, E. Toward “plug and play” prospective motion correction for MRI by combining observations of the time varying gradient and static vector fields. Magn. Reson. Med. 2019, 82, 1214–1228. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Zotev, V.; Misaki, M.; Phillips, R.; Luo, Q.; Bodurka, J. Automatic EEG-assisted retrospective motion correction for fMRI (aE-REMCOR). Neuroimage 2016, 129, 133–147. [Google Scholar] [CrossRef]
- Poulsen, C.; Wakeman, D.G.; Atefi, S.R.; Luu, P.; Konyn, A.; Bonmassar, G. Polymer thick film technology for improved simultaneous dEEG/MRI recording: Safety and MRI data quality. Magn. Reson. Med. 2017, 77, 895–903. [Google Scholar] [CrossRef]
- van der Kouwe, A.; Jeong, H.; Yang, Z.; Straney, D.; Frost, R.; Lewis, L.; Bonmassar, G. The MotoNet: An MRI-Compatible EEG Net with Embedded Motion Sensors. In Proceedings of the Joint annual Meeting ISMRM-ESMRMB-ISMRT, London, UK, 7–12 May 2022. [Google Scholar]
- Haykin, S.S. Adaptive Filter Theory, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; p. 920. [Google Scholar]
- Levitt, J.; Yang, Z.; Williams, S.; Espinosa, S.; Garcia-Casal, A.; Lewis, L. EEG-LLAMAS: An open source, low latency, EEG-fMRI neurofeedback platform. bioRxiv 2022. [Google Scholar] [CrossRef]
- Thrun, S.; Burgard, W.; Fox, D. Probabilistic Robotics; Intelligent Robotics and Autonomous Agents; MIT Press: Cambridge, MA, USA, 2005; 647p. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levitt, J.; van der Kouwe, A.; Jeong, H.; Lewis, L.D.; Bonmassar, G. The MotoNet: A 3 Tesla MRI-Conditional EEG Net with Embedded Motion Sensors. Sensors 2023, 23, 3539. https://doi.org/10.3390/s23073539
Levitt J, van der Kouwe A, Jeong H, Lewis LD, Bonmassar G. The MotoNet: A 3 Tesla MRI-Conditional EEG Net with Embedded Motion Sensors. Sensors. 2023; 23(7):3539. https://doi.org/10.3390/s23073539
Chicago/Turabian StyleLevitt, Joshua, André van der Kouwe, Hongbae Jeong, Laura D. Lewis, and Giorgio Bonmassar. 2023. "The MotoNet: A 3 Tesla MRI-Conditional EEG Net with Embedded Motion Sensors" Sensors 23, no. 7: 3539. https://doi.org/10.3390/s23073539
APA StyleLevitt, J., van der Kouwe, A., Jeong, H., Lewis, L. D., & Bonmassar, G. (2023). The MotoNet: A 3 Tesla MRI-Conditional EEG Net with Embedded Motion Sensors. Sensors, 23(7), 3539. https://doi.org/10.3390/s23073539






