The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis
Abstract
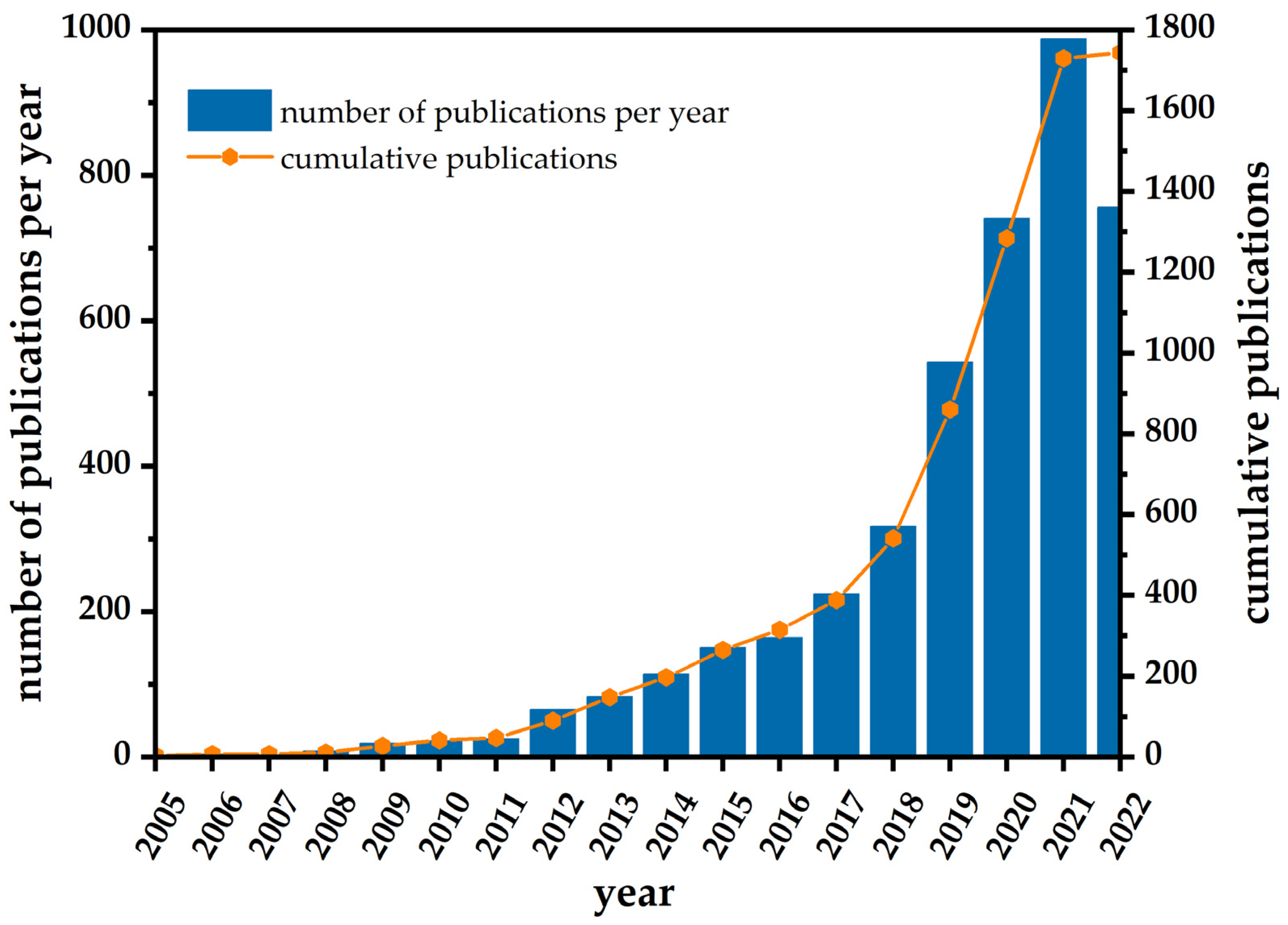
1. Introduction
2. General Methods for the Synthesis of Silver Nanoparticles
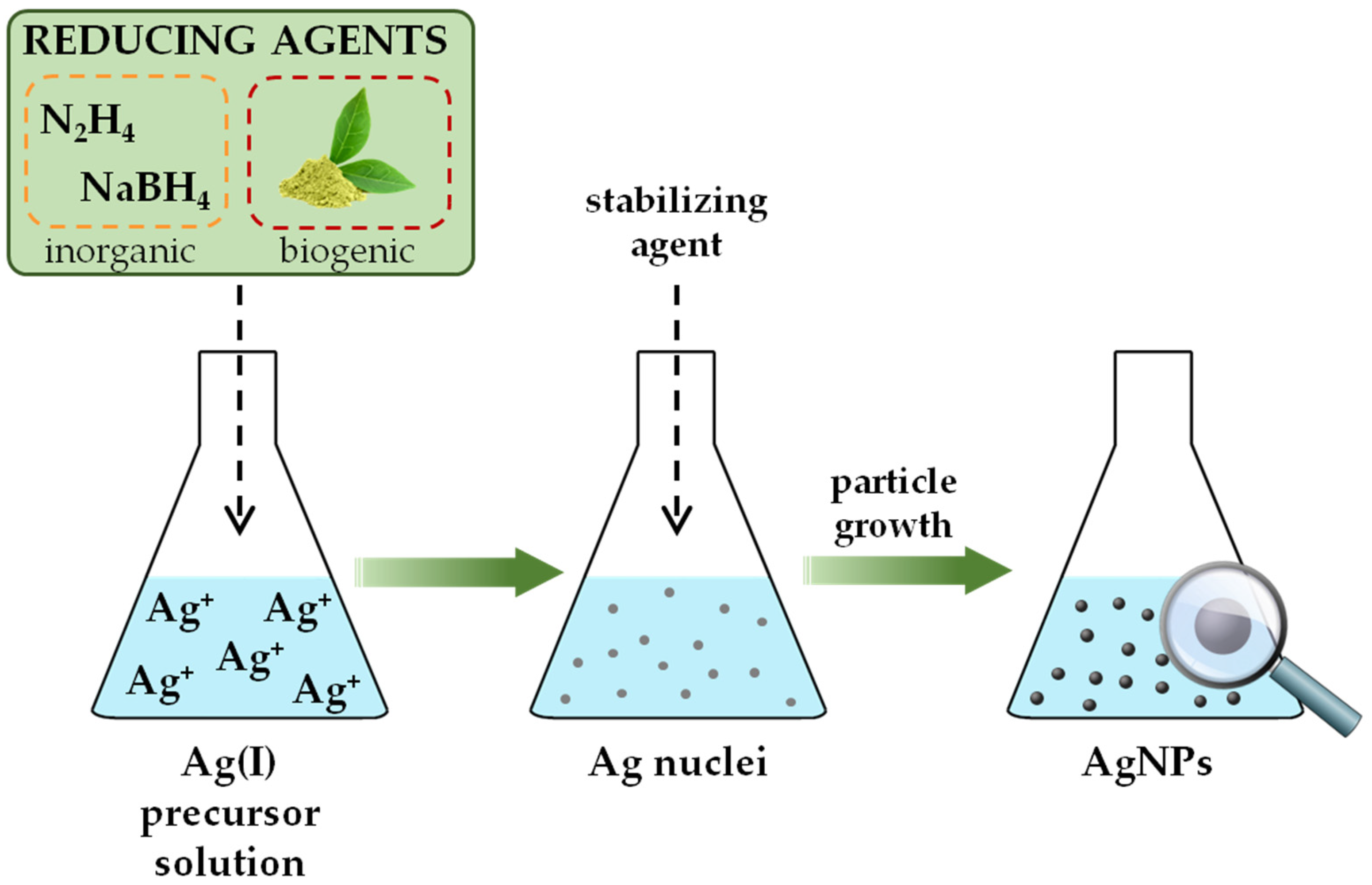
2.1. Bottom-Up Synthesis
2.1.1. Chemical Reduction
2.1.2. Green Methods
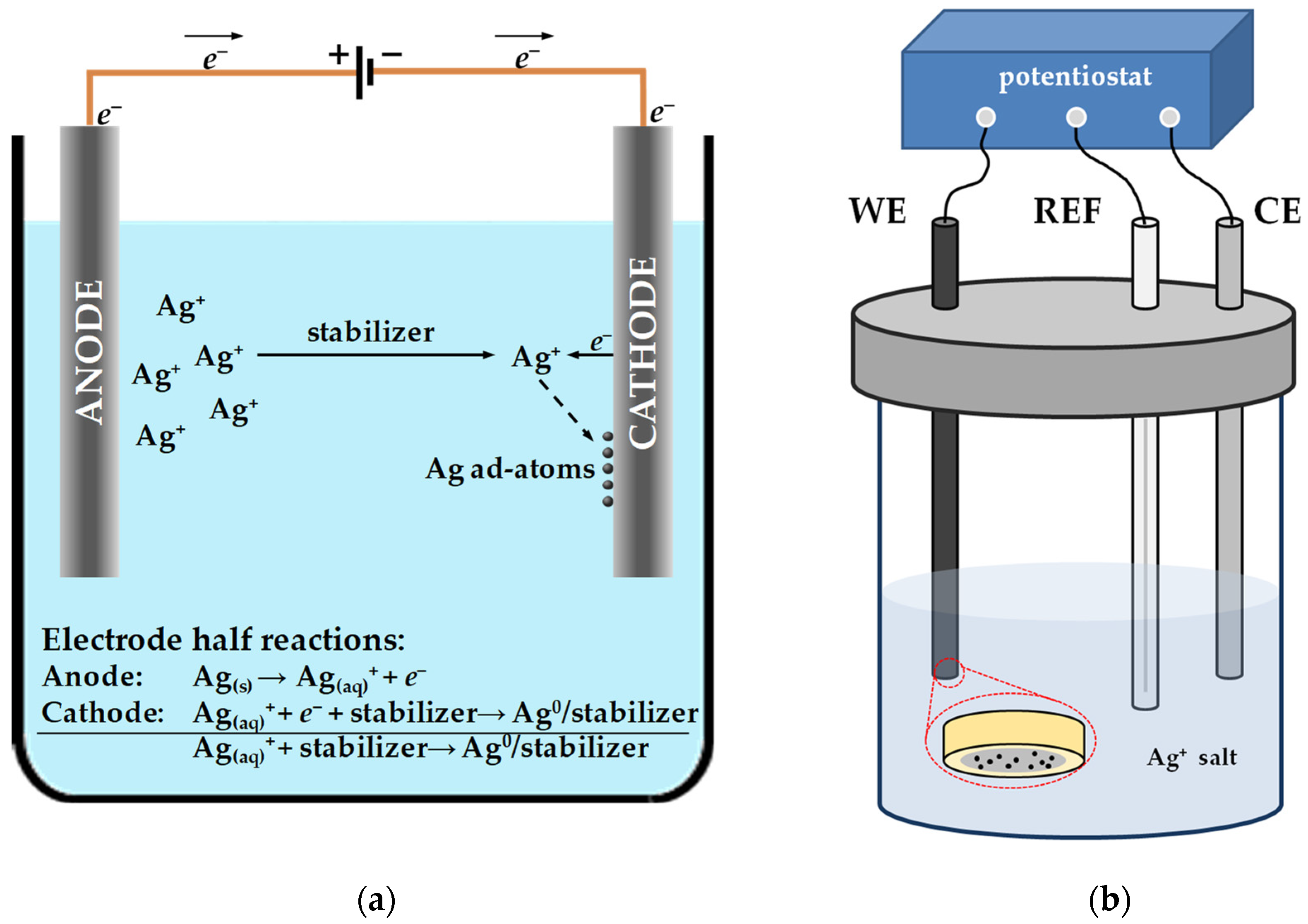
2.1.3. Electrochemical Synthesis
3. Application of Silver Nanoparticles in Voltammetric and Amperometric Sensors
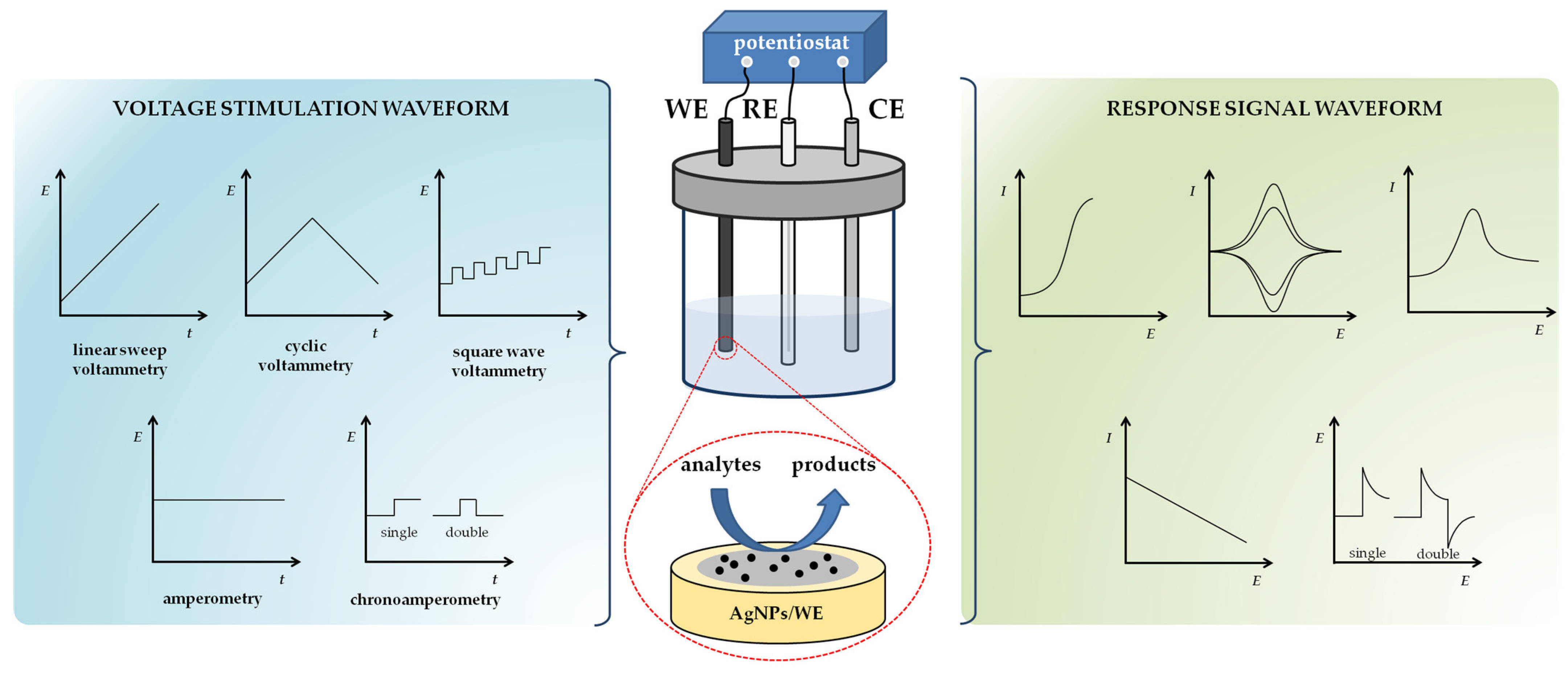
3.1. Working Principles of Voltammetric and Amperometric Sensing Techniques
3.2. Electrochemical Sensors for Detection of Heavy Metal Ions
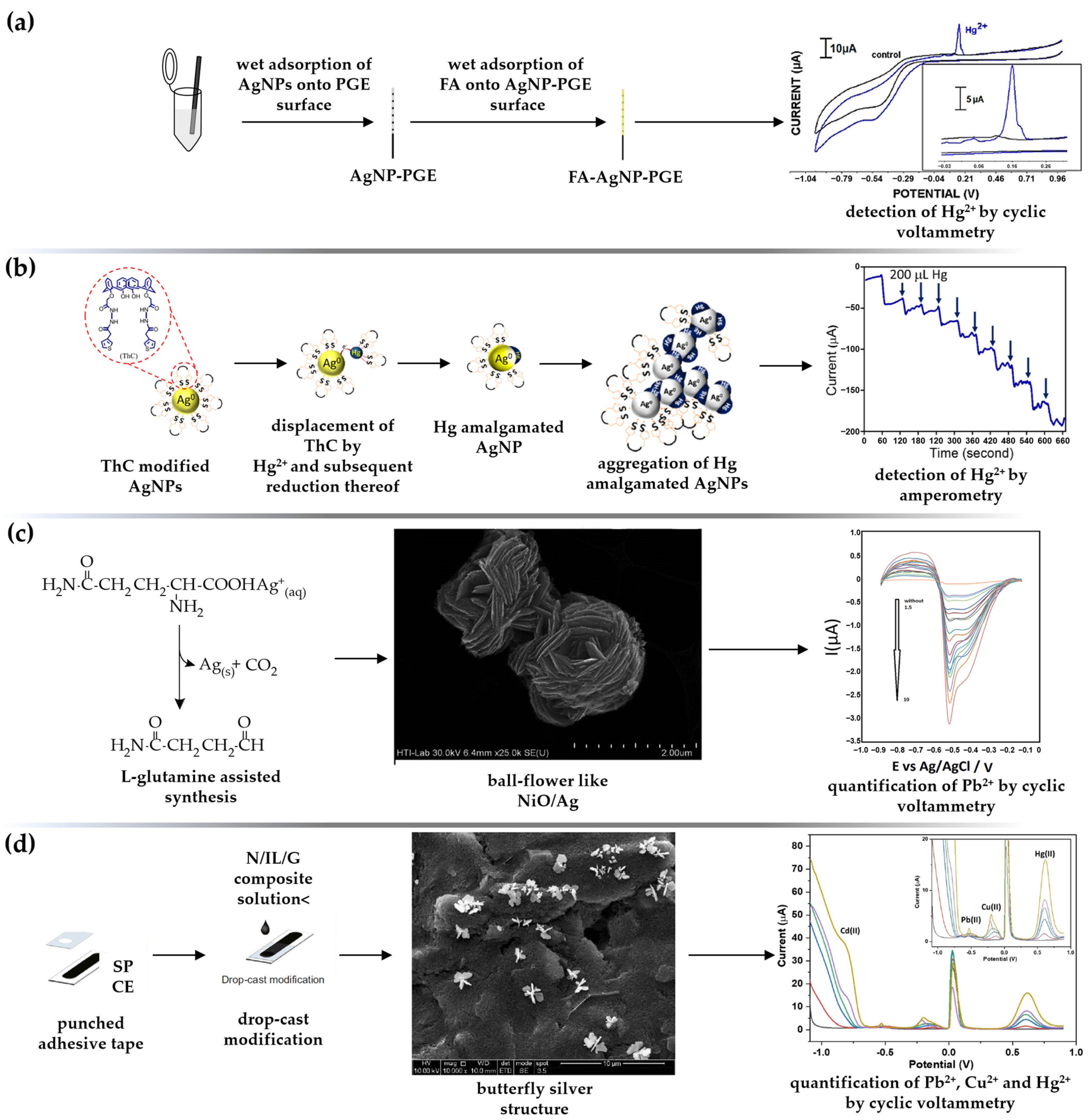
3.2.1. Electrochemical Sensors for Divalent HMs
3.2.2. Electrochemical Sensors for Trivalent and Hexavalent HMs
| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Refs. |
|---|---|---|---|---|---|---|
| Hg(II) | Tap water | AgNP-FA-PGE/CV | 10–25 μM | Green synthesis | 8.43 μM | [97] |
| Hg(II) | Lake water | AgNP-AB-Pt/DPV | 10–90 μM | Green synthesis | 2.1 μM | [98] |
| Hg(II) | Tap water, river water | AgNP-MD-Pt/DPV | 5.0–45 μM | Green synthesis | 1.46 μM | [99] |
| Hg(II) | Tap water, drinking water | ThC-AgNPs/Pt/Amp | 45–105 nM | Chemical reduction | 10.0 nM | [101] |
| Hg(II) | River water, well water | CLR-AgNP/AuE/DPV | 0.01–0.06 μM | Green synthesis | 6.50 nM | [102] |
| Hg(II) | Tap water, river water, lake water | CMC@AgNPs/GCE/DPASV | 5.0–75 μM | Chemical reduction | 0.19 nM | [103] |
| Hg(II) | Environmental water, drinking water | AgNPs/GCE/LSV | 100.0 pM–10.0 nM | Chemical reduction | 28.0 pM | [104] |
| Cd(II) | Lake water, water from pigment, cosmetics and fertilizer industries | AgNP-AS-Pt/DPV | 10–90 μM | Green synthesis | 0.277 μM | [106] |
| Pb(II) | Tap water, wastewater | AgNPs/PANI/CPE/SWV | 0.1–120 μM | Green synthesis | 0.04 μM | [108] |
| Pb(II) | River water, tap water, sweage water | Ag@Pt/GPE/SWASV | 0.25–10 μM | Green synthesis | 0.8 nM | [69] |
| Pb(II) | Pipe water, groundwater | BF-NiO-Ag/GCE/DPV | 1.5–10 nM | Green synthesis | 0.06 nM | [109] |
| Cu(II) | Lake water, waste water from electroplating unit | AgNP-MO/PE/DPV | 10–90 μM | Green synthesis | 0.530 μM | [111] |
| Cu(II) | Tap water, pool water | AgNPs-MBA-Cu2+-MBA-AuNPs-FTO/SWV | 0.1–100 nM | Borohydride reduction | 0.08 nM | [47] |
| Cu(II) Pb(II) | Groundwater (certified reference material) | AgNPs-SPCNFE/ASV | 7.6–130.7 μg/L 3.2–162.5 μg/L | Borohydride reduction Citrate reduction | 2.29 μg/L 0.96 μg/L | [113] |
| Cu(II) Pb(II) | Aqueous solution | Ag@MOF/ITO/CV | 1.0–50 μM 1.0–50 μM | Chemical reduction | 0.68 μM 0.64 μM | [114] |
| Pb(II) Cd(II) | Tap water | Polyrutin/AgNPs/GCE/DPASV | 29–140 nM 57–203 nM | Electrodeposition | 3.0 nM 10.0 nM | [77] |
| Cd(II) Pb(II) Cu(II) | Tap water | AgNPs@p-1,8-DAN/GCE/SWASV | 0.90 nM–9.0 μM 2.0 nM–24.0 μM 1.3 nM–9.0 μM | Electrodeposition | 0.17 nM 0.15 nM 0.09 nM | [73] |
| Cd(II) Cu(II) Pb(II) | Tap water | AgNPs/GrNPs/GE/SWASV | 0.5–120 μg/L | Electrodeposition | 5.0 ng/L 4.1 ng/L 1.0 ng/L | [78] |
| Cu(II) Cd(II) Hg(II) | Aqueous solution | rGO/AgNPs/CV | 1.0 nM–10 μM | Hydrothermal | 10−15 M 10−21 M 10−29 M | [85] |
| Pb(II) Cd(II) Cu(II) Hg(II) | Aqueous solution | AgNPs/rGO/MGCE/SWASV | 0.05–2.5 μM 0.05–3.5 μM 0.05–3.5 μM 0.05–3.0 μM | Chemical reduction | 0.141 μM 0.254 μM 0.178 μM 0.285 μM | [116] |
| Cd(II) Pb(II) Cu(II) Hg(II) | Tap water, rainwater, lake water, river water | AgNS/SPCE/DPSV | 5.0–300 ppb 5.0–300 ppb 50–500 ppb 5.0–100 ppb | Electrodeposition | 0.4 ppb 2.5 ppb 7.3 ppb 0.7 ppb | [79] |
| As(III) | River water | AgNPs/Au/DPASV | 0.05–0.2 μM | Chemical reduction | 0.0138 μM | [50] |
| As(III) | Real water | AgNPs/PpyNW/Au/ASV | 0.01–0.10 μM | Electrodeposition | 1.5 ppb | [119] |
| As(III) | River water, lake water, well water | GSH/DTT/Asn-AgNPs/AuE/DPV | 0.01–40 ppb | Green synthesis | 5.2 ppt | [120] |
| Cr(III) | Lake water, water from metal plating industry | AgNP-LE-Pt/DPV | 10–90 μM | Green synthesis | 0.804 μM | [122] |
| Cr(III) Cr(VI) | Tap water, wastewater | Ag-Au/SPCE/DPV | 0.05–1.0 ppm 0.05–5.0 ppm | Electrodeposition | 0.1 ppb | [81] |
| Cr(VI) | Tap water | Ag-plated/GCE/DPASV | 0.35–40 μM | Electrodeposition | 0.10 μM | [76] |
| Cr(VI) | Tap water, river water, electroplating wastewater | AgNPs-BPQ-BP-NRs/GPE/SWV | 1.0–100 μM 0.01–1.0 μM 0.08–10 nM | Chemical reduction | 2.0 pM | [123] |
3.3. Electrochemical Sensors for Nitrogen-Containing Inorganic Species
3.3.1. Electrochemical Sensors for Nitrite (NO2−) and Ammonium (NH4+) Detection

3.3.2. Electrochemical Sensors for Nitrate (NO3−) and Ammonia (NH3) Detection
| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Refs. |
|---|---|---|---|---|---|---|
| NO2− | Water | S.l-AgNPs/GCE/CV | 1.0–3.75 μM | Green synthesis | – | [65] |
| NH2OH NO2− | Drinking water, tap water | OAgNPs/GCE/DPV | 183.4–779.2 μM 15.3–64.9 μM | Electrodeposition | 57.8 μM 4.1 μM | [75] |
| NO2− | Tap water | AgNS/GCE/Amp | 0.1–8.0 μM | Green synthesis | 0.031 μM | [129] |
| NO2− I− | Drinking water, river water, sewage water | Au@Ag/CG/GCE/DPV | 2.5–1250 μM 3.5–1000 μM | Chemical reduction | 0.15 μM 0.1 μM | [86] |
| NO2− | Water | AgNP-GO/GCE/LSV | 1.0 μM–1.0 mM | 60Co γ-irradiation-assisted chemical reduction | 0.24 μM | [130] |
| NO2− | Pond water | Ag-rGO/GCE/DPV | 0.1–120 μM | Microwave-assisted chemical reduction | 0.012 μM | [131] |
| NO2− | Tap water, lake water, drinking water | AgNPs@PPy/rGO/GCE/DPV | 0.6–8.6 μM | Chemical reduction | 6.8 nM | [133] |
| SO32− NO2− | Drinking water, tap water, river water | AgNPs@PANI/rGO/GCE/DPV | 2.7–24.4 μM 1.0–28.2 μM | Chemical reduction | 77.0 nM 56.0 nM | [134] |
| NO2− | Tap water | Ag-AEfG100/GCE/Amp | 0.05–3000 μM | Chemical reduction | 0.023 μM | [135] |
| NO2− | Water | AgNP/MWCNTs/GCE/DPV | 1.0–100 μM | Electrodeposition | 0.095 μM | [74] |
| NO2− | Lake water, pickle water | Ag-ZnO/PGE/DPV | 30–1400 μM | Chemical reduction | 14.0 μM | [136] |
| NO2− | River water | Chit-AgNPs/ MWCNT/PE/CV | – | Borohydride reduction | 30.0 nM | [138] |
| NO2− | Water | Ag/HNT/MoS2/CPE/Amp | 2.0–425 μM | Green synthesis | 0.7 μM | [139] |
| NO2− | Tap water | Ag/H-C3N4/CC/Amp | 5.0–1000 μM | Green synthesis | 0.216 μM | [141] |
| NO2− | Tap water | Ag-CSA-PEDOT:PSS/glass/Amp | 0.5–3400 μM | Electrodeposition | 0.34 | [142] |
| NO2− NH4+ | Groundwater | Ag-CNT/DPV | 0.2–1.0 mM | Electrodeposition | 0.006 mM 0.003 mM | [144] |
| NO3− NH3 | Simulated water sample | Pt//Ag/ITO/CV | 0.27–10.9 mM Nonlinear (NH3) | Electrodeposition | 0.134 mM 3.946 μM | [148] |
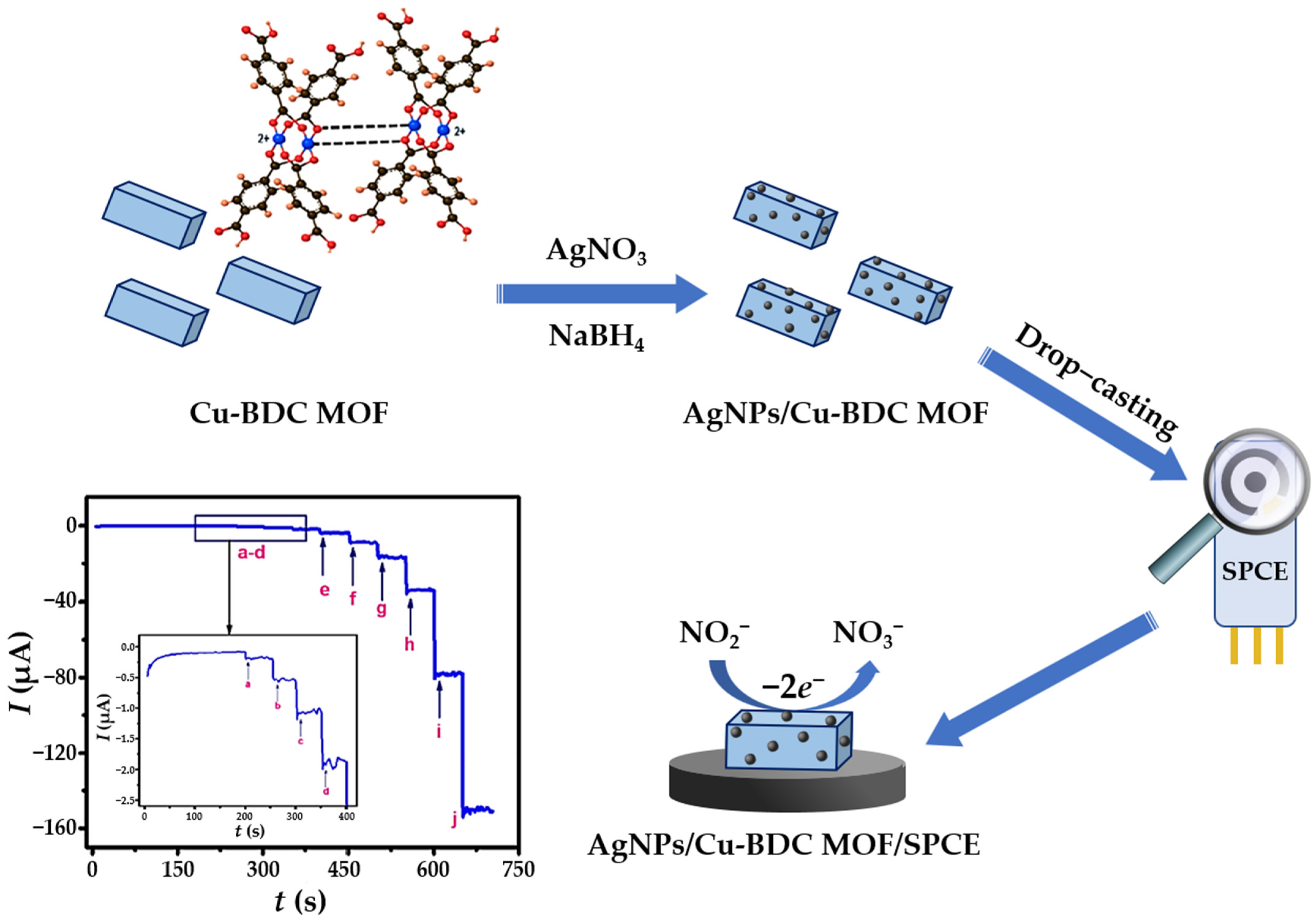
| NO3− | Water, soil extract | AgNPs/Cu-BDC/SPCE/Amp | 0.5–1000 μM | Borohydride reduction | 0.24 μM | [46] |
| NO3− | seawater | AgNPs/CTS/PVP/MNE/Amp | 5.0–2000 μM | Electrodeposition | 1.2 μM | [150] |
| NO3− | Artificial seawater | AgNPs/Au/SWV | 0.39–50 μM | Electrodeposition | – | [146] |
| NO3− | Artificial seawater | AgNPs/Au/SWV | 0.9 nM–1000 μM | In situ deposition | 0.9 nM | [149] |
3.4. Electrochemical Sensors for Phenolic Compounds
| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Ref. |
|---|---|---|---|---|---|---|
| Phenol | Tap water, mineral water | AgNPs-M/GCE/DPV | 0.8–20 μM | Green synthesis | 0.42 μM | [66] |
| Phenol o-cresol | Shale gas wastewater | AgNPs/CNTs/GCE/Amp | 10–160 μM 10–200 μM | Electrodeposition | 0.01 μM 0.01 μM | [80] |
| Phenol HQ CC BSA | Tap water | AgNPs/MWCNT/GCE/SWV | 2.4–152 μM 2.5–260 μM 20–260 μM 5.0–152 μM | Borohydride reduction | 3.1 μM 1.2 μM 1.6 μM 2.4 μM | [152] |
| HQ CC | Tap water | TACoPc/PANI/AgNPs/GCE/DPV | 10–100 μM 10–100 μM | Chemical reduction | 0.60 μM 0.46 μM | [153] |
| HQ CC | River water, tap water, rain water | Ag-rGO-Fe3O4/GCE/DPV | 10–50 μM 10–50 μM | Chemical reduction | 37.5 nM 335.4 nM | [87] |
| 4-NP | Aqueous solution | AgNCS/GCE/LSV | 0.1–0.6 mM | Borohydride reduction | – | [154] |
| 4-NP | Aqueous solution | Ag-rGO/GCE/Amp | 2–150 mM | Borohydride reduction | – | [155] |
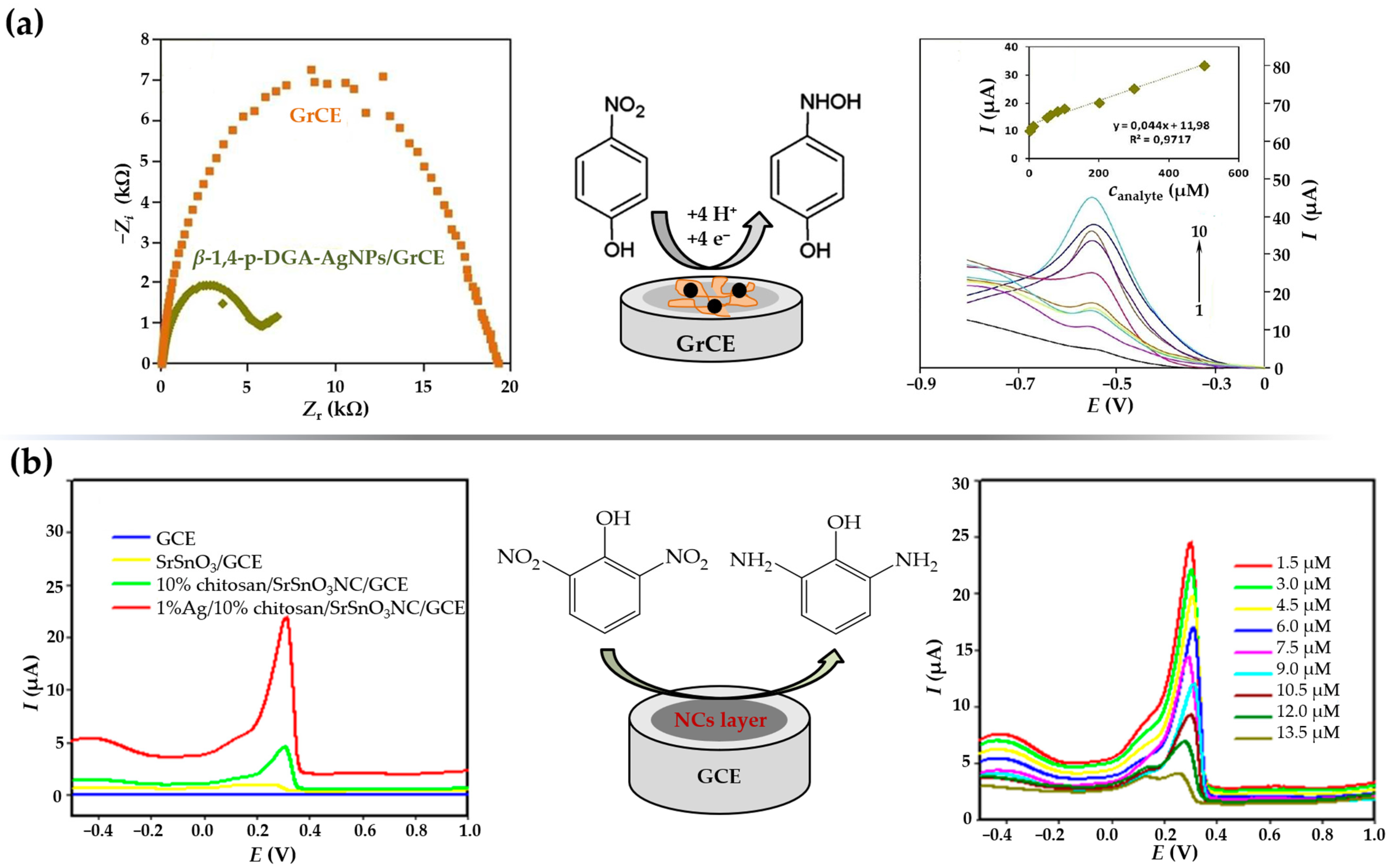
| 4-NP | Wastewater, river water | β-1,4-p-DGA-AgNPs/GrCE/DPV | 1–100 μM | Borohydride reduction | 0.6 μM | [156] |
| 4-NP | Tap water, drinking water, river water | TA@Fe3O4-AgNPs/GCE/DPV | 0.1–680.1 μM | Hydrothermal | 33.0 nM | [157] |
| 4-NP | Drinking water, tap water, industrial water, river water | rGO/HNT/AgNPs/SPCE/DPV | 0.1–363.9 μM | Chemical reduction | 48.6 nM | [158] |
| 4-NP N2H4 | River water, industrial water | CM-AgNPs/Gr-FeCo2O4/SPCE/ DPV | 2.5–1200 μM | Green synthesis | 18.0 nM 23.0 nM | [159] |
| 4-NP | Sewage, underground water | Ag-Co3O4NFs/CS-CNFs/SCPE/ DPV | 0.06–18.93 μM | UV-assisted chemical reduction | 0.4 nM | [160] |
| 2,6-DNP | Underground water, sea water, tap water | Ag-Chitosan/SrSnO3NC/GCE/ DPV | 1.5–13.5 μM | Chemical reduction | 0.18 μM | [161] |
| BPA | Drinking water | RGO-Ag/PLL/GCE/DPV | 1.0–80 μM | Hydrazine hydrate | 0.54 μM | [45] |
| BPA | Bottled water, well water, milk | AgNPs-rGO/ITO/DPV | 19 nM–0.820 μM | Green synthesis | 0.14 μM | [67] |
3.5. Electrochemical Sensors for Pharmaceuticals
3.6. Electrochemical Sensors for Nitroaromatics
3.7. Electrochemical Sensors for Natural and Synthetic Estrogens
| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Ref. |
|---|---|---|---|---|---|---|
| E2 | Environmental water | GN@Ag/g-C3N4/GCE/Amp | 0.005–8.0 μM | - | 0.002 μM | [181] |
| E2 | Tap water, bottled water | MIPAPh-AgNP/GCE/SWV | 10 pM–100 nM | Electrodeposition | 1.86 pM | [182] |
| E2 | River water | MIP-GO-AgNP/GCE/SWV | 10 fM–250 nM | Electrodeposition | 3.01 fM | [183] |
| E3 | Creek water | CNB-AgNP/GCE/DPV | 0.2–0.3 μM | Polyol method | 0.16 μM | [184] |
| E3 | Tap water, urine | rGO-AgNPs/GCE/DPV | – | Borohydride reduction | 21.0 nM | [185] |
| DNL | River water | AgNP/SWCNT-CPE/SWV | 29–543 μg L−1 | Chemical reduction | 43.7 nM | [84] |
4. Discussion
4.1. Electrochemical Sensor Technology
4.2. Wet Chemical Synthesis of AgNPs for Sensing Applications
4.3. Electroanalytical Techniques for Characterization of AgNPs-Modified Electrodes and Analyte Quantification
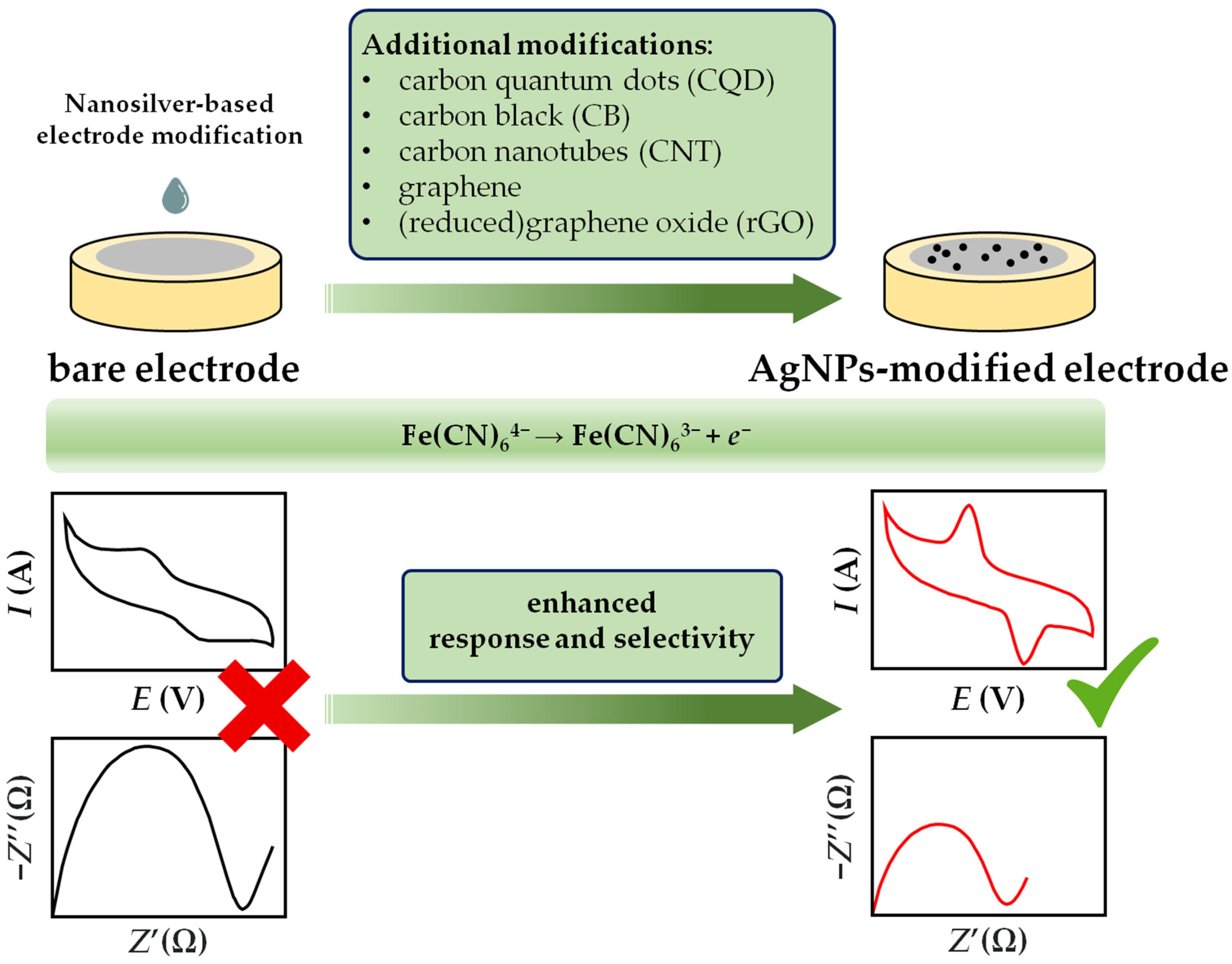
4.4. Mechanisms of AgNPs-based Voltammetric and Amperometric Sensors
- (i)
- Aggregation of AgNPs triggered by the addition of the analyte is a common optical sensing approach that can be converted to voltammetric [98] and amperometric sensors [101]. This strategy is feasible usually in the form of dual- [98,99] or multisensing [102,106,111,122] platforms, with optical, fluorescent, and electrochemical response towards the detection of HMs. In contrast to the colorimetric assay, electrochemical aggregation of functional silver material results in voltammetric/amperometric signal amplification or inhibition, depending on the chemistry of the nanoparticle stabilizer and the target analyte. For the aggregation-induced sensing mechanism, the role of biogenic synthesized AgNPs is highlighted because biogenic molecules are rich in electron-donor groups that successfully act as analyte-chelating ligands. AgNPs synthesized using fungus Agaricus bisporus [98] and the Mimosa diplotricha leaf extract [99] were found to be potent sensors towards hazardous mercury, boosting the DPV responses through strong metal–nanosilver stabilizer complexation. Green AgNPs also play a prominent role as multisensor probes for mercury [102], cadmium [106], arsenic [120], and chromium [122].
- (ii)
- Electrochemical sensors based on the displacement of functional nanomaterials are considered a novel tool for highly selective and sensitive detection of analytes. In two examples of the reviewed scientific papers, the mechanism of mercury detection was associated with the removal of the surface-bound stabilizer (calixarene moiety), leaving the AgNPs uncapped [101], or with galvanic displacement of silver by mercury [104]. As thermodynamically unstable species, the bare nanoparticles tend to aggregate into larger clusters, or form a silver—mercury amalgam [101]. This leads to signal inhibition, which is visible in the optical mode by a reduction in the SPR band in the UV-Vis spectrum, and in the amperometric mode by a reduction in current output. In the galvanic displacement method, the AgNPs interact with divalent mercury ions in the solution and convert the zero-valent silver into ionic silver species, resulting in loss of stripping signal. Since only small amounts of the analyte are required to displace measurable amounts of silver, this sensor can quantify inorganic mercury in the picomolar range.
- (iii)
- Aggravation or enhancement of the electron transfer pathway of AgNPs-modified WE leads to the amplification or decrement of voltammetric performance. Two voltammetric platforms based on MIPs supported by AgNPs-decorated GCE have been developed for highly selective and sensitive detection of natural estrogen in environmental and drinking water with picomolar and femtomolar detection limits [182,183]. The detection of the analyte is catalyzed by the AgNPs (signal amplification or inhibition), while the analyte capture and selective recognition were performed by the MIPs. In the presence of E2, more imprinted cavities of the poly(p-aminophenol) (MIP-pAPh) were occupied, resulting in direct amplification of the current response by AgNPs signal amplification [182]. The reverse sensing mechanism was proposed for the AgNP/GO/MIP (poly-imidazole; PImi) functional platform [183]. In addition to the imidazole moiety (p-type-electron acceptor), additional functional sites of GO (n-type electron donor) were available for analyte binding, which blocked the diffusion of the ferri/ferrocyanide redox probe at the electrode/solution interface, reducing the SWV current responses.
- (iv)
- Voltammetric and amperometric sensors, which detect inorganic/organic pollutants based on their OR response, are the most represented electrochemical sensor class. AgNPs increase the active surface area of the bare and/or modified electrode, facilitate electron transfer, and serve as active catalytic sites for oxidation/reduction of the aquatic pollutant. It has been reported that the reaction rate constant (k) is proportional to the total effective surface area of the nanosilver material [190]. Therefore, the homogeneity of particle size and shape of AgNPs anchored on sensing platforms is of utmost importance for catalytic efficiency. The adsorption of the analyte (the first step in the sensing mechanism) is more promoted on small particles with larger effective surface area, which facilitates the OR process of electroactive species on the modified electrodes [135]. The interactions between the AgNPs, electrode support and/or other electrode modifiers, and the target analyte, depends on the nature of the surface-active species. On the one hand, stabilizers can be interpreted as a barrier between the AgNPs and the electrode material, controlling the electron transfer kinetics and, thus, affecting the analytical performance of the sensor, especially in terms of achieving lower detection limits [103]. Therefore, clean and uncovered active surface sites of nanoclusters are preferred for catalytic and sensing applications [154]. However, since unprotected AgNPs tend to aggregate, the presence of a stabilizer is extremely important. The catalytic activity of green-capped AgNPs can be altered by the size of the stabilizer (bulky ligands or small molecules) [66,166], and by the adsorption of intermediary molecules (formed during the sensing mechanism) onto the biostabilizers, as was the case in the amperometric quantification of nitrobenzene [172]. The determination of nitrites based on their irreversible electrochemical oxidation via two-electron transfer was reported for various AgNPs-functionalized electrodes, i.e., nanospheres decorated GCE [129], core-shell Au@Ag structure anchored over carboxylated graphene/GCE [86], AgNPs-(r)GO platform [130,131], AgNPs/rGO nanohybrid in conjunction with PPy [133] and PANI [134] conducting polymers, AgNPs/ZnO nanocomposite [136], and AgNPs/HNT/MoS2 platform [139]. In contrast, one-electron transfer was demonstrated in nitrite sensing with a nanosilver–polymer composite (chitosan, PEDOT:PSS) [142], and also with an AgNPs-MWCNTs hybrid platform [74]. AgNP/SWCNT/CPE was used to detect DNL by the irreversible oxidation of phenolic groups to (semi)quinone species [84]. The overall chemical–electrochemical mechanism involves a transfer of two protons and two electrons, but the final product depends on the applied potential and the electrode modifier used. Oxidation products of E3, obtained using GCE coated with AgNP/CNB [184] and AgNPs-rGO [185], correspond to ketone derivatives. For ultrasensitive detection of AM and AT drugs, an oxidation mechanism involving rapid electron transfer from the analytes through the COOH-CNTs/Ag/NH2-CNTs ternary sandwich architecture to the GCE was proposed [177]. The overall electrochemical oxidation (single SWASV profile) of the electroactive center 1,4-dihydropyridine (AM) occurs through a two-electron/two-proton process, whereas the pyrrole center of AT exhibits two-electron/one-proton transfer. The reversible oxidation of CC and HQ phenolic compounds to o- and p-benzokinone species via a two-electron/two-proton transfer was mediated by the cobalt active site of the AgNPs/TACoPc/PANI ternary composite using the DPV technique [153] and with AgNPs/Fe3O4-rGO hybrid material [87]. Quantitative determination of BPA via electrooxidation to 2,2-bis(4-phenylquinone) was successfully performed with an AgNPs-rGO composite on a ITO substrate [67], and with an AgNPs-rGO/PLL-coated GCE [45]. Conversely, several phenolic compounds were detected using the reduction mechanism. The nitro groups of 4-nitrophenol [154,156,158], 2,6-dinitrophenol [161], 4-nitrotoluene [174], nitrofurantoin [176], and nitrobenzene [170,171,172] were irreversibly converted into hydroxylamine or aminophenol groups by an electrochemical mechanism involving four protons and four electrons. Moreover, nitrobenzene was detected on a silicate sol–gel matrix GCE functionalized with AgAu alloy, depending on the electrochemical reduction of the nitro group involving six protons and six electrons [83]. Gold electrode coated with AgNPs [146,149], in conjunction with the SWV technique, proved to be a highly efficient combination for the detection of nitrates in seawater due to their two-electron reduction to nitrites. In addition, nitrate was detected on a Cu(II)-terephthalate decorated SPCE via a Cu2+/Cu+ redox couple [46]. The adsorption of nitrates is promoted on the Cu(I) active site by copper–oxygen coordination interactions, which is further supported by the charge transferability of MOFs and the enhanced transfer of two electrons to the catalytic AgNP sites.
4.5. Design of AgNPs-based Voltammetric and Amperometric Sensors for Detection of Aqueous Pollutants
4.6. Summary and Future Perspective
5. Conclusions and Outlooks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, M.; Kadja, G.T.; Ilmi, M.M. Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. J. Ind. Eng. Chem. 2021, 93, 78–100. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Torrinha, A.; Oliveira, T.; Ribeiro, F.; Correia, A.; Lima-Neto, P.; Morais, S. Application of Nanostructured Carbon-Based Electrochemical (Bio)Sensors for Screening of Emerging Pharmaceutical Pollutants in Waters and Aquatic Species: A Review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Selvakumar, P.; Kumar, P.S.; Satheeskumar, V.; Vijaysunder, M.G.; Hariharan, S.; Antony, K. Recent advances in electrochemical sensor developments for detecting emerging pollutant in water environment. Chemosphere 2022, 304, 135331. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Kumar, P.; Ballesteros, E.; Kim, K.-H. Advances in functional nanomaterial-based electrochemical techniques for screening of endocrine disrupting chemicals in various sample matrices. TrAC Trends Anal. Chem. 2019, 113, 256–279. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water—A Review. Sensors 2020, 20, 54. [Google Scholar] [CrossRef]
- Su, S.; Chen, S.; Fan, C. Recent advances in two-dimensional nanomaterials-based electrochemical sensors for environmental analysis. Green Energy Environ. 2018, 3, 97–106. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 18. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, P.S.; Rangasamy, G.; Shankar, V.U.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef]
- Zahran, M.M.; Khalifa, Z.; Zahran, M.A.-H.; Azzem, M.A. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review. Mater. Adv. 2021, 2, 7350–7365. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 23. [Google Scholar] [CrossRef]
- Smiechowicz, E.; Niekraszewicz, B.; Kulpinski, P.; Dzitko, K. Antibacterial composite cellulose fibers modified with silver nanoparticles and nanosilica. Cellulose 2018, 25, 3499–3517. [Google Scholar] [CrossRef]
- Aboutorabi, S.N.; Nasiriboroumand, M.; Mohammadi, P.; Sheibani, H.; Barani, H. Biosynthesis of Silver Nanoparticles Using Safflower Flower: Structural Characterization, and Its Antibacterial Activity on Applied Wool Fabric. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2525–2532. [Google Scholar] [CrossRef]
- Srisod, S.; Motina, K.; Inprasit, T.; Pisitsak, P. A green and facile approach to durable antimicrobial coating of cotton with silver nanoparticles, whey protein, and natural tannin. Prog. Org. Coatings 2018, 120, 123–131. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Kassal, P. Recent Advances in (Bio)Chemical Sensors for Food Safety and Quality Based on Silver Nanomaterials. Food Technol. Biotechnol. 2021, 59, 216–237. [Google Scholar] [CrossRef]
- Salve, M.; Mandal, A.; Amreen, K.; Pattnaik, P.K.; Goel, S. Greenly synthesized silver nanoparticles for supercapacitor and electrochemical sensing applications in a 3D printed microfluidic platform. Microchem. J. 2020, 157, 104973. [Google Scholar] [CrossRef]
- Paul, D.; Sachan, D.; Das, G. Silver nanoparticles embedded on in-vitro biomineralized vaterite: A highly efficient catalyst with enhanced catalytic activity towards 4-Nitrophenol reduction. Mol. Catal. 2021, 504, 111433. [Google Scholar] [CrossRef]
- Majdoub, M.; Amedlous, A.; Anfar, Z.; Moussaoui, O. MoS2 nanosheets/silver nanoparticles anchored onto textile fabric as “dip catalyst” for synergistic p-nitrophenol hydrogenation. Environ. Sci. Pollut. Res. 2021, 28, 64674–64686. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Kant, T.; Shrivas, K.; Ganesan, V.; Mahipal, Y.K.; Devi, R.; Deb, M.K.; Shankar, R. Flexible printed paper electrode with silver nano-ink for electrochemical applications. Microchem. J. 2020, 155, 104687. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Pomal, N.C.; Bhatt, K.D.; Modi, K.M.; Desai, A.L.; Patel, N.P.; Kongor, A.; Kolivoška, V. Functionalized Silver Nanoparticles as Colorimetric and Fluorimetric Sensor for Environmentally Toxic Mercury Ions: An Overview. J. Fluoresc. 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, P.; Wang, Y.; Li, S.; Zhang, L.; Zhang, Y.; Ge, S.; Yu, J. Paper-based sandwich type SERS sensor based on silver nanoparticles and biomimetic recognizer. Sens. Actuators B Chem. 2020, 313, 127989. [Google Scholar] [CrossRef]
- Naqvi, T.K.; Srivastava, A.K.; Kulkarni, M.M.; Siddiqui, A.M.; Dwivedi, P.K. Silver nanoparticles decorated reduced graphene oxide (rGO) SERS sensor for multiple analytes. Appl. Surf. Sci. 2019, 478, 887–895. [Google Scholar] [CrossRef]
- Sudarman, F.; Shiddiq, M.; Armynah, B.; Tahir, D. Silver nanoparticles (AgNPs) synthesis methods as heavy-metal sensors: A review. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Abbas, A.; Amin, H.M. Silver nanoparticles modified electrodes for electroanalysis: An updated review and a perspective. Microchem. J. 2022, 175, 107166. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2020, 28, 58994–59002. [Google Scholar] [CrossRef]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2022, 22, 218. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 25. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic synthesis of gold and silver nanoparticles used in environmental applications: A review. Trends Environ. Anal. Chem. 2021, 30, e00129. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Nayak, S.; Goveas, L.C.; Kumar, P.S.; Selvaraj, R.; Vinayagam, R. Plant-mediated gold and silver nanoparticles as detectors of heavy metal contamination. Food Chem. Toxicol. 2022, 167, 113271. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles—Formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Gorup, L.F.; Longo, E.; Leite, E.R.; Camargo, E.R. Moderating effect of ammonia on particle growth and stability of quasimonodisperse silver nanoparticles synthesized by the Turkevich method. J. Colloid Interface Sci. 2011, 360, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Milardovicć, S. Synthesis and Electrochemical Characterization of AgNP Ink Suitable for Inkjet Printing. Int. J. Electrochem. Sci. 2018, 13, 11136–11149. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Yan, B.; Zhang, H. An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-l-lysine nanocomposites. J. Electroanal. Chem. 2017, 805, 39–46. [Google Scholar] [CrossRef]
- Amali, R.; Lim, H.; Ibrahim, I.; Zainal, Z.; Ahmad, S. Silver nanoparticles-loaded copper (II)-terephthalate framework nanocomposite as a screen-printed carbon electrode modifier for amperometric nitrate detection. J. Electroanal. Chem. 2022, 918, 116440. [Google Scholar] [CrossRef]
- Zhou, M.; Han, L.; Deng, D.; Zhang, Z.; He, H.; Zhang, L.; Luo, L. 4-mercaptobenzoic acid modified silver nanoparticles-enhanced electrochemical sensor for highly sensitive detection of Cu2+. Sens. Actuators B Chem. 2019, 291, 164–169. [Google Scholar] [CrossRef]
- Cheng, Z.-P.; Chu, X.-Z.; Wu, X.-Q.; Xu, J.-M.; Zhong, H.; Yin, J.-Z. Controlled synthesis of silver nanoplates and nanoparticles by reducing silver nitrate with hydroxylamine hydrochloride. Rare Met. 2017, 36, 799–805. [Google Scholar] [CrossRef]
- Mohaghegh, S.; Osouli-Bostanabad, K.; Nazemiyeh, H.; Javadzadeh, Y.; Parvizpur, A.; Barzegar-Jalali, M.; Adibkia, K. A comparative study of eco-friendly silver nanoparticles synthesis using Prunus domestica plum extract and sodium citrate as reducing agents. Adv. Powder Technol. 2020, 31, 1169–1180. [Google Scholar] [CrossRef]
- Sonkoue, B.M.; Tchekwagep, P.M.S.; Nanseu-Njiki, C.P.; Ngameni, E. Electrochemical Determination of Arsenic Using Silver Nanoparticles. Electroanalysis 2018, 30, 2738–2743. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Kassal, P.; Zlatar, M. Electrochemical and spectroscopic characterization of AgNP suspension stability influenced by strong inorganic acids. Electrochim. Acta 2021, 377, 138126. [Google Scholar] [CrossRef]
- Şenol, A.M.; Metin, O.; Onganer, Y. A facile route for the preparation of silver nanoparticles-graphene oxide nanocomposites and their interactions with pyronin Y dye molecules. Dye. Pigment. 2019, 162, 926–933. [Google Scholar] [CrossRef]
- Wolf, J.B.; Stawski, T.M.; Smales, G.J.; Thünemann, A.F.; Emmerling, F. Towards automation of the polyol process for the synthesis of silver nanoparticles. Sci. Rep. 2022, 12, 5769. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, C.; Wang, Y.; Mao, L.; Sun, Q.; Zhang, L.; Song, H.; Huang, F.; Zuo, C. Low content and low-temperature cured silver nanoparticles/silver ion composite ink for flexible electronic applications with robust mechanical performance. Appl. Surf. Sci. 2021, 564, 150447. [Google Scholar] [CrossRef]
- Polte, J.; Tuaev, X.; Wuithschick, M.; Fischer, A.; Thuenemann, A.F.; Rademann, K.; Kraehnert, R.; Emmerling, F. Formation Mechanism of Colloidal Silver Nanoparticles: Analogies and Differences to the Growth of Gold Nanoparticles. ACS Nano 2012, 6, 5791–5802. [Google Scholar] [CrossRef]
- Ivanišević, I.; Rukavina, V.; Kassal, P.; Milardović, S. Impact of Weak Organic Acids on Precipitation of Poly(acrylic acid) Stabilized Silver Nanoparticles; an Electrochemical Approach. Croat. Chem. Acta 2018, 91, 491–499. [Google Scholar] [CrossRef]
- Saldías, C.; Bonardd, S.; Quezada, C.; Radi´c, D.; Leiva, A. The Role of Polymers in the Synthesis of Noble Metal Nanoparticles: A Review. J. Nanosci. Nanotechnol. 2017, 17, 87–114. [Google Scholar] [CrossRef]
- Leopold, N.; Stefancu, A.; Herman, K.; Tódor, I.S.; Iancu, S.D.; Moisoiu, V.; Leopold, L.F. The role of adatoms in chloride-activated colloidal silver nanoparticles for surface-enhanced Raman scattering enhancement. Beilstein J. Nanotechnol. 2018, 9, 2236–2247. [Google Scholar] [CrossRef]
- Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, Ľ.; Devínsky, F.; Horáková, R.; Opravil, T. Silver nanoparticles stabilised with cationic single-chain surfactants: Structure-physical properties-biological activity relationship study. J. Mol. Liq. 2018, 272, 60–72. [Google Scholar] [CrossRef]
- Banerjee, S.; Loza, K.; Meyer-Zaika, W.; Prymak, O.; Epple, M. Structural Evolution of Silver Nanoparticles during Wet-Chemical Synthesis. Chem. Mater. 2014, 26, 951–957. [Google Scholar] [CrossRef]
- de Souza, T.A.J.; Souza, L.R.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Gao, R.; Knowles, B.; Wang, J.; Wang, P.; Sun, G.; Cui, Y. Formation of silver nanoparticles by human gut microbiota. Sci. Total. Environ. 2019, 651, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Mahdavi, B.; Abadi, Z.H.; Farhadi, S. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem. 2018, 32, 9. [Google Scholar] [CrossRef]
- Jebril, S.; Fdhila, A.; Dridi, C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci. Rep. 2021, 11, 22060. [Google Scholar] [CrossRef]
- Verma, D.; Dhiman, T.K.; Das Mukherjee, M.; Solanki, P.R. Electrophoretically Deposited Green Synthesized Silver Nanoparticles Anchored in Reduced Graphene Oxide Composite Based Electrochemical Sensor for Detection of Bisphenol A. J. Electrochem. Soc. 2021, 168, 097504. [Google Scholar] [CrossRef]
- Zamarchi, F.; Vieira, I.C. Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. J. Pharm. Biomed. Anal. 2021, 196, 113912. [Google Scholar] [CrossRef]
- Dash, S.R.; Bag, S.S.; Golder, A.K. Synthesis of highly structured spherical Ag@Pt core-shell NPs using bio-analytes for electrocatalytic Pb(II) sensing. Sens. Actuators B Chem. 2020, 314, 128062. [Google Scholar] [CrossRef]
- Soliwoda, K.; Tomaszewska, E.; Małek, K.; Celichowski, G.; Orlowski, P.; Krzyzowska, M.; Grobelny, J. The synthesis of monodisperse silver nanoparticles with plant extracts. Colloids Surfaces B Biointerfaces 2019, 177, 19–24. [Google Scholar] [CrossRef]
- Singaravelan, R.; Alwar, S.B.S. Electrochemical synthesis, characterisation and phytogenic properties of silver nanoparticles. Appl. Nanosci. 2015, 5, 983–991. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Dinh, N.X.; Pham, T.N.; Hoang, T.V.; Tuan, P.A.; Huy, T.Q.; Le, A.-T. Scalable Electrochemical Synthesis of Novel Biogenic Silver Nanoparticles and Its Application to High-Sensitive Detection of 4-Nitrophenol in Aqueous System. Adv. Polym. Technol. 2021, 2021, 6646219. [Google Scholar] [CrossRef]
- Hassan, K.M.; Elhaddad, G.M.; AbdelAzzem, M. Voltammetric determination of cadmium(II), lead(II) and copper(II) with a glassy carbon electrode modified with silver nanoparticles deposited on poly(1,8-diaminonaphthalene). Microchim. Acta 2019, 186, 440. [Google Scholar] [CrossRef]
- Wan, Y.; Zheng, Y.F.; Wan, H.T.; Yin, H.Y.; Song, X.C. A novel electrochemical sensor based on Ag nanoparticles decorated multi-walled carbon nanotubes for applied determination of nitrite. Food Control. 2017, 73, 1507–1513. [Google Scholar] [CrossRef]
- Hajisafari, M.; Nasirizadeh, N. An electrochemical nanosensor for simultaneous determination of hydroxylamine and nitrite using oxadiazole self-assembled on silver nanoparticle-modified glassy carbon electrode. Ionics 2017, 23, 1541–1551. [Google Scholar] [CrossRef]
- Stojanović, Z.; Koudelkova, Z.; Sedlackova, E.; Hynek, D.; Richtera, L.; Adam, V. Determination of chromium(vi) by anodic stripping voltammetry using a silver-plated glassy carbon electrode. Anal. Methods 2018, 10, 2917–2923. [Google Scholar] [CrossRef]
- Liuzhu, Z.; Sekar, S.; Chen, J.; Lee, S.; Kim, D.Y.; Manikandan, R. A polyrutin/AgNPs coated GCE for simultaneous anodic stripping voltammetric determination of Pb(II) and Cd(II)ions in environmental samples. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129082. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Rahimnejad, M.; Asghary, M.; Younesi, H. Simultaneous electro-determination of trace copper, lead, and cadmium in tap water by using silver nanoparticles and graphene nanoplates as nanocomposite modified graphite electrode. Microchem. J. 2022, 175, 107137. [Google Scholar] [CrossRef]
- Naseri, M.; Mohammadniaei, M.; Ghosh, K.; Sarkar, S.; Sankar, R.; Mukherjee, S.; Pal, S.; Dezfouli, E.A.; Halder, A.; Qiao, J.; et al. A Robust Electrochemical Sensor Based on Butterfly-shaped Silver Nanostructure for Concurrent Quantification of Heavy Metals in Water Samples. Electroanalysis 2022, 35, e202200114. [Google Scholar] [CrossRef]
- Zhu, Y. Synthesis of Ag Nanoparticles Decorated Carbon Nanotubes as an Electrochemical Sensor for Determination of Phenolic Compounds in Shale Gas Wastewater. Int. J. Electrochem. Sci. 2021, 16, 21074. [Google Scholar] [CrossRef]
- Zhao, K.; Ge, L.; Wong, T.I.; Zhou, X.; Lisak, G. Gold-silver nanoparticles modified electrochemical sensor array for simultaneous determination of chromium(III) and chromium(VI) in wastewater samples. Chemosphere 2021, 281, 130880. [Google Scholar] [CrossRef] [PubMed]
- Hanko, M.; Švorc, L.; Planková, A.; Mikuš, P. Overview and recent advances in electrochemical sensing of glutathione—A review. Anal. Chim. Acta 2019, 1062, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Jeong, J.; Kang, D.-K.; Kim, K. One-step Synthesis of AuAg Alloy Nanodots and its Electrochemical Studies towards Nitrobenzene Reduction and Sensing. Electroanalysis 2018, 30, 57–66. [Google Scholar] [CrossRef]
- Bezerra, A.P.; Santos, A.O.; Abrantes-Coutinho, V.E.; Filho, E.D.S.; Soares, J.M.; Castro, S.S.; Oliveira, T.M. Theoretical and experimental findings regarding the electroanalysis of dienestrol in natural waters using a silver nanoparticles/single-walled carbon nanotubes-based amperometric sensor. J. Electroanal. Chem. 2021, 880, 114821. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Fang, C.; Ai, L.; Chen, J.; Su, J.; Zhang, Q.; Fu, Q. Facile synthesis of reduced graphene oxide/silver nanoparticles composites and their application for detecting heavy metal ions. J. Alloys Compd. 2019, 787, 683–693. [Google Scholar] [CrossRef]
- Bao, Z.-L.; Zhong, H.; Li, X.-R.; Zhang, A.-R.; Liu, Y.-X.; Chen, P.; Cheng, Z.-P.; Qian, H.-Y. Core-shell Au@Ag nanoparticles on carboxylated graphene for simultaneous electrochemical sensing of iodide and nitrite. Sens. Actuators B Chem. 2021, 345, 130319. [Google Scholar] [CrossRef]
- Abraham, T.; Rejil, K.R.; George, J.M.; Antony, A.; Pillai, S.C.; Hinder, S.J.; Mathew, B. Magnetic Fe3O4–reduced graphene oxide composite decorated with Ag nanoparticles as electrochemical sensor and self-cleaning material for organic pollutants. J. Porous Mater. 2020, 27, 303–318. [Google Scholar] [CrossRef]
- Meng, L.; Bian, R.; Guo, C.; Xu, B.; Liu, H.; Jiang, L. Aligning Ag Nanowires by a Facile Bioinspired Directional Liquid Transfer: Toward Anisotropic Flexible Conductive Electrodes. Adv. Mater. 2018, 30, 9. [Google Scholar] [CrossRef]
- Terán-Alcocer, A.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Paixao, T. Measuring Electrochemical Surface Area of Nanomaterials versus the Randles−Ševčík Equation. Chemelectrochem 2020, 7, 3414–3415. [Google Scholar] [CrossRef]
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical sensors for nitrogen species: A review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Dehghan, Z.; Hosseinzadeh, R. Electrochemical determination of ascorbic acid, uric acid and folic acid using carbon paste electrode modified with novel synthesized ferrocene derivative and core-shell magnetic nanoparticles in aqueous media. Appl. Organomet. Chem. 2018, 32, e4551. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- The Council of the European Union. Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption (OJ L 330 05.12.1998 p. 32). In Documents in European Community Environmental Law, 2nd ed.; Galizzi, P., Sands, P., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 865–878. [Google Scholar]
- Ashrafi, A.M.; Koudelkova, Z.; Sedlackova, E.; Richtera, L.; Adam, V. Review—Electrochemical Sensors and Biosensors for Determination of Mercury Ions. J. Electrochem. Soc. 2018, 165, B824–B834. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A.; Fafal, T.; Kıvçak, B. Eco-friendly Sensors Developed by Herbal Based Silver Nanoparticles for Electrochemical Detection of Mercury (II) Ion. Electroanalysis 2019, 31, 1075–1082. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green silver-nanoparticle-based dual sensor for toxic Hg(II) ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef]
- Punnoose, M.S.; Bijimol, D.; Abraham, T.; Plathanam, N.J.; Mathew, B. Green Synthesized Unmodified Silver Nanoparticles as Reproducible Dual Sensor for Mercuric Ions and Catalyst to Abate Environmental Pollutants. Bionanoscience 2021, 11, 739–754. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The role of calix[n]arenes and pillar[n]arenes in the design of silver nanoparticles: Self-assembly and application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef]
- Vyas, G.; Bhatt, S.; Paul, P. Synthesis of Calixarene-Capped Silver Nanoparticles for Colorimetric and Amperometric Detection of Mercury (HgII, Hg0). ACS Omega 2019, 4, 3860–3870. [Google Scholar] [CrossRef]
- George, J.M.; Mathew, B. Curcuma longa rhizome extract mediated unmodified silver nanoparticles as multisensing probe for Hg(II) ions. Mater. Res. Express 2019, 6, 1150h5. [Google Scholar] [CrossRef]
- Meenakshi, S.; Devi, S.; Pandian, K.; Chitra, K.; Tharmaraj, P. Aniline-mediated synthesis of carboxymethyl cellulose protected silver nanoparticles modified electrode for the differential pulse anodic stripping voltammetry detection of mercury at trace level. Ionics 2019, 25, 3431–3441. [Google Scholar] [CrossRef]
- Suherman, A.L.; Ngamchuea, K.; Tanner, E.E.L.; Sokolov, S.V.; Holter, J.; Young, N.P.; Compton, R.G. Electrochemical Detection of Ultratrace (Picomolar) Levels of Hg2+ Using a Silver Nanoparticle-Modified Glassy Carbon Electrode. Anal. Chem. 2017, 89, 7166–7173. [Google Scholar] [CrossRef] [PubMed]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Aravind, A.; Sebastian, M.; Mathew, B. Green silver nanoparticles as a multifunctional sensor for toxic Cd(ii) ions. New J. Chem. 2018, 42, 15022–15031. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Ganash, A.A.; Alghamdi, R.A. Fabrication of a novel polyaniline/green-synthesized, silver-nanoparticle-modified carbon paste electrode for electrochemical sensing of lead ions. JCCS 2021, 68, 2312–2325. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Zalnezhad, E.; Ladan, M.; Alias, Y. l-Glutamine-assisted synthesis of flower-like NiO and ball-flower-like NiO/Ag as an electrochemical sensor for lead(ii) detection. RSC Adv. 2017, 7, 30870–30878. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green Silver Nanoparticles Based Multi-Technique Sensor for Environmental Hazardous Cu(II) Ion. Bionanoscience 2019, 9, 373–385. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Bastos-Arrieta, J.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; De Pablo, J.; Esteban, M. Ag Nanoparticles Drop-Casting Modification of Screen-Printed Electrodes for the Simultaneous Voltammetric Determination of Cu(II) and Pb(II). Sensors 2017, 17, 1458. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J. Ag metal organic frameworks nanocomposite modified electrode for simultaneous electrochemical detection of copper (II) and lead (II). J. Appl. Electrochem. 2021, 51, 1207–1216. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef]
- Thakkar, S.; Dumée, L.F.; Gupta, M.; Singh, B.R.; Yang, W. Nano–Enabled sensors for detection of arsenic in water. Water Res. 2021, 188, 116538. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Deep, A.; Kim, K.-H.; Kailasa, S.K.; Yoon, H.-O. Nanomaterial-based electrochemical sensors for arsenic—A review. Biosens. Bioelectron. 2017, 95, 106–116. [Google Scholar] [CrossRef]
- Salunke, R.S.; Chavan, P.G.; Shirale, D.J. Anodic stripping voltammetry studies of electrochemically engineered silver nanoparticles over single polypyrrole nanowire device for tracing of arsenic(III): An environmental perspective. Nanotechnol. Environ. Eng. 2018, 3, 12. [Google Scholar] [CrossRef]
- Wen, S.-H.; Liang, R.-P.; Zhang, L.; Qiu, J.-D. Multimodal Assay of Arsenite Contamination in Environmental Samples with Improved Sensitivity through Stimuli-Response of Multiligands Modified Silver Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 6223–6232. [Google Scholar] [CrossRef]
- Hilali, N.; Mohammadi, H.; Amine, A.; Zine, N.; Errachid, A. Recent Advances in Electrochemical Monitoring of Chromium. Sensors 2020, 20, 5153. [Google Scholar] [CrossRef]
- Aravind, A.; Sebastian, M.; Mathew, B. Green synthesized unmodified silver nanoparticles as a multi-sensor for Cr(iii) ions. Environ. Sci. Water Res. Technol. 2018, 4, 1531–1542. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Noroozifar, M. Ultra-trace determination of hexavalent chromium by novel two dimensional biphenol-biphenoquinone nanoribbons/silver nanoparticles. Sens. Actuators B Chem. 2019, 281, 1023–1033. [Google Scholar] [CrossRef]
- Ikhsan, N.I.; Rameshkumar, P.; Pandikumar, A.; Shahid, M.M.; Huang, N.M.; Kumar, S.V.; Lim, H.N. Facile synthesis of graphene oxide–silver nanocomposite and its modified electrode for enhanced electrochemical detection of nitrite ions. Talanta 2015, 144, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Liu, X.; Guo, L.; Ji, X.; Wang, L.; Tian, D.; Yang, X. Nonenzymatic nitrite sensor based on a titanium dioxide nanoparticles/ionic liquid composite electrode. J. Electroanal. Chem. 2014, 719, 35–40. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Wang, F.; Liu, C.; Yu, C.; Jiang, G. In-situ insertion of carbon nanotubes into metal-organic frameworks-derived α-Fe2O3 polyhedrons for highly sensitive electrochemical detection of nitrite. Electrochim. Acta 2018, 285, 128–138. [Google Scholar] [CrossRef]
- Li, X.; Ping, J.; Ying, Y. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. Trends Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, X.; Yin, Y.; Fang, W. Determination of Nitrite by Noble Metal Nanomaterial-Based Electrochemical Sensors: A Minireview. Anal. Lett. 2021, 54, 2826–2850. [Google Scholar] [CrossRef]
- Shivakumar, M.; Nagashree, K.L.; Manjappa, S.; Dharmaprakash, M.S. Electrochemical Detection of Nitrite Using Glassy Carbon Electrode Modified with Silver Nanospheres (AgNS) Obtained by Green Synthesis Using Pre-hydrolysed Liquor. Electroanalysis 2017, 29, 1434–1442. [Google Scholar] [CrossRef]
- Zhao, X.; Li, N.; Jing, M.; Zhang, Y.; Wang, W.; Liu, L.; Xu, Z.; Liu, L.; Li, F.; Wu, N. Monodispersed and spherical silver nanoparticles/graphene nanocomposites from gamma-ray assisted in-situ synthesis for nitrite electrochemical sensing. Electrochim. Acta 2019, 295, 434–443. [Google Scholar] [CrossRef]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.-S.; Yoo, J.-Y.; Hahn, Y.-B. Fabrication of sensitive non-enzymatic nitrite sensor using silver-reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2018, 516, 67–75. [Google Scholar] [CrossRef]
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-doped conducting polymers for electrochemical sensors and biosensors. J. Mater. Chem. B 2018, 6, 4173–4190. [Google Scholar] [CrossRef]
- Kaladevi, G.; Meenakshi, S.; Pandian, K.; Wilson, P. Synthesis of Well-Dispersed Silver Nanoparticles on Polypyrrole/Reduced Graphene Oxide Nanocomposite for Simultaneous Detection of Toxic Hydrazine and Nitrite in Water Sources. J. Electrochem. Soc. 2017, 164, B620–B631. [Google Scholar] [CrossRef]
- Kaladevi, G.; Wilson, P.; Pandian, K. Simultaneous and Selective Electrochemical Detection of Sulfite and Nitrite in Water Sources Using Homogeneously Dispersed Ag Nanoparticles over PANI/rGO Nanocomposite. J. Electrochem. Soc. 2020, 167, 027514. [Google Scholar] [CrossRef]
- Ma, C.; Qian, Y.; Zhang, S.; Song, H.; Gao, J.; Wang, S.; Liu, M.; Xie, K.; Zhang, X. Temperature-controlled ethanolamine and Ag-nanoparticle dual-functionalization of graphene oxide for enhanced electrochemical nitrite determination. Sens. Actuators B Chem. 2018, 274, 441–450. [Google Scholar] [CrossRef]
- Salagare, S.; Adarakatti, P.S.; Yarradoddappa, V. Facile synthesis of silver nanoparticle-decorated zinc oxide nanocomposite-based pencil graphite electrode for selective electrochemical determination of nitrite. Carbon Lett. 2021, 31, 1273–1286. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical sensors and biosensors for the determination of diclofenac in pharmaceutical, biological and water samples. Talanta Open 2020, 3, 100026. [Google Scholar] [CrossRef]
- Bibi, S.; Zaman, M.I.; Niaz, A.; Rahim, A.; Nawaz, M.; Arian, M.B. Voltammetric determination of nitrite by using a multiwalled carbon nanotube paste electrode modified with chitosan-functionalized silver nanoparticles. Microchim. Acta 2019, 186, 595. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing. Biosens. Bioelectron. 2018, 109, 279–285. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Materiomics 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, C.; Zhang, S.; Li, P.; Zhu, W.; Zhang, X.; Gao, J.; Song, H.; Chen, D.; Pang, D.; et al. Nanosilver and protonated carbon nitride co-coated carbon cloth fibers based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite. Sci. Total. Environ. 2020, 742, 140622. [Google Scholar] [CrossRef]
- Pang, D.; Ma, C.; Chen, D.; Shen, Y.; Zhu, W.; Gao, J.; Song, H.; Zhang, X.; Zhang, S. Silver nanoparticle-functionalized poly (3, 4-ethylenedioxythiophene): Polystyrene film on glass substrate for electrochemical determination of nitrite. Org. Electron. 2019, 75, 105374. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Ressler, A.; Kassal, P. Fabrication of an All-Solid-State Ammonium Paper Electrode Using a Graphite-Polyvinyl Butyral Transducer Layer. Chemosensors 2021, 9, 333. [Google Scholar] [CrossRef]
- Baciu, A.; Manea, F.; Pop, A.; Pode, R.; Schoonman, J. Simultaneous voltammetric detection of ammonium and nitrite from groundwater at silver-electrodecorated carbon nanotube electrode. Process. Saf. Environ. Prot. 2017, 108, 18–25. [Google Scholar] [CrossRef]
- Jiang, C.; He, Y.; Liu, Y. Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices. Anal. 2020, 145, 5400–5413. [Google Scholar] [CrossRef]
- Legrand, D.C.; Barus, C.; Garçon, V. Square Wave Voltammetry Measurements of Low Concentrations of Nitrate Using Au/AgNPs Electrode in Chloride Solutions. Electroanalysis 2017, 29, 2882–2887. [Google Scholar] [CrossRef]
- Amali, R.; Lim, H.; Ibrahim, I.; Huang, N.; Zainal, Z.; Ahmad, S. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Wang, J.; Diao, P. Simultaneous detection of ammonia and nitrate using a modified electrode with two regions. Microchem. J. 2020, 154, 104649. [Google Scholar] [CrossRef]
- Lebon, E.; Fau, P.; Comtat, M.; Kahn, M.L.; Sournia-Saquet, A.; Temple-Boyer, P.; Dubreuil, B.; Behra, P.; Fajerwerg, K. In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water. Chemosensors 2018, 6, 50. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Pan, D.; Han, H.; Zhang, P. Self-assembly of silver nanoparticles on chitosan/polyvinylpyrrolidone modified micro-needle electrode for amperometric detection of nitrate in seawater. Microchem. J. 2021, 164, 105965. [Google Scholar] [CrossRef]
- Aliabadi, M.H.; Esmaeili, N.; Jahromi, H.S. An electrochemical composite sensor for phenol detection in waste water. Appl. Nanosci. 2020, 10, 597–609. [Google Scholar] [CrossRef]
- Goulart, L.A.; Gonçalves, R.; Correa, A.A.; Pereira, E.C.; Mascaro, L.H. Synergic effect of silver nanoparticles and carbon nanotubes on the simultaneous voltammetric determination of hydroquinone, catechol, bisphenol A and phenol. Microchim. Acta 2018, 185, 9. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, S.M.; Devendrachari, M.C.; Kotresh, H.M.N.; Khan, F. Silver nanoparticles decorated phthalocyanine doped polyaniline for the simultaneous electrochemical detection of hydroquinone and catechol. J. Electroanal. Chem. 2021, 884, 115071. [Google Scholar] [CrossRef]
- Kalaiyarasi, G.M.; Elakkiya, R.; Kundu, M.; Jin, W.; Sasidharan, M.; Maduraiveeran, G. Uncapped Silver Nanoclusters as Potential Catalyst for Enhanced Direct-Electrochemical Oxidation of 4-Nitrophenol. J. Clust. Sci. 2019, 30, 393–402. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Fatesh, A.S.; Wahab, R.; Alam, M.; Fakeeha, A.H. Synthesis of silver nanoparticles decorated on reduced graphene oxide nanosheets and their electrochemical sensing towards hazardous 4-nitrophenol. J. Mater. Sci. Mater. Electron. 2020, 31, 11927–11937. [Google Scholar] [CrossRef]
- Laghrib, F.; Houcini, H.; Khalil, F.; Liba, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Synthesis of Silver Nanoparticles Using Chitosan as Stabilizer Agent: Application towards Electrocatalytical Reduction of p-Nitrophenol. Chemistryselect 2020, 5, 1220–1227. [Google Scholar] [CrossRef]
- Sangili, A.; Annalakshmi, M.; Chen, S.-M.; Balasubramanian, P.; Sundrarajan, M. Synthesis of silver nanoparticles decorated on core-shell structured tannic acid-coated iron oxide nanospheres for excellent electrochemical detection and efficient catalytic reduction of hazardous 4-nitrophenol. Compos. B Eng. 2019, 162, 33–42. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Sharma, T.S.K.; Ganguly, A. Design strategy of rGO–HNT–AgNPs based hybrid nanocomposite with enhanced performance for electrochemical detection of 4-nitrophenol. Inorg. Chem. Front. 2020, 7, 1981–1994. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Voltammetric simultaneous quantification of p-nitrophenol and hydrazine by using magnetic spinel FeCo2O4 nanosheets on reduced graphene oxide layers modified with curcumin-stabilized silver nanoparticles. Microchim. Acta 2019, 186, 561. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Bi-functional renewable biopolymer wrapped CNFs/Ag doped spinel cobalt oxide as a sensitive platform for highly toxic nitroaromatic compound detection and degradation. Chemosphere 2022, 291, 132998. [Google Scholar] [CrossRef]
- Faisal, M.; Alam, M.M.; Ahmed, J.; Asiri, A.M.; Alsaiari, M.; Alruwais, R.S.; Madkhali, O.; Rahman, M.M.; Harraz, F.A. Efficient Detection of 2,6-Dinitrophenol with Silver Nanoparticle-Decorated Chitosan/SrSnO3 Nanocomposites by Differential Pulse Voltammetry. Biosensors 2022, 12, 976. [Google Scholar] [CrossRef]
- Dao, K.C.; Yang, C.-C.; Chen, K.-F.; Tsai, Y.-P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Simultaneous determination of paracetamol and levofloxacin using a glassy carbon electrode modified with carbon black, silver nanoparticles and PEDOT:PSS film. Sens. Actuators B Chem. 2018, 255, 2264–2273. [Google Scholar] [CrossRef]
- Dou, N.; Zhang, S.; Qu, J. Simultaneous detection of acetaminophen and 4-aminophenol with an electrochemical sensor based on silver–palladium bimetal nanoparticles and reduced graphene oxide. RSC Adv. 2019, 9, 31440–31446. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Y.; Hu, J.; Zheng, S.; Wang, X.; Zhou, H.; Jin, B. Co-based metal-organic framework nanopinnas composite doped with Ag nanoparticles: A sensitive electrochemical sensing platform for simultaneous determination of dopamine and acetaminophen. Microchem. J. 2020, 155, 104759. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Y.; Ni, K.; Liu, T.; Gu, M.; Wu, Y.; Du, G.; Ran, X. Construction of a novel electrochemical sensor based on biomass material nanocellulose and its detection of acetaminophen. RSC Adv. 2022, 12, 27736–27745. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2020, 2, 20200102. [Google Scholar] [CrossRef]
- Sookhakian, M.; Basirun, W.; Goh, B.T.; Woi, P.M.; Alias, Y. Molybdenum disulfide nanosheet decorated with silver nanoparticles for selective detection of dopamine. Colloids Surfaces B Biointerfaces 2019, 176, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef]
- Pandiyarajan, C.; Rameshkumar, P.; Murugesan, S.; Selvaraj, M. Silver nanoparticles-supported graphitic-like carbon nitride for the electrochemical sensing of nitrobenzene and its derivatives. J. Mater. Sci. Mater. Electron. 2021, 32, 19912–19924. [Google Scholar] [CrossRef]
- Shivakumar, M.; Dharmaprakash, M.S.; Manjappa, S.; Nagashree, K.L. Green synthesis of silver nanoparticles (SNPs)-modified electrode for electrochemical detection of nitrobenzene. J. Iran. Chem. Soc. 2020, 17, 893–900. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Cheng, Y.-H.; Muthukrishnan, P.; Padmavathy, S.; Elangovan, A. Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. J. Photochem. Photobiol. B Biol. 2017, 170, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kappen, J.; Aravind, M.K.; Varalakshmi, P.; Ashokkumar, B.; John, S.A. Quantitative removal of Hg(II) as Hg(0) using carbon cloths coated graphene quantum dots and their silver nanoparticles composite and application of Hg(0) for the sensitive determination of nitrobenzene. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 641, 128542. [Google Scholar] [CrossRef]
- Rani, S.; Dilbaghi, N.; Kumar, S.; Varma, R.S.; Malhotra, R. Rapid redox sensing of p-nitrotoluene in real water samples using silver nanoparticles. Inorg. Chem. Commun. 2020, 120, 108157. [Google Scholar] [CrossRef]
- Laghrib, F.; Farahi, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M. Chemical synthesis of nanosilver on chitosan and electroanalysis activity against the p-nitroaniline reduction. J. Electroanal. Chem. 2019, 845, 111–118. [Google Scholar] [CrossRef]
- Amalraj, A.J.J.; Murthy, U.N.; Wang, S.-F. Silver-capped selenium explored as an electro-catalyst for simultaneous detection of nitro-aromatic drugs in different aqueous samples. J. Ind. Eng. Chem. 2022, 108, 243–253. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.A.; Nisar, J.; Ashiq, M.N. Electrochemical sensing platform for the simultaneous femtomolar detection of amlodipine and atorvastatin drugs. RSC Adv. 2021, 11, 27135–27151. [Google Scholar] [CrossRef]
- Kaya, S.I.; Yıldırım, S.; Cetinkaya, A.; Erkmen, C.; Uslu, B.; Ozkan, S.A. Nanomaterial-based electroanalytical sensors for the selected prohibited anabolic agents, hormones and metabolic modulators and their sensitive assays. Trends Anal. Chem. 2021, 145, 116457. [Google Scholar] [CrossRef]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Recent progress in screen-printed electrochemical sensors and biosensors for the detection of estrogens. Trends Anal. Chem. 2021, 139, 116254. [Google Scholar] [CrossRef]
- Manjunatha, J. Electroanalysis of estriol hormone using electrochemical sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Singh, A.K.; Agrahari, S.; Gautam, R.K.; Tiwari, I. Fabrication of an innovative electrochemical sensor based on graphene-coated silver nanoparticles decorated over graphitic carbon nitride for efficient determination of estradiol. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Regasa, M.B.; Nyokong, T. Design and fabrication of electrochemical sensor based on molecularly imprinted polymer loaded onto silver nanoparticles for the detection of 17-β-estradiol. J. Mol. Recognit. 2022, 35, e2978. [Google Scholar] [CrossRef] [PubMed]
- Regasa, M.B.; Nyokong, T. Synergistic recognition and electrochemical sensing of 17β-Estradiol using ordered molecularly imprinted polymer-graphene oxide-silver nanoparticles composite films. J. Electroanal. Chem. 2022, 922, 116713. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Vicentini, F.C.; Janegitz, B.C.; Mendonça, C.D.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Constantino, C.J.; Machado, S.A.; et al. Sensitive detection of estriol hormone in creek water using a sensor platform based on carbon black and silver nanoparticles. Talanta 2017, 174, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Donini, C.A.; da Silva, M.K.L.; Simões, R.P.; Cesarino, I. Reduced graphene oxide modified with silver nanoparticles for the electrochemical detection of estriol. J. Electroanal. Chem. 2018, 809, 67–73. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Shimizu, F.M.; Scagion, V.P.; Correa, D.S. Ternary nanocomposites based on cellulose nanowhiskers, silver nanoparticles and electrospun nanofibers: Use in an electronic tongue for heavy metal detection. Sens. Actuators B Chem. 2019, 290, 387–395. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Chen, W. Recent advances in electrochemical sensors for the detection of 2,4 6-trinitrotoluene. Curr. Opin. Electrochem. 2019, 17, 16–22. [Google Scholar] [CrossRef]
- Alahi, E.E.; Mukhopadhyay, S. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Wani, I.A. Review—Recent Advances in Biogenic Silver Nanoparticles & NanoComposite Based Plasmonic-Colorimetric and Electrochemical Sensors. ECS J. Solid State Sci. Technol. 2021, 10, 047003. [Google Scholar] [CrossRef]
- Liao, G.; Fang, J.; Li, Q.; Li, S.; Xu, Z.; Fang, B. Ag-Based nanocomposites: Synthesis and applications in catalysis. Nanoscale 2019, 11, 7062–7096. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Hoang, S.A.; Bolan, N.S.; Kirkham, M.; Liu, J.; Xia, X.; Li, Y. Silver nanoparticles in aquatic sediments: Occurrence, chemical transformations, toxicity, and analytical methods. J. Hazard. Mater. 2021, 418, 126368. [Google Scholar] [CrossRef]
- Navratil, R.; Kotzianova, A.; Halouzka, V.; Opletal, T.; Triskova, I.; Trnkova, L.; Hrbac, J. Polymer lead pencil graphite as electrode material: Voltammetric, XPS and Raman study. J. Electroanal. Chem. 2016, 783, 152–160. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, R.; Paul, R. Graphene-Based Electrochemical Sensors for Psychoactive Drugs. Nanomaterials 2022, 12, 2250. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, J.; Ervin, E.; Olafson, C. Development of a Novel, Low-Cost, Disposable Wooden Pencil Graphite Electrode for Use in the Determination of Antioxidants and Other Biological Compounds. Sensors 2015, 15, 18887–18900. [Google Scholar] [CrossRef]

| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Ref. |
|---|---|---|---|---|---|---|
| AP | Water, medicine samples | AgNPs-xGnP/GCE/SWV | 4.98–33.8 μM | Green synthesis | 0.85 nM | [68] |
| AP LEV | River water | AgNPs-CB-PEDOT:PSS/GCE/ SWV | 0.62–7.1 μM 0.67–12 μM | Borohydride reduction | 0.012 μM 0.014 μM | [163] |
| AP | Water samples | CS/Ag-Pd@rGO/GCE/DPV | 0.50–300.0 μM | Chemical reduction | 0.23 μM | [164] |
| DA AP | Aqueous pharmaceutical solution | Ag-ZIF-67p/GCE/DVP | 0.1–100 μM 0.5–200 μM | Borohydride reduction | 0.05 μM 0.2 μM | [165] |
| AP | Aqueous solution | CNCs@CP5–AgNPs/GCE/DPV | 0.5–500 μM | Borohydride reduction | 90.0 nM | [166] |
| DA | Aqueous solution | Ag@MoS2/GCE/DPV | 1.0–500 μM | Green synthesis | 0.2 μM | [168] |
| DA | Aqueous solution | Pt-Ag/Gr/GCE/DPV | 0.1–60 μM | Hydrazine reduction | 0.012 μM | [169] |
| Analyte | Sample | Sensor Design/ Detection Method | Linear Range | AgNP Synthetic Approach | LOD | Ref. |
|---|---|---|---|---|---|---|
| NB | Aqueous solution | EDAS/(g-C3N4-Ag)/GCE/SWV | 5.0–50 μM | Borohydride reduction | 2.0 μM | [170] |
| NB | Aqueous solution | SSG-AuAgNDs/GCE/SWV | 1.0–80 μM | Chemical reduction | - | [83] |
| NB | Tap water, lake water | AgNPs/GCE/DPV | 5.0–40 μM | Green synthesis | 0.027 μM | [171] |
| NB | Aqueous solution | AgNPs/GCE/Amp | 0.05–21 μM 23–2593 μM | Green synthesis | 12.0 nM | [172] |
| NB | Lake water, tap water, river water, sea water | CC/Ag@GQDs/GCE/DPV | 500 nM–1.0 mM | – | 30.0 pM | [173] |
| p-NT | Tap water | Ag/AuE/Amp | 0.01–0.10 μM | Borohydride reduction | 0.092 μM | [174] |
| p-NA | Wastewater | CS-SNPs/CPE/DPV | 7.0 nM–1.0 μM | Borohydride reduction | 5.0 nM | [175] |
| NFT 4-NP | Tap water, river water, drinking water | Ag/Se/GCE/DPV | 0.1–210 μM 0.1–150 μM | Chemical reduction | 23.87 nM 7.82 nM | [176] |
| AM AT | Tap water, drinking water | COOH-CNTs/Ag/NH2-CNT/GCE/SWASV | 6 nM–50 pM 9 nM–75 pM | Commercial AgNPs | 77.6 fM 83.2 fM | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanišević, I. The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis. Sensors 2023, 23, 3692. https://doi.org/10.3390/s23073692
Ivanišević I. The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis. Sensors. 2023; 23(7):3692. https://doi.org/10.3390/s23073692
Chicago/Turabian StyleIvanišević, Irena. 2023. "The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis" Sensors 23, no. 7: 3692. https://doi.org/10.3390/s23073692
APA StyleIvanišević, I. (2023). The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis. Sensors, 23(7), 3692. https://doi.org/10.3390/s23073692






