First Ex Vivo Animal Study of a Biological Heart Valve Prosthesis Sensorized with Intravalvular Impedance
Abstract
1. Introduction
2. Materials and Methods
2.1. IVI Sensing Applied to a BHV
2.2. Impedance Measurement Unit
2.3. Ex Vivo Animal Testing
2.3.1. Cardiac BioSimulator Platform
2.3.2. Test Conditions
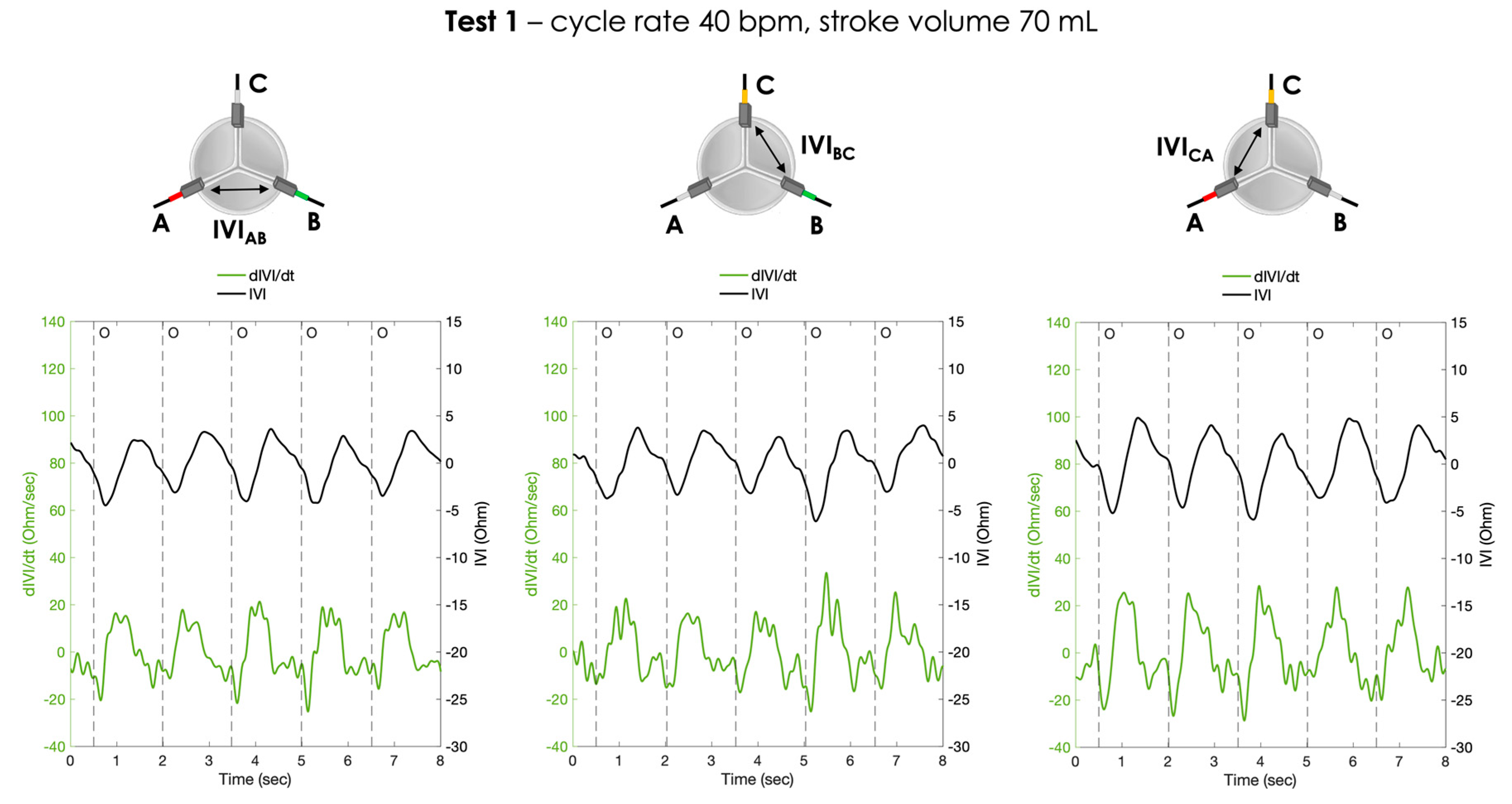
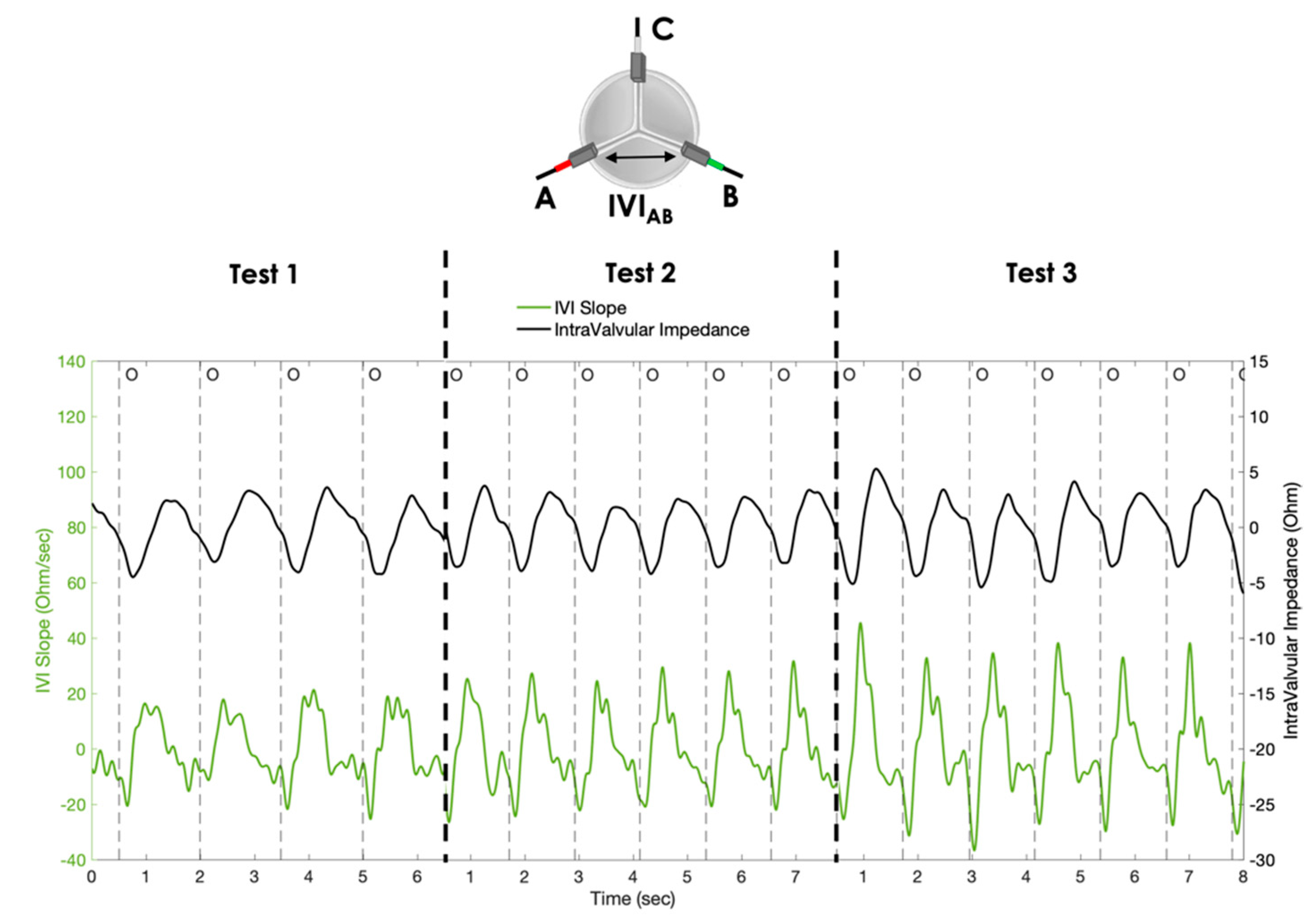
- Test 1: cycle rate 40 bpm, stroke volume 70 mL;
- Test 2: cycle rate 50 bpm, stroke volume 70 mL;
- Test 3: cycle rate 50 bpm, stroke volume 80 mL.
2.4. Data Analysis and Statistics
3. Results
4. Discussion
Study Limitations and Future Directions
5. Conclusions
6. Patents
- WO2015EP58201 20150415. Heart valve prosthesis with integrated electronic circuit for measuring intravalvular electrical impedance, and system for monitoring functionality of the prosthesis. E. Marcelli (Inventor); Alma Mater Studiorum (Applicant). Filed: 15 April 2015.
- Also published as: EP3131502 (A1); CN106456043 (A); US9987129 (B2)—Issued: 5 June 2018.
- N. 0001423344 Protesi valvolare cardiaca con circuito elettronico integrato per effettuare misure di impedenza elettrica intravalvolare e sistema per monitorare la funzionalità di tale protesi—E. Marcelli (Inventor); Alma Mater Studiorum (Applicant). Filed: 16 April 2014. Issued: 22 July 2016.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwinlll, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, E.J.J. ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 36, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; Backer, O.D.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Kanjanauthai, S.; Pirelli, L.; Nalluri, N.; Kliger, C.A. Subclinical leaflet thrombosis following transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 640–647. [Google Scholar] [CrossRef] [PubMed]
- BYanagawa, B.; Mazine, A.; Bhatt, D.L.; Clavel, M.-A.; Côté, N.; Cheema, A.N.; Pibarot, P.; Verma, S. Subclinical bioprosthetic aortic valve thrombosis: Clinical and translational implications. Curr. Opin. Cardiol. 2017, 32, 137–146. [Google Scholar] [CrossRef]
- Bogyi, M.; Schernthaner, R.E.; Loewe, C.; Gager, G.M.; Dizdarevic, A.M.; Kronberger, C.; Postula, M.; Legutko, J.; Velagapudi, P.; Hengstenberg, C.; et al. Subclinical Leaflet Thrombosis After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 2643–2656. [Google Scholar] [CrossRef]
- De Backer, O.; Dangas, G.D.; Jilaihawi, H.; Leipsic, J.A.; Terkelsen, C.J.; Makkar, R.; Kini, A.S.; Veien, K.T.; Abdel-Wahab, M.; Kim, W.-K.; et al. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 130–139. [Google Scholar] [CrossRef]
- Rashid, H.N.; Brown, A.J.; McCormick, L.M.; Amiruddin, A.S.; Be, K.K.; Cameron, J.D.; Nasis, A.; Gooley, R.P. Subclinical Leaflet Thrombosis in Transcatheter Aortic Valve Replacement Detected by Multidetector Computed Tomography―A Review of Current Evidence. Circ. J. 2018, 82, 1735–1742. [Google Scholar] [CrossRef]
- Martín, M.; Cuevas, J.; Cigarrán, H.; Calvo, J.; Morís, C. Transcatheter Aortic Valve Implantation and Subclinical and Clinical Leaflet Thrombosis: Multimodality Imaging for Diagnosis and Risk Stratification. Eur. Cardiol. Rev. 2021, 16, e35. [Google Scholar] [CrossRef]
- Makkar, R.R.; Blanke, P.; Leipsic, J.; Thourani, V.; Chakravarty, T.; Brown, D.; Trento, A.; Guyton, R.; Babaliaros, V.; Williams, M.; et al. Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves. J. Am. Coll. Cardiol. 2020, 75, 3003–3015. [Google Scholar] [CrossRef]
- Rosseel, L.; De Backer, O.; Sondergaard, L. Clinical Valve Thrombosis and Subclinical Leaflet Thrombosis Following Transcatheter Aortic Valve Replacement: Is There a Need for a Patient-Tailored Antithrombotic Therapy? Front. Cardiovasc. Med. 2019, 6, 44. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Okutucu, S.; Russo, G.; Martins, E.C.C. The Issue of Subclinical Leaflet Thrombosis after Transcatheter Aortic Valve Implantation. Cardiol. Res. 2020, 11, 269–273. [Google Scholar] [CrossRef]
- AVerstraete, A.; Herregods, M.C.; Verbrugghe, P.; Lamberigts, M.; Vanassche, T.; Meyns, B.; Oosterlinck, W.; Rega, F.; Adriaenssens, T.; Van Hoof, L.; et al. Antithrombotic Treatment After Surgical and Transcatheter Heart Valve Repair and Replacement. Front. Cardiovasc. Med. 2021, 8, 702780. [Google Scholar] [CrossRef]
- Bhogal, S.; Waksman, R.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Levitt, R.; Parikh, P.; Bilfinger, T.; Hanna, N.; Buchbinder, M.; et al. Subclinical leaflet thrombosis and antithrombotic therapy post-TAVI: An LRT substudy. Int. J. Cardiol. 2023, 371, 305–311. [Google Scholar] [CrossRef]
- Lanning, C.; Shandas, R. Development and validation of implantable sensors for monitoring function of prosthetic heart valves: In vitro studies. Med. Biol. Eng. Comput. 2003, 41, 416–424. [Google Scholar] [CrossRef]
- Bailoor, S.; Seo, J.-H.; Dasi, L.; Schena, S.; Mittal, R. Prosthetic Valve Monitoring via In Situ Pressure Sensors: In Silico Concept Evaluation using Supervised Learning. Cardiovasc. Eng. Technol. 2022, 13, 90–103. [Google Scholar] [CrossRef]
- Rivero, G.; García-Páez, J.M.; Álvarez, L.; Multigner, M.; Valdes, J.; Carabias, I.; Spottorno, J.; Hernando, A. Magnetic Sensor for Early Detection of Heart Valve Bioprostheses Failure. Sens. Lett. 2007, 5, 263–266. [Google Scholar] [CrossRef]
- Vennemann, B.; Obrist, D.; Rösgen, T. A smartphone-enabled wireless and batteryless implantable blood flow sensor for remote monitoring of prosthetic heart valve function. PLoS ONE 2020, 15, e0227372. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, E. Protesi Valvolare Cardiaca con Circuito Elettronico Integrato per Effettuare Misure di Impedenza Elettrica Intravalvolare e Sistema per Monitorare la Funzionalità di tale Protesi. IT Patent IT2014BO00217, 22 July 2016. [Google Scholar]
- EMarcelli, E.; Bortolani, B.; Corazza, I.; Cercenelli, L. A Novel Sensorized Heart Valve Prosthesis: Preliminary In Vitro Evaluation. Sensors 2018, 18, 3905. [Google Scholar] [CrossRef] [PubMed]
- Gironi, C.; Cercenelli, L.; Bortolani, B.; Emiliani, N.; Tartarini, L.; Marcelli, E. Innovative IntraValvular Impedance Sensing Applied to Biological Heart Valve Prostheses: Design and In Vitro Evaluation. Sensors 2022, 22, 8297. [Google Scholar] [CrossRef] [PubMed]
- Ely, K.J.; Hall, P.; Zhou, Y. Microwelding methods in medical components and devices. In Joining and Assembly of Medical Materials and Devices, 1st ed.; Zhou, Y.N., Breyen, M., Eds.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2013; pp. 47–78. [Google Scholar] [CrossRef]
- Leopaldi, A.M.; Wrobel, K.; Speziali, G.; van Tuijl, S.; Drasutiene, A.; Chitwood, W.R. The dynamic cardiac biosimulator: A method for training physicians in beating-heart mitral valve repair procedures. J. Thorac. Cardiovasc. Surg. 2018, 155, 147–155. [Google Scholar] [CrossRef]
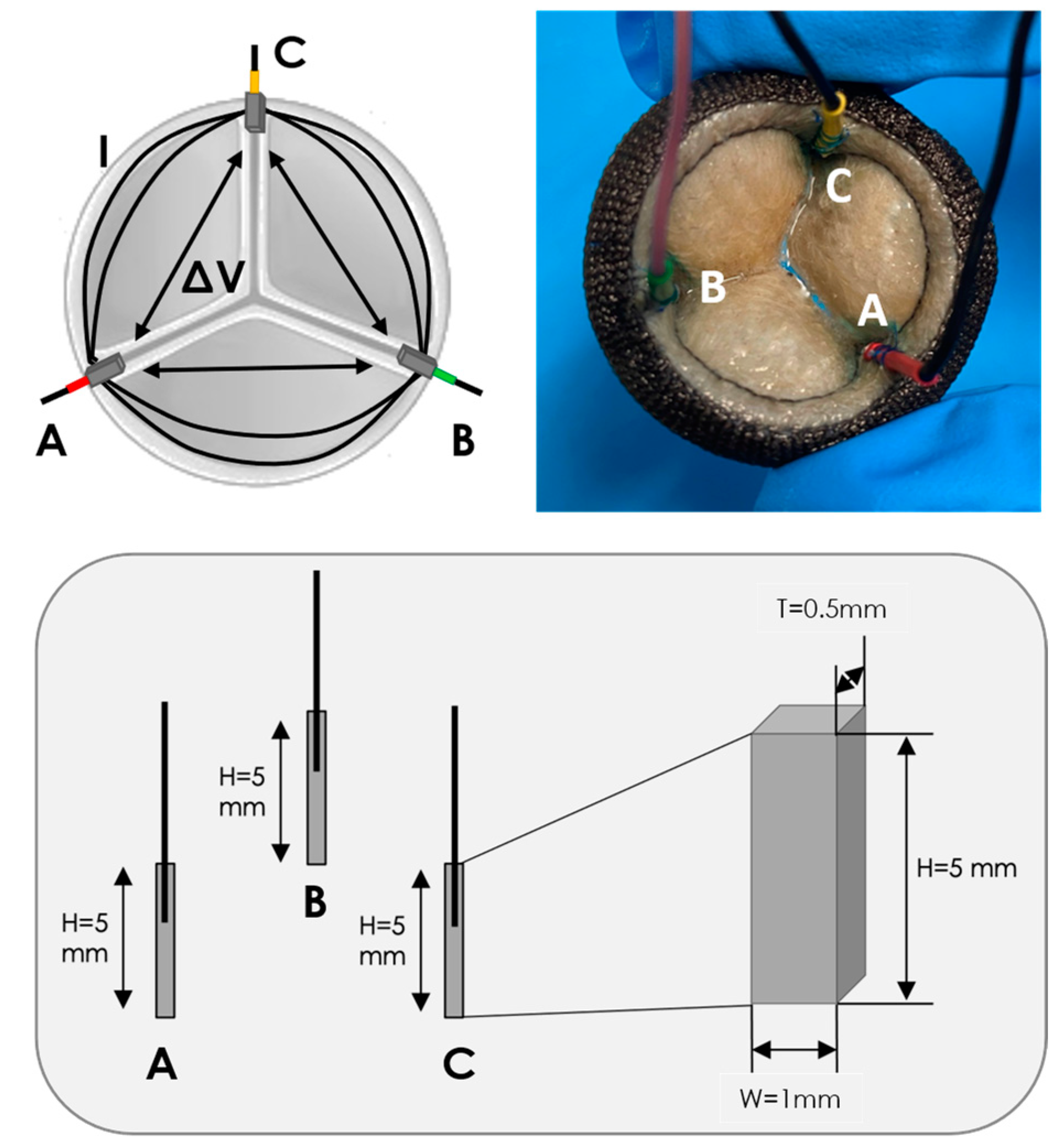

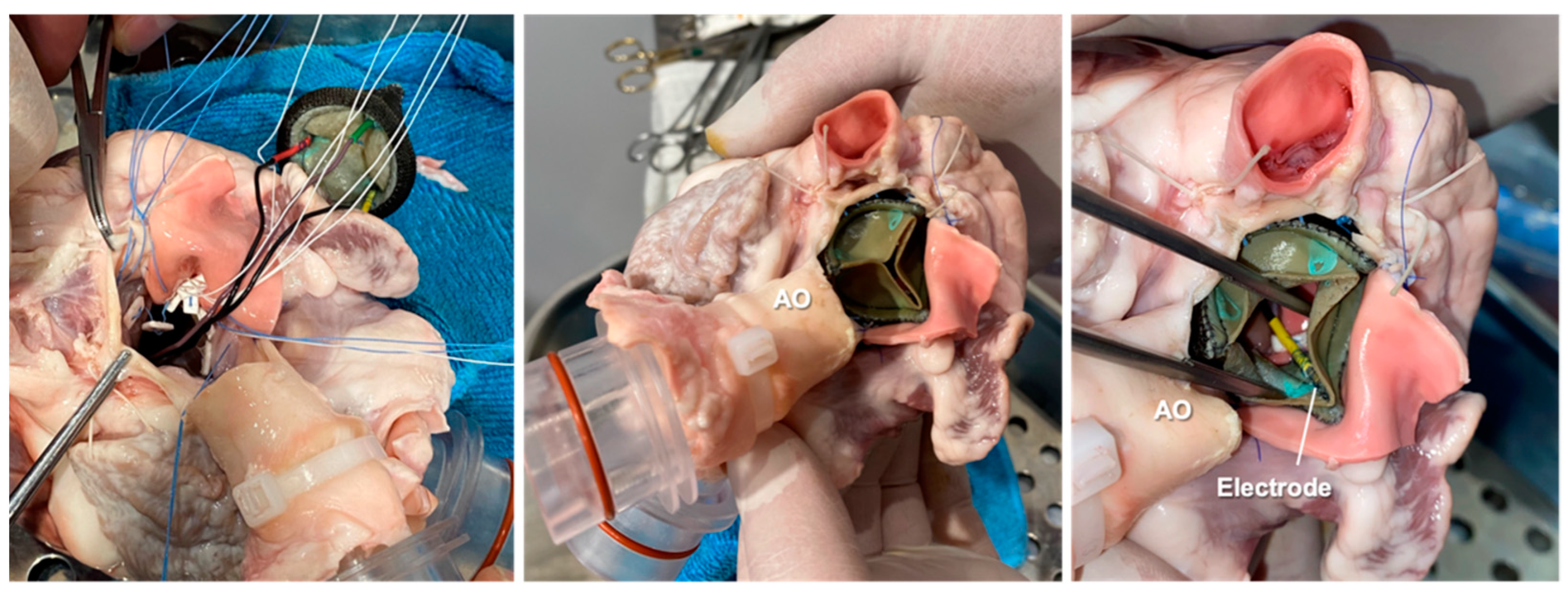
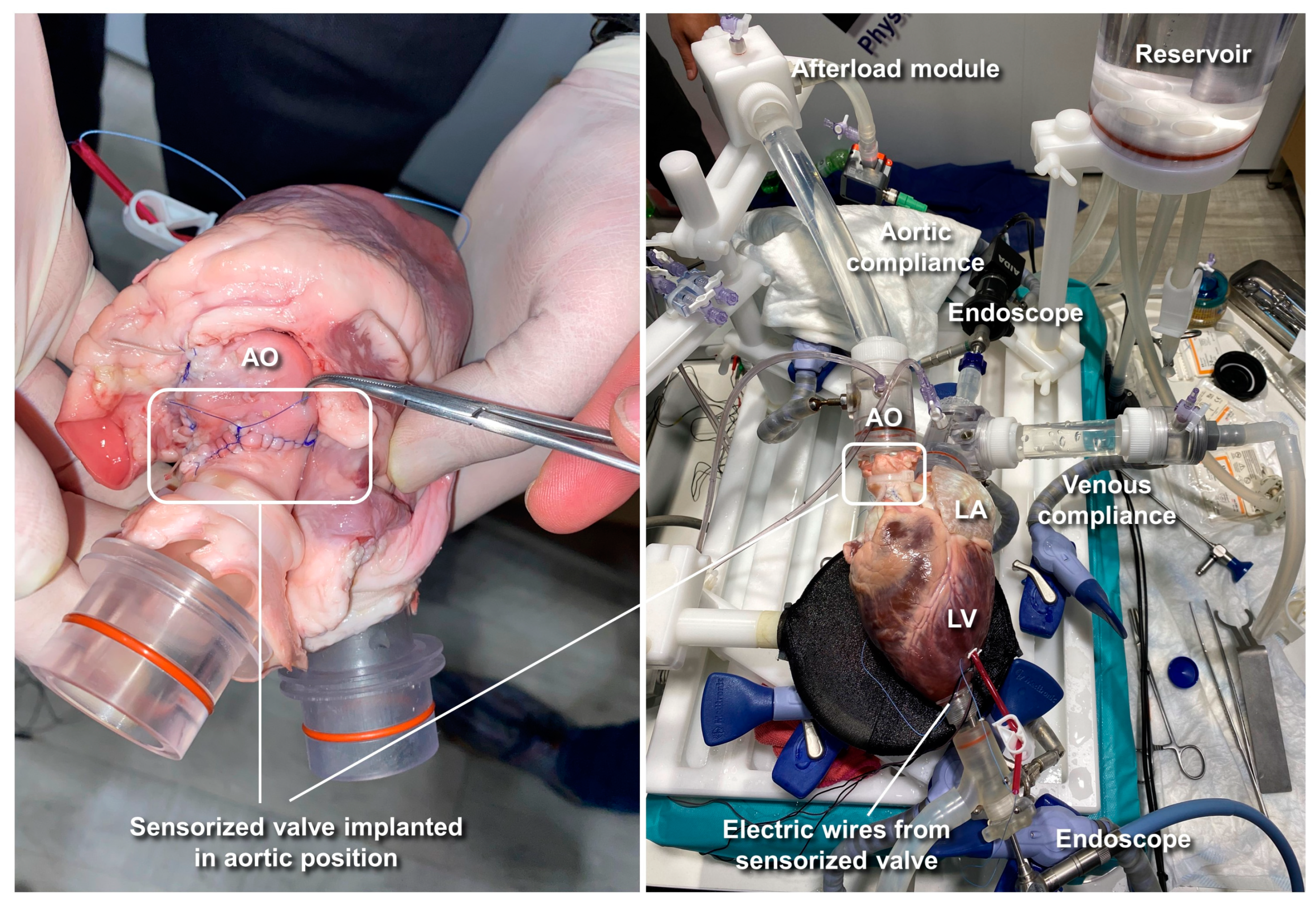
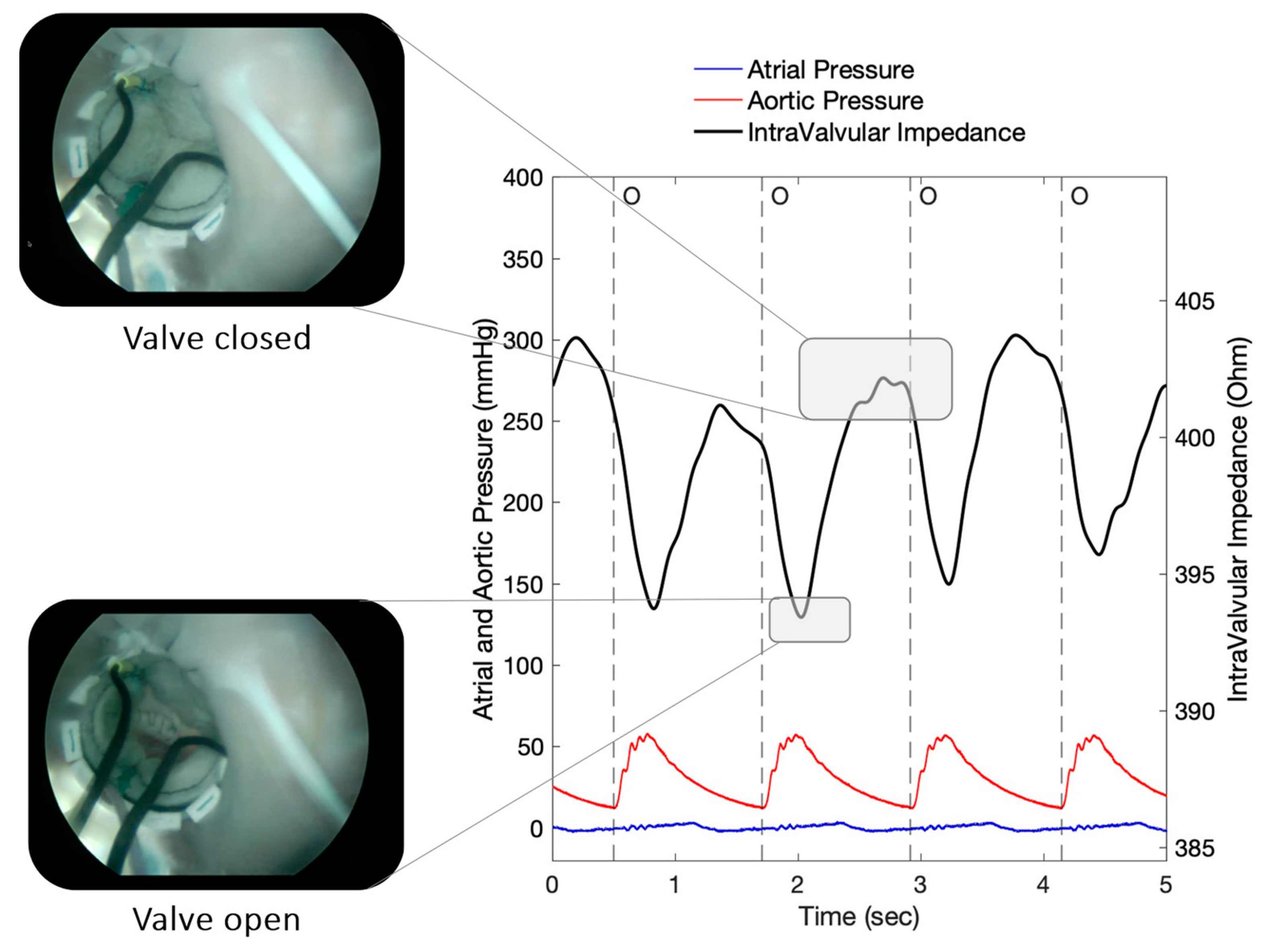





| Test 1 | Test 2 | Test 3 | |
|---|---|---|---|
| Electrodes | Test 1 vs. Test 2 | Test 2 vs. Test 3 | Test 1 vs. Test 3 |
|---|---|---|---|
| * | * | ||
| * | * | ||
| * | * |
| Test 1 | Test 2 | Test 3 | |
|---|---|---|---|
| [Ohm/s] | |||
| [Ohm/s] | |||
| [Ohm/s] |
| Electrodes | Test 1 vs. Test 2 | Test 2 vs. Test 3 | Test 1 vs. Test 3 |
|---|---|---|---|
| * <0.001 | * 0.002 | * 0.001 | |
| * 0.01 | 0.125 | * 0.001 | |
| 0.483 | * <0.001 | * 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercenelli, L.; Gironi, C.; Bortolani, B.; Marcelli, E. First Ex Vivo Animal Study of a Biological Heart Valve Prosthesis Sensorized with Intravalvular Impedance. Sensors 2023, 23, 3829. https://doi.org/10.3390/s23083829
Cercenelli L, Gironi C, Bortolani B, Marcelli E. First Ex Vivo Animal Study of a Biological Heart Valve Prosthesis Sensorized with Intravalvular Impedance. Sensors. 2023; 23(8):3829. https://doi.org/10.3390/s23083829
Chicago/Turabian StyleCercenelli, Laura, Camilla Gironi, Barbara Bortolani, and Emanuela Marcelli. 2023. "First Ex Vivo Animal Study of a Biological Heart Valve Prosthesis Sensorized with Intravalvular Impedance" Sensors 23, no. 8: 3829. https://doi.org/10.3390/s23083829
APA StyleCercenelli, L., Gironi, C., Bortolani, B., & Marcelli, E. (2023). First Ex Vivo Animal Study of a Biological Heart Valve Prosthesis Sensorized with Intravalvular Impedance. Sensors, 23(8), 3829. https://doi.org/10.3390/s23083829







