An Open-Source, Interoperable Architecture for Generating Real-Time Surgical Team Cognitive Alerts from Heart-Rate Variability Monitoring
Abstract
1. Introduction
2. Methods
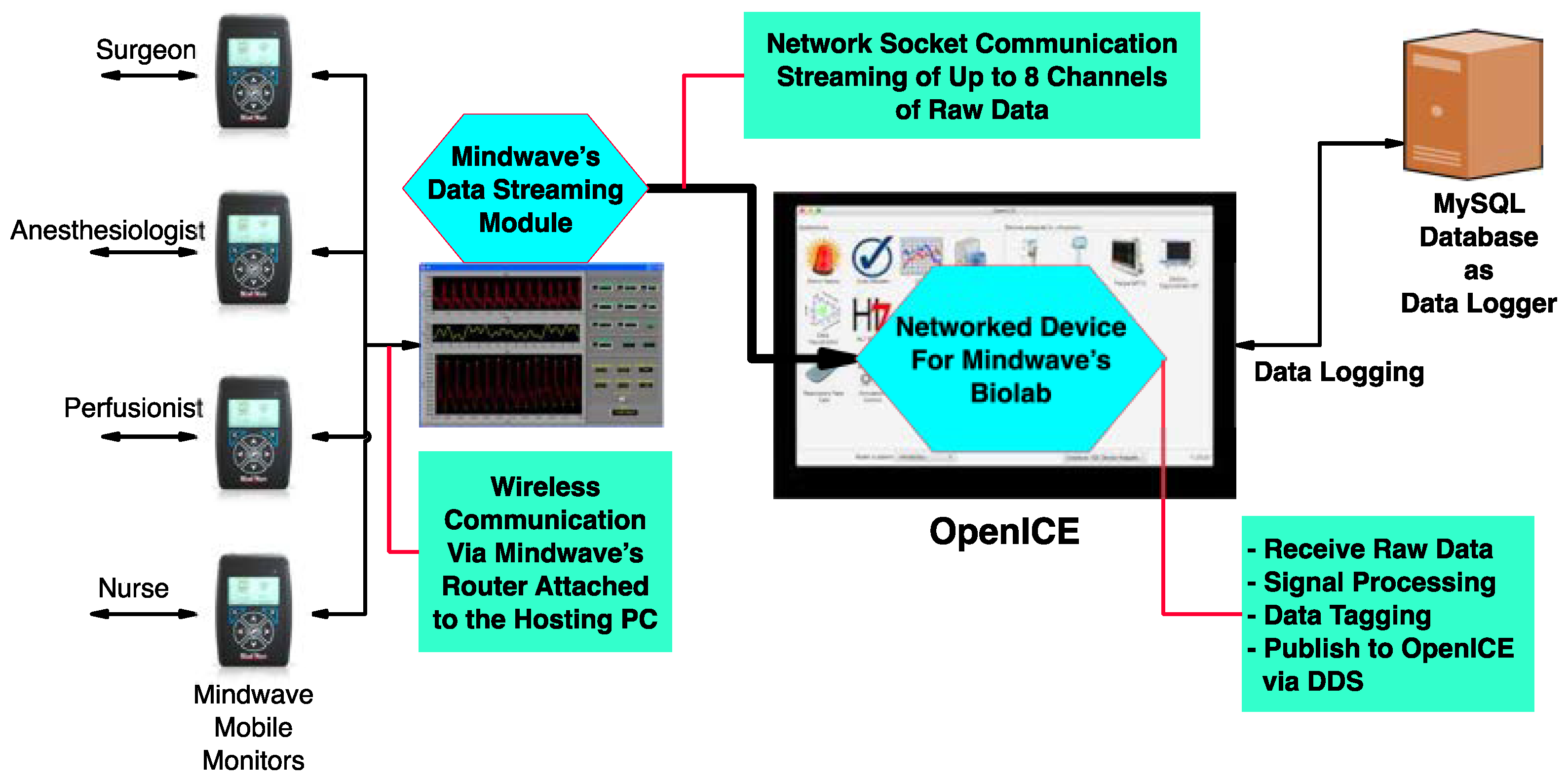
2.1. COR-ICE: OpenICE-Centered Context-Aware Operating Room
2.2. Deriving Cognitive Load Estimates and Alerts from Heart-Rate Variability Metrics
2.3. Processing Pipeline
3. Results
System Implementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahr, J.A.; Prager, R.L.; Abernathy, J.; Martinez, E.A.; Salas, E.; Seifert, P.C.; Groom, R.C.; Spiess, B.D.; Searles, B.E.; Sundt, T.M.; et al. Patient safety in the cardiac operat ing room: Human factors and teamwork. Circulation 2013, 128, 1139–1169. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, L.; Vedula, S.S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Giannarou, S.; et al. Surgical data science for next-generation interventions. Nat. Biomed. Eng. 2017, 1, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Arney, D.; Rance, G.; Rithy, S.; Goldman, J.M.; Zenati, M.A. A Novel Interoperable Safety System for Improved Coordination and Communication in Cardiac Surgery. In OR 2.0 Context Aware Oper Theaters; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11041, pp. 39–45. [Google Scholar] [CrossRef]
- Zenati, M.A.; Kennedy-Metz, L.; Dias, R.D. Cognitive engineering to im- prove patient safety and outcomes in cardiothoracic surgery. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 7. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.D.; Conboy, H.M.; Gabany, J.M.; Clarck, L.A.; Osterwei, L.J.; Avrunin, G.S.; Arney, D.; Goldman, J.M.; Riccardi, G.; Yule, S.J.; et al. Development of an Interactive Dashboard to Analyze Cognitive Workload of Surgical Teams During Complex Procedural Care. IEEE Int. Interdiscip. Conf. Cogn. Methods Situat. Aware Decis. Support. 2018, 2018, 77–82. [Google Scholar] [CrossRef] [PubMed]
- AAMI 2700-01; AAMI American National Standard ANSI/ AAMI 2700-1:2019 Medical Devices and Medical Systems—Essential Safety and Performance Requirements for Equipment Comprising the Patient-Centric Integrated Clinical Environment (ICE)—Part 1: General Requirements and Conceptual Model. 2019. Available online: https://webstore.ansi.org/standards/aami/ansiaami27002019 (accessed on 10 January 2023).
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Szi-Wen, C.; Hsiao-Chen, C.; Hsiao-Lung, C. A real-time QRS detection method based on moving-averaging incorporating with wavelet denoising. Comput. Methods Programs Biomed. 2006, 82, 187–195. [Google Scholar] [CrossRef]
- Cutmore, T.R.H.; James, D.A. Sensors and sensor systems for psychophysiological monitoring: A review of current trends. J. Psychophysiol. 2007, 21, 51–71. [Google Scholar] [CrossRef]
- Schwartz, M.S. A new improved universally accepted official definition of biofeedback: Where did it come from? Why? Who did it? Who is it for? What’s next? Biofeedback 2010, 38, 88–90. [Google Scholar] [CrossRef]
- Vasilyev, V.; Borisov, V.; Syskov, A. Biofeedback Methodology: A Narrative Review. In Proceedings of the 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON), Novosibirsk, Russia, 21–27 October 2019; pp. 11–16. [Google Scholar]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable devices for biofeedback rehabilitation: A systematic review and meta-analysis to design application rules and estimate the effectiveness on balance and gait outcomes in neurological diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef]
- Melnikov, M.Y. The Current Evidence Levels for Biofeedback and Neurofeedback Interventions in Treating Depression: A Narrative Review. Neural Plast. 2021, 2021, 8878857. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Gutierrez-Osuna, R. Using Heart Rate Monitors to Detect Mental Stress. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 219–223. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements. Biofeedback 2017, 44, 229–233. [Google Scholar] [CrossRef]
- Salahuddin, L.; Cho, J.; Jeong, M.G.; Kim, D. Ultra Short Term Analysis of Heart Rate Variability for Monitoring Mental Stress in Mobile Settings. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 4656–4659. [Google Scholar]
- Baek, H.J.; Cho, C.-H.; Cho, J.; Woo, J.-M. Reliability of Ultra-Short-Term Analysis as a Surrogate of Standard 5-Min Analysis of Heart Rate Variability. Telemed. E-Health 2015, 21, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Schaaff, K.; Adam, M.T.P. Measuring emotional arousal for online applications: Evaluation of ultra-short term heart rate variability measures. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; pp. 362–368. [Google Scholar]
- Kennedy-Metz, L.R.; Bizzego, A.; Dias, R.D.; Zenati, M.A.; Furlanello, C. Autonomic Activity and Surgical Flow Disruptions in Healthcare Providers during Cardiac Surgery. In Proceedings of the IEEE Conference on Cognitive and Computational Aspects of Situation Management, Victoria, BC, Canada, 24–29 August 2020; pp. 200–204. [Google Scholar]
- Ishaque, S.; Rueda, A.; Nguyen, B.; Khan, N.; Krishnan, S. Physiological Signal Analysis and Classification of Stress from Virtual Reality Video Game. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 867–870. [Google Scholar]
- Böhm, B.; Rötting, N.; Schwenk, W.; Grebe, S.; Mansmann, U. A prospective randomized trial on heart rate variability of the surgical team during laparoscopic and conventional sigmoid resection. Arch. Surg. 2001, 136, 305–310. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Maniyeri, J.; Guan, C. Detection of variations in cognitive workload using multi-modality physiological sensors and a large margin unbiased regression machine. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2985–2988. [Google Scholar]
- Daglius Dias, R.; Ngo-Howard, M.; Boskovski, M.; Zenati, M.; Yule, S. Systematic review of measurement tools to assess surgeons’ intraoperative cognitive workload. Br. J. Surg. 2018, 105, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Metz, L.R.; Dias, R.D.; Conboy, H.M.; Nudel, J.; Stock, E.M.; Zenati, M.A. Quantifying intraoperative team cognitive workload in complex surgical environments. In Proceedings of the International Conference on Robotics and Automation, Montreal, QC, Canada, 20–24 May 2019. [Google Scholar]
- Zenati, M.A.; Leissner, K.; Zorca, S.; Kennedy-Metz, L.; Yule, S.J.; Dias, R.D. First reported use of team cognitive workload for root cause analysis in cardiac surgery. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, F. Mental Workload Monitoring: New Perspectives from Neuroscience. In Human Mental Workload: Models and Applications. H-WORKLOAD 2019. Communications in Computer and Information Science; Longo, L., Leva, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 1107. [Google Scholar] [CrossRef]
- Suliburk, J.; Buck, Q.; Pirko, C.; Massarweh, N.; Barshes, N.; Singh, H.; Rosengart, T. Analysis of human performance deficiencies associated with surgical adverse events. JAMA Netw. Open 2019, 2, e198067. [Google Scholar] [CrossRef]
- Stevens, R.; Galloway, T.; Wang, P.; Berka, C.; Tan, V.; Wohlgemuth, T.; Lamb, J.; Buckles, R. Modeling the neurodynamic complexity of submarine navigation teams. Comput. Math. Organ. Theory 2013, 19, 346–369. [Google Scholar] [CrossRef]
- Dias, R.D.; Zenati, M.A.; Stevens, R.H.; Gabany, J.M.; Yule, S.J. Physiological synchronization and entropy as measures of team cognitive load. J. Biomed. Inform. 2019, 96, 103250. [Google Scholar] [CrossRef]
- Kennedy-Metz, L.R.; Dias, R.D.; Stevens, R.H.; Yule, S.J.; Zenati, M.A. Analysis of Mirrored Psychophysiological Change of Cardiac Surgery Team Members During Open Surgery. J. Surg. Educ. 2020, 78, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kasparick, M.; Schmitz, M.; Golatowski, F.; Timmermann, D. Dynamic remote control through service orchestration of point-of-care and surgical devices based on ieee 11073 sdc. In Proceedings of the IEEE-NIH 2016 Special Topics Conference on Healthcare Innovations and Point-of-Care Technologies, Cancun, Mexico, 9–11 November 2016. [Google Scholar]
- Kasparick, M.; Schlichting, S.; Golatowski, F.; Timmermann, D. New IEEE 11073 standards for interoperable, networked point-of-care medical devices. In Proceedings of the 2015 37th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Voume 2015. [Google Scholar]
- Weininger, S.; Jaffe, M.B.; Robkin, M.; Rausch, T.; Arney, D.; Goldman, J.M. The importance of state and context in safe interoperable medical systems. IEEE J. Transl. Eng. Health Med. 2016, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.D.; Yule, S.J.; Zenati, M.A. Augmented Cognition in the Operating Room. In Digital Surgery; Atallah, S., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- ISO/IEEE 11073-10101:2020(en); Health informatics—Device interoperability—Part 10101: Point-of-care medical device communication—Nomenclature. International Organization for Standardization: Geneva, Switzerland, 2020.
- Emina, A.; Subasi, A. Effect of Multiscale PCA de-noising in ECG beat classification for diagnosis of cardiovascular diseases. Circuits Syst. Signal Process. 2015, 34, 513–533. [Google Scholar]
- Hammad, M.; Iliyasu, A.; Subasi, A.; Edmond, H.; El-Latif, A.A. A Multi-tier Deep Learning Model for Arrhythmia Detection. IEEE Trans. Instrum. Measurement. 2020, 70, 1–9. [Google Scholar] [CrossRef]
- Qaisar, S.M.; Subasi, A. Cloud-based ECG Monitoring using Event-Driven ECG Acquisition and Machine Learning Techniques. Phys. Eng. Sci. Med. 2020, 43, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Patro, K.K.; Prakash, A.J.; Samantray, S.; Pławiak, J.; Tadeusiewicz, R.; Pławiak, P. A Hybrid Approach of a Deep Learning Technique for Real–Time ECG Beat Detection. Int. J. Appl. Math. Comput. Sci. 2022, 32, 455–465. [Google Scholar] [CrossRef]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors 2022, 2, 6536. [Google Scholar] [CrossRef] [PubMed]
- AAMI 2700-2-1: 2022; Medical devices and medical systems-Essential Safety and Performance Requirements for Equipment Comprising the Patient-Centric Integrated Clinical Environment (ICE): Part 2-1: Particular Requirements for Forensic Data Logging. Association for the Advancement of Medical Instrumentation: Washington, DC, USA, 2022.



| Stage | Input | Output |
|---|---|---|
| ECG Acquisition | Physiologic measurement | Standardized ECG waveform |
| ECG Filtering | Standardized ECG waveform | Standardized ECG waveform |
| Beat Detection | Standardized ECG waveform | IBI Series |
| Metric Calculation | IBI Series | HRV Metrics Series |
| CL Estimation | HRV Metrics Series | CL Estimate Series |
| Alerting | CL Estimate Series | Team CL Alerts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arney, D.; Zhang, Y.; Kennedy-Metz, L.R.; Dias, R.D.; Goldman, J.M.; Zenati, M.A. An Open-Source, Interoperable Architecture for Generating Real-Time Surgical Team Cognitive Alerts from Heart-Rate Variability Monitoring. Sensors 2023, 23, 3890. https://doi.org/10.3390/s23083890
Arney D, Zhang Y, Kennedy-Metz LR, Dias RD, Goldman JM, Zenati MA. An Open-Source, Interoperable Architecture for Generating Real-Time Surgical Team Cognitive Alerts from Heart-Rate Variability Monitoring. Sensors. 2023; 23(8):3890. https://doi.org/10.3390/s23083890
Chicago/Turabian StyleArney, David, Yi Zhang, Lauren R. Kennedy-Metz, Roger D. Dias, Julian M. Goldman, and Marco A. Zenati. 2023. "An Open-Source, Interoperable Architecture for Generating Real-Time Surgical Team Cognitive Alerts from Heart-Rate Variability Monitoring" Sensors 23, no. 8: 3890. https://doi.org/10.3390/s23083890
APA StyleArney, D., Zhang, Y., Kennedy-Metz, L. R., Dias, R. D., Goldman, J. M., & Zenati, M. A. (2023). An Open-Source, Interoperable Architecture for Generating Real-Time Surgical Team Cognitive Alerts from Heart-Rate Variability Monitoring. Sensors, 23(8), 3890. https://doi.org/10.3390/s23083890







