A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Procedures
2.2. Physical Measurements
2.3. Synthesis of Complex 3
2.4. Fabrication of Paper-Based Sensors and Sensing Experiments
3. Results and Discussion
3.1. Photophysical Properties of Substituted Zn(salmal) Complexes
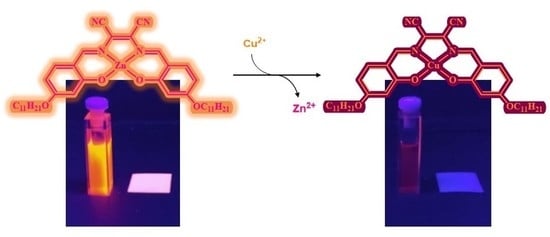
3.2. Transmetalation Studies
3.3. Detection of Cu2+ Ions: Studies in Solution
3.4. Detection of Cu2+ Ions: Paper-Based Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbhijnaKrishna, R.; Velmathi, S. A review on fluorimetric and colorimetric detection of metal ions by chemodosimetric approach 2013–2021. Coord. Chem. Rev. 2022, 459, 214401. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Zhu, Q.; Tang, B.Z.; Xu, J.W. Recent advances in cation sensing using aggregation-induced emission. Mater. Chem. Front. 2021, 5, 659–708. [Google Scholar] [CrossRef]
- Jin, J.; Xue, J.; Liu, Y.; Yang, G.; Wang, Y.-Y. Recent progresses in luminescent metal–organic frameworks (LMOFs) as sensors for the detection of anions and cations in aqueous solution. Dalton Trans. 2021, 50, 1950–1972. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chen, X.; Almahri, A.; Allehyani, E.S.; Alhumaydhi, F.A.; Ibrahim, M.M.; Ali, S. Recent developments in fluorescent and colorimetric chemosensors based on Schiff bases for metallic cations detection: A review. J. Environ. Chem. Eng. 2021, 9, 106381. [Google Scholar] [CrossRef]
- Patil, N.S.; Dhake, R.B.; Ahamed, M.I.; Fegade, U. A Mini Review on Organic Chemosensors for Cation Recognition. J. Fluoresc. 2020, 30, 1295–1330. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, A.; Xu, Q.; Pandey, D.S. Zinc(II), copper(II) and cadmium(II) complexes as fluorescent chemosensors for cations. Dalton Trans. 2020, 49, 542–568. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A review of the applications of Schiff bases as optical chemical sensors. TrAC. Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Smith, D.G.; Topolnicki, I.L.; Zwicker, V.E.; Jolliffe, K.A.; New, E.J. Fluorescent sensing arrays for cations and anions. Analyst 2017, 142, 3549–3563. [Google Scholar] [CrossRef]
- Yeung, M.C.-L.; Yam, V.W.-W. Luminescent cation sensors: From host–guest chemistry, supramolecular chemistry to reaction-based mechanisms. Chem. Soc. Rev. 2015, 44, 4192–4202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, X.; Xiang, H. Fluorescent metal ion chemosensors via cation exchange reactions of complexes, quantum dots, and metal–organic frameworks. Analyst 2015, 140, 7082–7115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef]
- Sharma, S.; Ghosh, K.S. Recent advances (2017–20) in the detection of copper ion by using fluorescence sensors working through transfer of photo-induced electron (PET), excited-state intramolecular proton (ESIPT) and Förster resonance energy (FRET). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119610. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.M.; Han, J. Fluorescent chemosensors for copper(II) ion: Structure, mechanism and application. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 78–103. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnlund, J.R.; Jacob, R.A.; Keen, C.L.; Strain, J.J.; Kelley, D.S.; Domek, J.M.; Keyes, W.R.; Ensunsa, J.L.; Lykkesfeldt, J.; Coulter, J. Long-term high copper intake: Effects on indexes of copper status, antioxidant status, and immune function in young men. Am. J. Clin. Nutr. 2004, 79, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafique, M.; Hajra, S.; Tahir, M.B.; Gillani, S.S.A.; Irshad, M. A review on sources of heavy metals, their toxicity and removal technique using physico-chemical processes from wastewater. Environ. Sci. Pollut. Res. Int. 2022, 29, 16772–16781. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diimine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of Zinc Complexes: Easily Tunable Optical Properties by Variation of the Bridge Between the Imido Groups of Schiff Base Ligands. Eur. J. Inorg. Chem. 2014, 2014, 4186–4198. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis Acidic Zinc(II) Complexes of Tetradentate Ligands as Building Blocks for Responsive Assembled Supramolecular Structures. Chemistry 2023, 5, 119–137. [Google Scholar] [CrossRef]
- Di Bella, S. Lewis acidic zinc(II) salen-type Schiff-base complexes: Sensing properties and responsive nanostructures. Dalton Trans. 2021, 50, 6050–6063. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff–Base Complexes of Salen–Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef] [Green Version]
- Strianese, M.; Ferrara, G.; Vykhovanets, V.; Blal, N.; Guarnieri, D.; Landi, A.; Lamberti, M.; Peluso, A.; Pellecchia, C. Sol-Gel Dipping Devices for H2S Visualization. Sensors 2023, 23, 2023. [Google Scholar] [CrossRef]
- Munzi, G.; Consiglio, G.; Failla, S.; Di Bella, S. Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines. Inorganics 2021, 9, 49. [Google Scholar] [CrossRef]
- Munzi, G.; Failla, S.; Di Bella, S. Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines. Analyst 2021, 146, 2144–2151. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Cacciola, S.; Maccarrone, G.; Failla, S.; Di Bella, S. Dinuclear zinc(II) salen-type Schiff-base complexes as molecular tweezers. Dalton Trans. 2020, 49, 5121–5133. [Google Scholar] [CrossRef]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) complexes as probes for detecting hydrogen sulfide and its anion: Bioimaging applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Di Bella, S. Synthesis, Characterization, Optical Absorption/Fluorescence Spectroscopy, and Second-Order Nonlinear Optical Properties of Aggregate Molecular Architectures of Unsymmetrical Schiff-Base Zinc(II) Complexes. Dalton Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Highly Sensitive Fluorescent Probe for Detection of Alkaloids. Tetrahedron 2011, 67, 9446–9449. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Sensitive Fluorescent Detection and Lewis Basicity of Aliphatic Amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Di Bella, S. New Molecular Architectures by Aggregation of Tailored Zinc(II) Schiff-Base Complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Consiglio, G.; Munzi, G.; Failla, S.; Di Bella, S. Deaggregation properties and transmetalation studies of a zinc(II) salen-type Schiff-base complex. Dalton Trans. 2022, 51, 11859–11867. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Munzi, G.; Di Bella, S. A simple approach based on transmetalation for the selective and sensitive colorimetric/fluorometric detection of copper(II) ions in drinking water. New J. Chem. 2022, 46, 18018–18024. [Google Scholar] [CrossRef]
- Kurahashi, T. Variation of the Emission Efficiency and Wavelength from Fluorescent Zinc Salen Complexes upon Systematic Structural Modifications. ACS Omega 2022, 7, 30642–30654. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef]
- Fox, M.A.; Whitesell, J.K. Organic Chemistry, 2nd ed.; Jones and Bartlett: Sudbury, MA, USA, 1997; ISBN 9780763704131. [Google Scholar]
- Salassa, G.; Salassa, L. Unconventional Approaches in Coordination Chemistry and Organometallic Reactivity. ACS Omega 2021, 6, 7240–7247. [Google Scholar] [CrossRef]
- Carnes, M.E.; Collins, M.S.; Johnson, D.W. Transmetalation of self-assembled, supramolecular complexes. Chem. Soc. Rev. 2014, 43, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to metal exchange within metal–organic frameworks. J. Mater. Chem. A 2013, 1, 5453–5468. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S.; Bertolo, L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Coord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, X.; Zhang, Y.; Liu, J.; Zhou, X.; Xiang, H. Optical Chemosensors Based on Transmetalation of Salen-Based Schiff Base Complexes. Inorg. Chem. 2014, 53, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Expedient Method for the Transmetalation of Zn(II)-Centered Salphen Complexes. Inorg. Chem. 2007, 46, 7265–7267. [Google Scholar] [CrossRef]
- San Felices, L.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Isolation and Structural Characterization of a Binuclear Intermediate Species Pertinent to Transmetalation of Zn(salphen) Complexes and the Formation of Polynuclear Salen Structures. Inorg. Chem. 2009, 48, 846–853. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Maccarrone, G.; Di Bella, S. A Lewis Basicity Scale in Dichloromethane for Amines and Common Nonprotogenic Solvents Using a Zinc(II) Schiff-Base Complex as Reference Lewis Acid. J. Org. Chem. 2011, 76, 8879–8884. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chem. 1928, 9, 113–203. [Google Scholar]
- Gil, V.M.S.; Olivieira, N.C. On the use of the method of continuous variations. J. Chem. Educ. 1990, 67, 473. [Google Scholar] [CrossRef]
- Currie, L.A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta 1999, 391, 127–134. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://data.europa.eu/eli/dir/2020/2184/oj (accessed on 12 January 2021).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Copper in Drinking-Water; Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Guo, X.; Zhao, Y.-R.; Zhang, L.-L.; Yan, X.-J.; Liu, H.-B.; Li, Q.-Z.; Xie, C.-Z.; Xu, J.-Y. Highly selective fluorescent probe in aqueous solution based on coumarin Schiff base for detecting Cu2+ and specific biosensing of glutathione in mitochondria. J. Photochem. Photobiol. A Chem. 2023, 435, 114350. [Google Scholar] [CrossRef]
- Shi, J.; Wang, M.; Pang, X.; Liu, Y.; Liu, W.; Huo, Y.; Shen, F.; Li, S.; Zhao, L.; Cao, D. A highly sensitive coumarin-based fluorescent probe for visual detection of Cu2+ in aqueous solution and its bioimaging in living cells. J. Mol. Struct. 2023, 1281, 135062. [Google Scholar] [CrossRef]
- Shruthi, B.; Revanasiddappa, H.D.; Jayalakshmi, B.; Syed, A.; Elgorban, A.M.; Eswaramoorthy, R.; Amachawadi, R.G.; Shivamallu, C.; Kollur, S.P. Hydrobenzoic acid-based ‘Turn-Off’ fluorescent sensor for selective detection of Cu2+ ions: Chemical preparation, characterization and photophysical studies. Inorg. Chem. Comm. 2023, 150, 110467. [Google Scholar] [CrossRef]
- Saedi, Z.; Roushani, M.; Khaleghian-Moghadam, R.; Darabi, A. Selective and sensitive Detection of Cu2+ in aqueous solution based on cation exchange by Metal−Organic framework TMU-16 as a fluorescent sensor. J. Lumin. 2022, 251, 119165. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, B.; Zhao, L.; Zhao, L.; Wang, Q.; Wang, C.; Xu, B. A dansyl-based fluorescent probe for sensing Cu2+ in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120009. [Google Scholar] [CrossRef]
- Patel, S.; Jamunkar, R.; Sinha, D.; Monisha; Patle, T.K.; Kant, T.; Dewangan, K.; Shrivas, K. Recent development in nanomaterials fabricated paper-based colorimetric and fluorescent sensors: A review. Trends Environ. Anal. Chem. 2021, 31, e00136. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveri, I.P.; Attinà, A.; Di Bella, S. A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution. Sensors 2023, 23, 3925. https://doi.org/10.3390/s23083925
Oliveri IP, Attinà A, Di Bella S. A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution. Sensors. 2023; 23(8):3925. https://doi.org/10.3390/s23083925
Chicago/Turabian StyleOliveri, Ivan Pietro, Agostino Attinà, and Santo Di Bella. 2023. "A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution" Sensors 23, no. 8: 3925. https://doi.org/10.3390/s23083925
APA StyleOliveri, I. P., Attinà, A., & Di Bella, S. (2023). A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution. Sensors, 23(8), 3925. https://doi.org/10.3390/s23083925