Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Chemicals
2.2. Standard Solutions and Urine Samples
2.3. Visible Spectroscopy
2.4. LC–MS/MS
3. Results and Discussion
3.1. LC–MS/MS Analysis
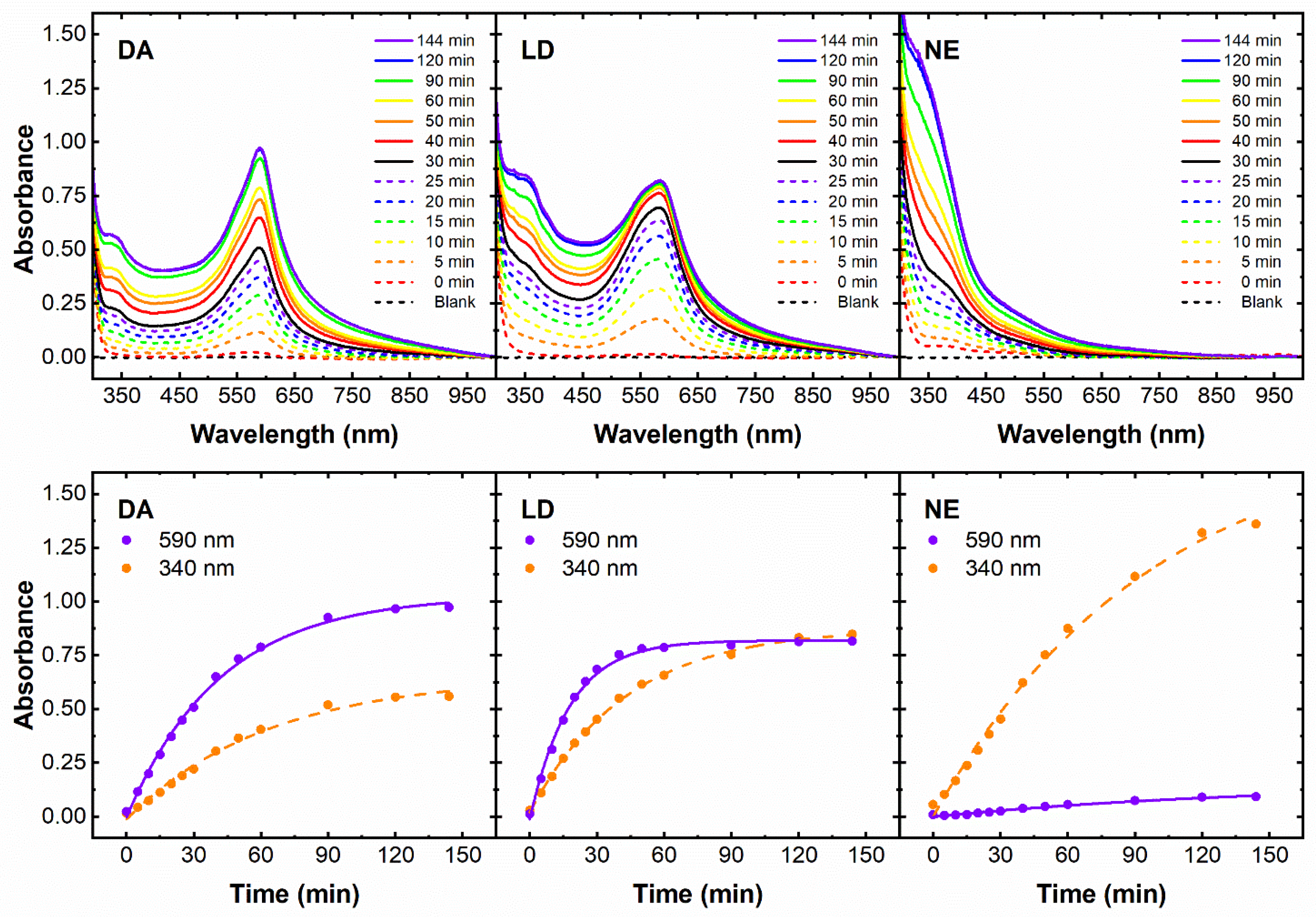
3.2. Spectroscopic Characterization of Products
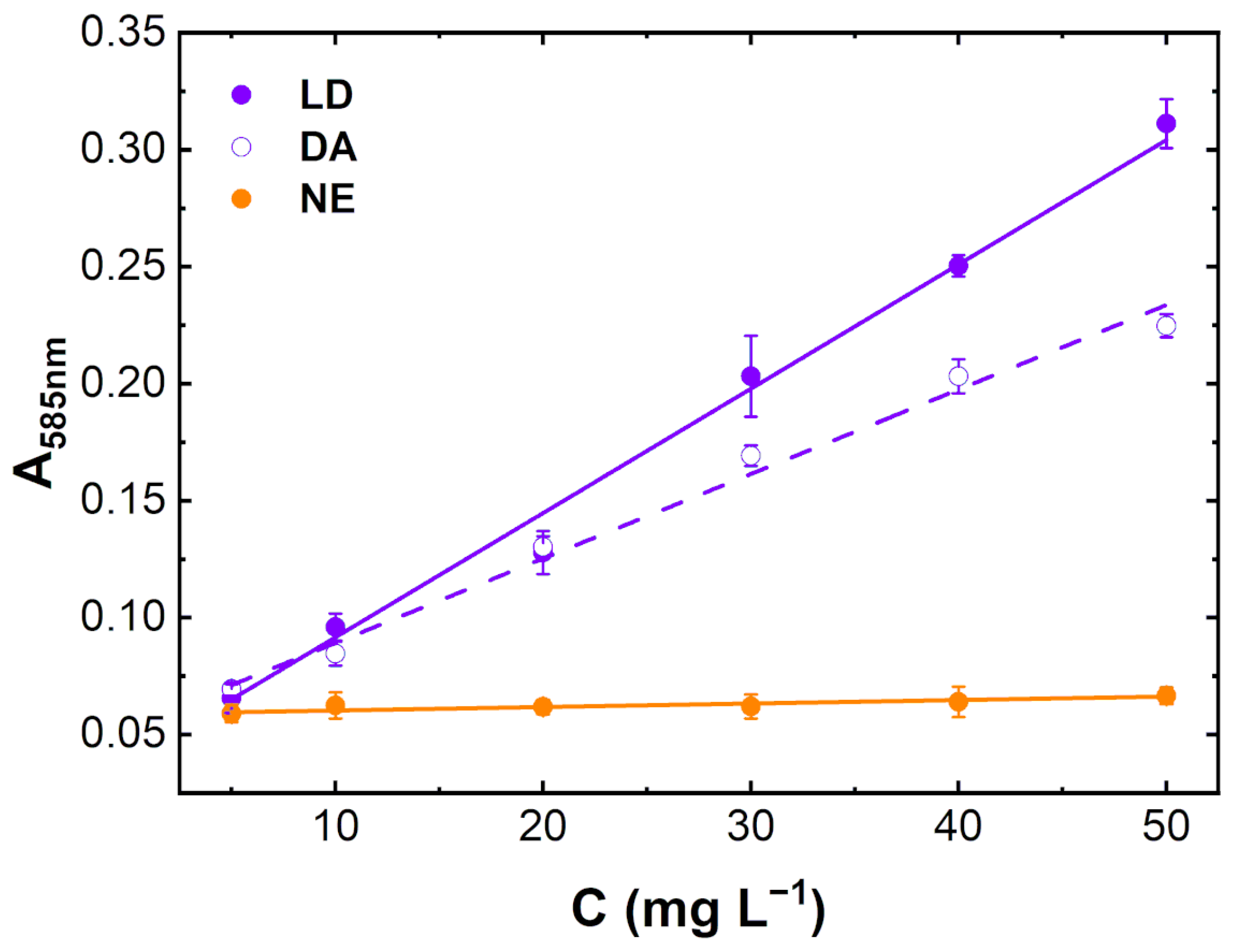
3.3. Quantification of Catecholamines in Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, T.; Zhang, G.; Chai, H.; Qu, L.; Zhang, X. Flexible biosensors based on colorimetry, fluorescence, and electrochemistry for point-of-care testing. Front. Bioeng. Biotechnol. 2021, 9, 753692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Hu, X.; Tong, W.; Leng, Y.; Xiong, Y. Recent advances in colorimetry/fluorimetry-based dual-modal sensing technologies. Biosens. Bioelectron. 2021, 190, 113386. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Doménech-Carbó, A. Spot tests: Past and present. ChemTexts 2022, 8, 4. [Google Scholar] [CrossRef]
- Lettieri, M.; Scarano, S.; Palladino, P.; Minunni, M. Colorimetric determination of carbidopa in anti-Parkinson drugs based on 4-hydroxy-3-methoxybenzaldazine formation by reaction with vanillin. Anal. Bioanal. Chem. 2022, 414, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, M.; Emanuele, R.; Scarano, S.; Palladino, P.; Minunni, M. Melanochrome-based colorimetric assay for quantitative detection of levodopa in co-presence of carbidopa and its application to relevant anti-Parkinson drugs. Anal. Bioanal. Chem. 2022, 414, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological Treatment of Parkinson Disease. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H. Recent advances in treating Parkinson’s disease. F1000Research 2017, 6, 260. [Google Scholar] [CrossRef]
- Mason, H.S.; Peterson, E.W. Melanoproteins I. reactions between enzyme-generated quinones and amino acids. Biochim. Biophys. Acta 1965, 111, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Duchoň, J.; Matouš, B. A spectrophotometric assay for mammalian tyrosinase utilizing the formation of melanochrome from L-dopa. Anal. Biochem. 1985, 146, 405–410. [Google Scholar] [CrossRef]
- Fatibello-Filho, O.; da Cruz, V.I. Flow Injection Spectrophotometric Determination of L-Dopa and Carbidopa in Pharmaceutical Formulations Using a Crude Extract of Sweet Potato Root [Ipomoea batatas (L.) Lam.] as Enzymatic Source. Analyst 1997, 122, 345–350. [Google Scholar] [CrossRef]
- Napolitano, A.; Corradini, M.G.; Prota, G. A reinvestigation of the structure of melanochrome. Tetrahedron Lett. 1985, 26, 2805–2808. [Google Scholar] [CrossRef]
- Pezzella, A.; Panzella, L.; Crescenzi, O.; Napolitano, A.; Navaratman, S.; Edge, R.; Land, E.J.; Barone, V.; d’Ischia, M. Short-Lived Quinonoid Species from 5.;6-Dihydroxyindole Dimers en Route to Eumelanin Polymers: Integrated Chemical.; Pulse Radiolytic.; and Quantum Mechanical Investigation. J. Am. Chem. Soc. 2006, 128, 15490–15498. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin broadband absorption develops from aggregation-modulated chromophore interactions under structural and redox control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.J.S.; Broadhurst, T.; Robertson, A. The chemistry of the melanins. Part V. The autoxidation of 5:6-dihydroxyindoles. J. Chem. Soc. 1954, 1947–1953. [Google Scholar] [CrossRef]
- Napolitano, A.; Pezzella, A.; d’Ischia, M.; Prota, G. The first characterisation of a transient 5.;6-indolequinone. Tetrahedron Lett. 1996, 37, 4241–4242. [Google Scholar] [CrossRef]
- Napolitano, A.; Palumbo, A.; d’Ischia, M.; Prota, G. Mechanism of Selective Incorporation of the Melanoma Seeker 2-Thiouracil into Growing Melanin. J. Med. Chem. 1996, 39, 5192–5201. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and Indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 37. [Google Scholar] [CrossRef]
- Pezzella, A.; Crescenzi, O.; Natangelo, A.; Panzella, L.; Napolitano, A.; Navaratnam, S.; Edge, R.; Land, E.J.; d’Ischia, M. Chemical, Pulse Radiolysis and Density Functional Studies of a New, Labile 5,6-Indolequinone and Its Semiquinone. J. Org. Chem. 2007, 72, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Passian, A.; Imam, N. Nanosystems, edge computing, and the next generation computing systems. Sensors 2019, 19, 4048. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, P.; Männistö, P.T. Optimization of the Hydrolysis of Conjugated L-DOPA, Dopamine and Dihydroxyphenylacetic Acid in Human Urine for Assay by High-Performance Liquid Chromatography with Electrochemical Detection. Clin. Chem. Lab. Med. 1997, 35, 229–235. [Google Scholar] [CrossRef]
- Baranowska, I.; Płonka, J. Determination of levodopa and biogenic amines in urine samples using high-performance liquid chromatography. J. Chromatogr. Sci. 2008, 46, 30–34. [Google Scholar] [CrossRef]
- Okuda, H.; Wakamatsu, K.; Ito, S.; Sota, T. Possible Oxidative Polymerization Mechanism of 5,6-Dihydroxyindole from ab Initio Calculations. J. Phys. Chem. A 2008, 112, 11213–11222. [Google Scholar] [CrossRef]
- Antidormi, A.; Melis, C.; Canadell, E.; Colombo, L. Understanding the Polymerization Process of Eumelanin by Computer Simulations. J. Phys. Chem. C 2018, 122, 28368–28374. [Google Scholar] [CrossRef]
- Alves, G.G.; Lavarda, F.C.; Graeff, C.F.; Batagin-Neto, A. Reactivity of eumelanin building blocks: A DFT study of monomers and dimers. J. Mol. Graph. Model. 2020, 98, 107609. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Panzella, L.; Napolitano, A.; d’Ischia, M. Oxidation Chemistry of Norepinephrine: Partitioning of the O-Quinone between Competing Cyclization and Chain Breakdown Pathways and Their Roles in Melanin Formation. Chem. Res. Toxicol. 2007, 20, 1549–1555. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of Quinone Methides versus o-Quinones in Catecholamine Metabolism and Eumelanin Biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J.; Copeland, L.G.; Playfer, J.R.; Roberts, N.B. Measuring L-dopa in plasma and urine to monitor therapy of elderly patients with Parkinson disease treated with L-dopa and a dopa decarboxylase inhibitor. Clin. Chem. 1993, 39, 629–634. [Google Scholar] [CrossRef]
- Shariatgorji, M.; Nilsson, A.; Fridjonsdottir, E.; Vallianatou, T.; Källback, P.; Katan, L.; Sävmarker, J.; Mantas, I.; Zhang, X.; Bezard, E.; et al. Comprehensive mapping of neurotransmitter networks by MALDI–MS imaging. Nat. Methods 2019, 16, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Ajith, A.; Sthanikam, Y.; Banerjee, S. Chemical analysis of the human brain by imaging mass spectrometry. Analyst 2021, 146, 5451–5473. [Google Scholar] [CrossRef] [PubMed]
| Molecule | Precursor Ion [M+H]+ | Product Ions | Collision Energy (eV) |
|---|---|---|---|
| DA | 154.1 | 137.2 | 10 |
| 91.1 | 25 | ||
| 65.2 | 35 | ||
| LD | 198.1 | 152.1 | 10 |
| 139.0 | 15 | ||
| 135.0 | 15 | ||
| 107.0 | 35 | ||
| 79.0 | 35 | ||
| 1 MC | 293.1 | 214.9 | 15 |
| 136.9 | 15 | ||
| 121.9 | 25 | ||
| 58.9 | 30 | ||
| NE | 170.1 | 107.1 | 15 |
| 135.0 | 15 | ||
| 151.8 | 15 | ||
| 2 IQ | 147.2 | 103.2 | 10 |
| 73.9 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, M.; Spinelli, M.; Caponi, L.; Scarano, S.; Palladino, P.; Amoresano, A.; Minunni, M. Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation. Sensors 2023, 23, 3971. https://doi.org/10.3390/s23083971
Lettieri M, Spinelli M, Caponi L, Scarano S, Palladino P, Amoresano A, Minunni M. Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation. Sensors. 2023; 23(8):3971. https://doi.org/10.3390/s23083971
Chicago/Turabian StyleLettieri, Mariagrazia, Michele Spinelli, Laura Caponi, Simona Scarano, Pasquale Palladino, Angela Amoresano, and Maria Minunni. 2023. "Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation" Sensors 23, no. 8: 3971. https://doi.org/10.3390/s23083971
APA StyleLettieri, M., Spinelli, M., Caponi, L., Scarano, S., Palladino, P., Amoresano, A., & Minunni, M. (2023). Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation. Sensors, 23(8), 3971. https://doi.org/10.3390/s23083971









