Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers
Abstract
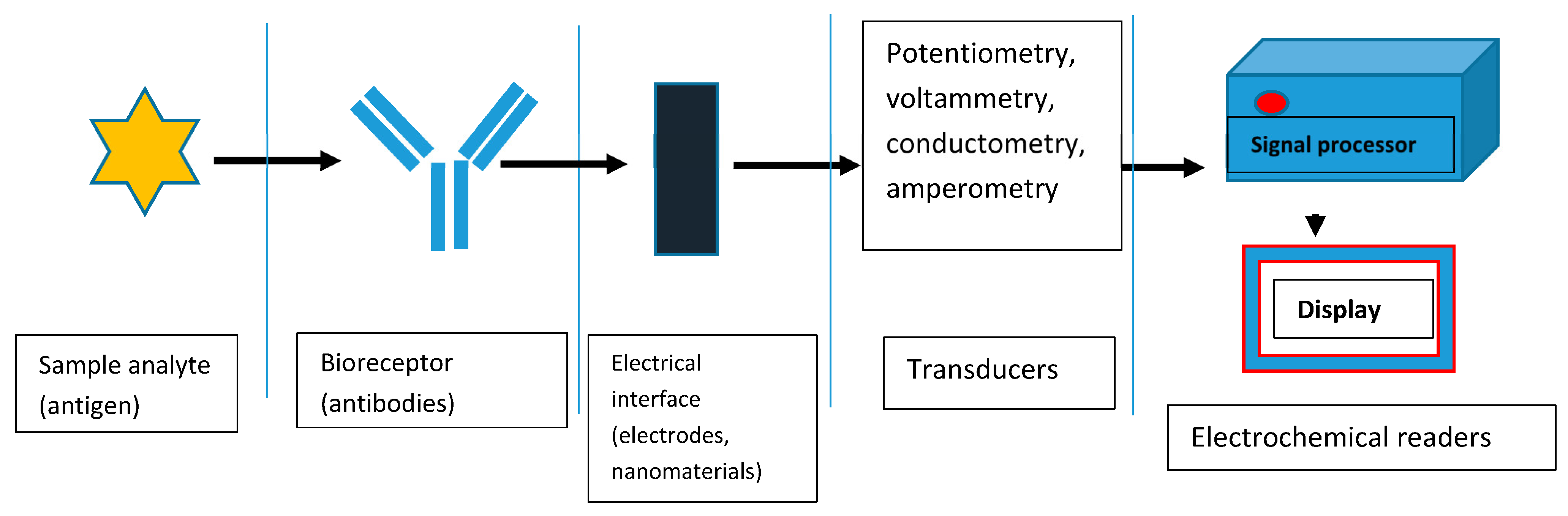
1. Introduction
2. EC and PEC Immunosensors for the Determination of Carcinoembryonic Antigen (CEA)
2.1. EC Immunosensors for OC Biomarkers Detection
2.1.1. Label–Free EC Immunosensors for OC Biomarkers Detection
Label–Free EC Immunosensors for Carcinoembryonic Antigen (CEA) Detection
Label–Free EC Immunosensors for Cancer Antigen 19–9 (CA 19–9) Detection
Label–Free EC Immunosensors for Alpha–Fetoprotein (AFP) Detection
Label–Free EC Immunosensors for p53 Detection
Label–Free EC Immunosensors for Cancer Antigen 15–3 (CA 15–3) Detection
Label–Free EC Immunosensors for CA 125 Detection
Label–Free EC Immunosensors for HER2 Detection
Label–Free EC Immunosensors for Human Epididymis Protein 4 (HE4) Detection
2.1.2. Sandwich–Type EC Immunosensors for OC Biomarkers Detection
Sandwich–Type EC Immunosensors for CEA Detection
Sandwich–Type EC Immunosensors for CA 19–9 Detection
Sandwich–Type EC Immunosensors for AFP Detection
Sandwich–Type EC Immunosensors for P53 Detection
Sandwich–Type EC Immunosensors for CA 153 Detection
Sandwich–Type EC Immunosensors for CA125 Detection
Sandwich–Type EC Immunosensors for HER2 Detection
Sandwich–Type EC Immunosensors for HE4 Detection
2.2. PEC Immunosensors for OC Biomarkers Detection
2.2.1. PEC Immunosensors for CEA Detection
2.2.2. PEC Immunosensors for AFP Detection
2.2.3. PEC Immunosensors for CA 125 Detection
2.2.4. PEC Immunosensors for CA 199 Detection
2.2.5. PEC Immunosensors for HE4 Detection
3. Multiplex Immunosensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arjmand, M.; Avval, F. Clinical biomarkers for detection of ovarian cancer. J. Mol. Cancer 2019, 2, 3–7. [Google Scholar]
- Centers for Disease Control and Prevention. Basic Information About Ovarian Cancer. 2021. Available online: www.cdc.gov/cancer/ovarian/basic_info/index.htm (accessed on 26 June 2022).
- Kamal, R.; Hamed, S.; Mansour, S.M.; Mounir, Y.; Sallam, S.A. Ovarian cancer screening—Ultrasound; impact on ovarian cancer mortality. Br. J. Radiol. 2018, 91, 20170571. [Google Scholar] [CrossRef] [PubMed]
- Manasa, G.; Mascarenhas, R.J.; Shetti, N.P.; Malode, S.J.; Aminabhavi, T.M. Biomarkers for Early Diagnosis of Ovarian Carcinoma. ACS Biomater. Sci. Eng. 2022, 8, 2726–2746. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, M.; Parkin, D.M.; Stöter, O.; Korir, A.; Kamate, B.; Liu, B.; Bojang, L.; N’Da, G.; Manraj, S.S.; Bukirwa, P.; et al. Trends in the incidence of ovarian cancer in sub-Saharan Africa. Int. J. Cancer 2023, 152, 1328–1336. [Google Scholar] [CrossRef]
- Qu, J.; Yu, F. Fabrication of a highly sensitive electrochemical immunosensor for human epididymis protein 4 (HE4) detection. Int. J. Electrochem. Sci. 2018, 13, 11193–11202. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Xiao, Z.; Liu, M.; Zhu, Y.; Chen, Q.; Li, Y.; Ai, K. Nanomaterial-based biosensor developing as a route toward in vitro diagnosis of early ovarian cancer. Mater. Today Biol. 2022, 13, 100218. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Della Corte, L.; Di Filippo, C.; Iorio, G.; Bifulco, G. Role of biomarkers for early detection of ovarian cancer recurrence. Gland. Surg. 2020, 9, 1102–1111. [Google Scholar] [CrossRef]
- Atallah, G.A.; Abd Aziz, N.H.; Teik, C.K.; Shafiee, M.N.; Kampan, N.C. New Predictive Biomarkers for Ovarian Cancer. Diagnostics 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef]
- Mavrič, T.; Benčina, M.; Imani, R.; Junkar, I.; Valant, M.; Kralj-Iglič, V.; Iglič, A. Electrochemical Biosensor Based on TiO2 Nanomaterials for Cancer Diagnostics. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–105. [Google Scholar] [CrossRef]
- Li, Y.-J.; Ma, M.-J.; Zhu, J.-J. Dual-Signal Amplification Strategy for Ultrasensitive Photoelectrochemical Immunosensing of α-Fetoprotein. Anal. Chem. 2012, 84, 10492–10499. [Google Scholar] [CrossRef]
- Jiao, L.; Mu, Z.; Zhu, C.; Wei, Q.; Li, H.; Du, D.; Lin, Y. Graphene loaded bimetallic Au@Pt nanodendrites enhancing ultrasensitive electrochemical immunoassay of AFP. Sens. Actuators B Chem. 2016, 231, 513–519. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2011, 4, 1. [Google Scholar]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-S.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar] [CrossRef]
- Islam, M.T.; Uddin, M.A. Biosensors, the emerging tools in the identification and detection of cancer markers. J. Gynecol. Women’s Health 2017, 5, 555667. [Google Scholar]
- Mercan, H.; Inam, R.; Aboul-Enein, H.Y. Square Wave Adsorptive Stripping Voltammetric Determination of Cyromazine Insecticide with Multi-Walled Carbon Nanotube Paste Electrode. Anal. Lett. 2011, 44, 1392–1404. [Google Scholar] [CrossRef]
- Isildak, I.; Attar, A.; Demir, E.; Kemer, B.; Aboul-Enein, H.Y. A Novel all Solid-State Contact PVC-Membrane Beryllium-Selective Electrode Based on 4-Hydroxybenzo-15-Crown-5 Ether Ionophore. Curr. Anal. Chem. 2018, 14, 43–48. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Das, S.; Lee, S.Y.; Terashima, C.; Nakata, K.; Fujishima, A.; Choi, W.; Kang, Y.S.; Paik, U. Synergistic Metal-Metal Oxide Nanoparticles Supported Electrocatalytic Graphene for Improved Photoelectrochemical Glucose Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 4864–4871. [Google Scholar] [CrossRef] [PubMed]
- Haddour, N.; Chauvin, J.; Gondran, C.; Cosnier, S. Photoelectrochemical Immunosensor for Label-Free Detection and Quantification of Anti-cholera Toxin Antibody. J. Am. Chem. Soc. 2006, 128, 9693–9698. [Google Scholar] [CrossRef]
- Ikeda, A.; Nakasu, M.; Ogasawara, S.; Nakanishi, H.; Nakamura, M.; Kikuchi, J.-I. Photoelectrochemical Sensor with Porphyrin-Deposited Electrodes for Determination of Nucleotides in Water. Org. Lett. 2009, 11, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Victorious, A.; Saha, S.; Pandey, R.; Didar, T.F.; Soleymani, L. Affinity-Based Detection of Biomolecules Using Photo-Electrochemical Readout. Front. Chem. 2019, 7, 617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jia, Y.; Wang, J.; Hu, X.; Zhao, Z.; Cheng, Y. Cysteine-assisted photoelectrochemical immunoassay for the carcinoembryonic antigen by using an ITO electrode modified with C3N4–BiOCl semiconductor and CuO nanoparticles as antibody labels. Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, G.; Zhang, Y.; Du, B.; Wei, Q. Ultrasensitive photoelectrochemical immunosensor based on Cu-doped TiO2 and carbon nitride for detection of carcinoembryonic antigen. Carbon 2019, 146, 276–283. [Google Scholar] [CrossRef]
- Quinchia, J.; Echeverri, D.; Cruz-Pacheco, A.F.; Maldonado, M.E.; Orozco, J. Electrochemical Biosensors for Determination of Colorectal Tumor Biomarkers. Micromachines 2020, 11, 411. [Google Scholar] [CrossRef]
- Samuel, V.R.; Rao, K. A review on label free biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Benjamin, S.R.; de Lima, F. Current and Prospective of Breast Cancer Biomarkers. In Molecular Biotechnology; IntechOpen: London, UK, 2020. [Google Scholar]
- Xie, Y.; Zhang, M.; Bin, Q.; Xie, S.; Cheng, F.; Lv, W. Photoelectrochemical immunosensor based on CdSe@ BiVO4 Co-sensitized TiO2 for carcinoembryonic antigen. Biosens. Bioelectron. 2020, 150, 111949. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.; Vashist, S.K. Immunosensing procedures for carcinoembryonic antigen using graphene and nanocomposites. Biosens. Bioelectron. 2017, 89, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; She, Z.; Ma, T.; Tian, S.; Kraatz, H.-B. Electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 102, 610–616. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, Y.-J.; Bao, T.; Xiong, H.-Y.; Zhang, X.-H.; Wang, S.-F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Gajdosova, V.; Lorencova, L.; Kasak, P.; Tkac, J. Electrochemical Nanobiosensors for Detection of Breast Cancer Biomarkers. Sensors 2020, 20, 4022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Luo, J.; Liu, J.; Wang, L.; Fan, Y.; Yan, S.; Yang, Y.; Cai, X. A novel label-free microfluidic paper-based immunosensor for highly sensitive electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2016, 83, 319–326. [Google Scholar] [CrossRef]
- Shamsuddin, S.H.; Gibson, T.D.; Tomlinson, D.C.; McPherson, M.J.; Jayne, D.G.; Millner, P.A. Reagentless Affimer- and antibody-based impedimetric biosensors for CEA-detection using a novel non-conducting polymer. Biosens. Bioelectron. 2021, 178, 113013. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-S.; Zhu, X.-F.; Xu, J.-K.; Lu, L.-M.; Wang, W.-M.; Yang, T.-T.; Xing, H.-K.; Yu, Y.-F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Wei, Q. Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 255, 125–132. [Google Scholar] [CrossRef]
- Lin, L.P.; Tham, S.-Y.; Loh, H.-S.; Tan, M.T. Biocompatible graphene-zirconia nanocomposite as a cyto-safe immunosensor for the rapid detection of carcinoembryonic antigen. Sci. Rep. 2021, 11, 22536. [Google Scholar] [CrossRef]
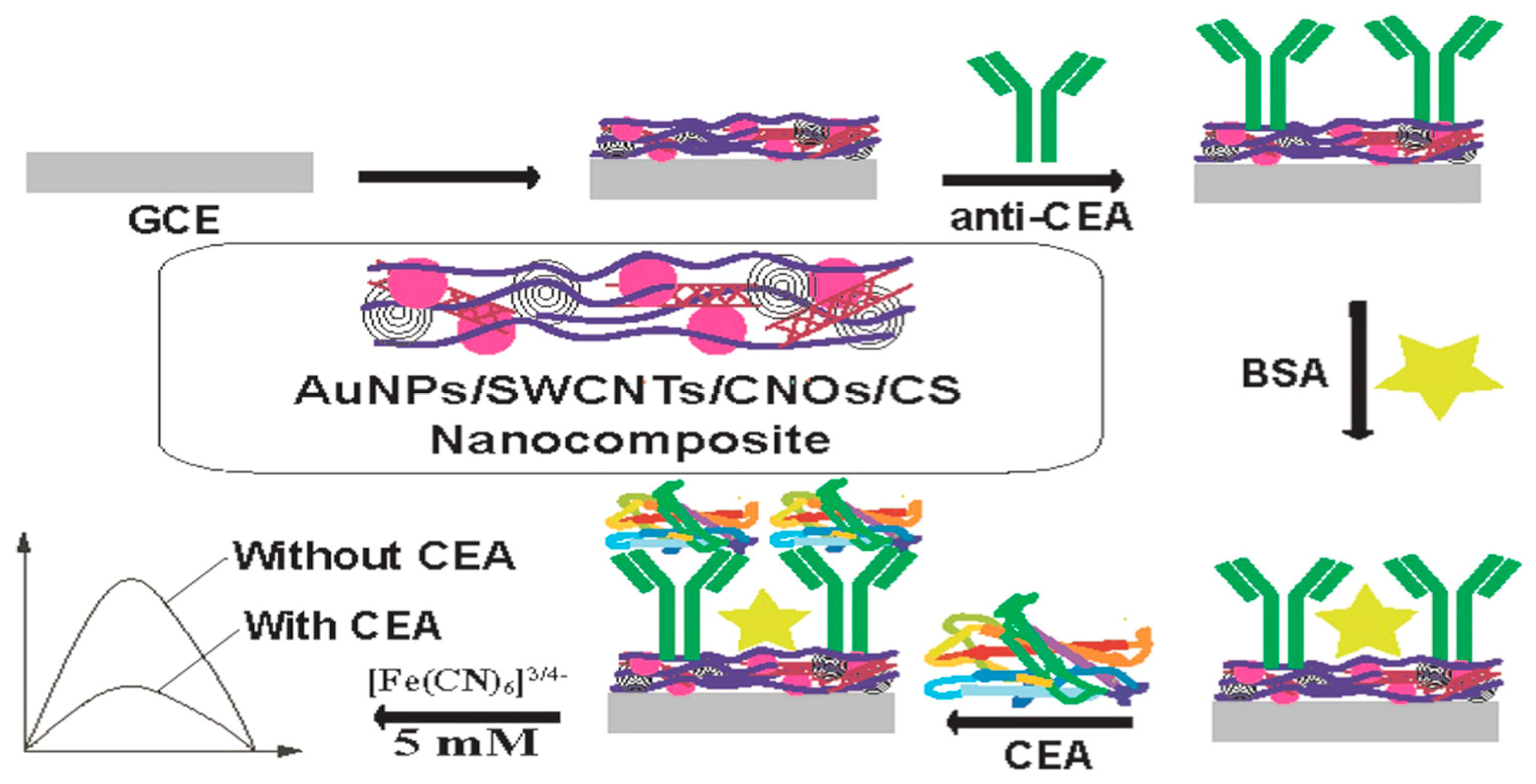
- Rizwan, M.; Elma, S.; Lim, S.A.; Ahmed, M.U. AuNPs/CNOs/SWCNTs/chitosan-nanocomposite modified electrochemical sensor for the label-free detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 107, 211–217. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, X.; Sun, D.; Cao, P.; Ding, M.; Li, Y.; Miao, Y. A new Bi 2 MoO 6 nano-tremella-based electrochemical immunosensor for the sensitive detection of a carcinoembryonic antigen. RSC Adv. 2020, 10, 15870–15880. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, W.; Lu, S.; Guo, C.; Wang, P.; Zhang, D.; Ma, W. A Label-Free Electrochemical Immunosensor for CEA Detection on a Novel Signal Amplification Platform of Cu2S/Pd/CuO Nanocomposites. Front. Bioeng. Biotechnol. 2021, 9, 767717. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Mabuba, N.; Arotiba, O.A. An Exfoliated Graphite-Based Electrochemical Immunosensor on a Dendrimer/Carbon Nanodot Platform for the Detection of Carcinoembryonic Antigen Cancer Biomarker. Biosensors 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Paimard, G.; Shahlaei, M.; Moradipour, P.; Akbari, H.; Jafari, M.; Arkan, E. An Impedimetric Immunosensor modified with electrospun core-shell nanofibers for determination of the carcinoma embryonic antigen. Sens. Actuators B Chem. 2020, 311, 127928. [Google Scholar] [CrossRef]
- Singh, P.; Katkar, P.K.; Patil, U.M.; Bohara, R.A. A robust electrochemical immunosensor based on core-shell nanostructured silica-coated silver for cancer (carcinoembryonic-antigen-CEA) diagnosis. RSC Adv. 2021, 11, 10130–10143. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, H.; Ma, Z. A nanocomposite containing Prussian Blue, platinum nanoparticles and polyaniline for multi-amplification of the signal of voltammetric immunosensors: Highly sensitive detection of carcinoma antigen 125. Microchim. Acta 2017, 184, 4269–4277. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, M.; Cao, K.; Wu, M.; Zhao, C.; Li, H.; Hong, C. An electrochemical immunosensor for CEA detection based on Au–Ag/rGO@ PDA nanocomposites as integrated double signal amplification strategy. Microchem. J. 2019, 151, 104223. [Google Scholar] [CrossRef]
- Song, Y.; Cao, K.; Li, W.; Ma, C.; Qiao, X.; Li, H.; Hong, C. Optimal film thickness of rGO/MoS2 @ polyaniline nanosheets of 3D arrays for carcinoembryonic antigen high sensitivity detection. Microchem. J. 2020, 155, 104694. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Z.; Zhao, C.; Han, W.; Lin, L.; Liu, A.; Weng, S.; Lin, X. Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19–9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int. J. Nanomed. 2017, 12, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-W.; Tian, J.-H.; Ye, J.-J.; Chang, H.-W.; Tsai, Y.-C. Construction of a Label-Free Electrochemical Immunosensor Based on Zn–Co–S/Graphene Nanocomposites for Carbohydrate Antigen 19–9 Detection. Nanomaterials 2021, 11, 1475. [Google Scholar] [CrossRef]
- Du, S.; Zhao, Y.; Lv, C.; Wei, M.; Gao, Z.; Meng, X. Applying Serum Proteins and MicroRNA as Novel Biomarkers for Early-Stage Cervical Cancer Detection. Sci. Rep. 2020, 10, 9033. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Jana, S.K. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Hu, B.; Guo, C.; Song, Y.; Jia, Q.; He, L.; Zhang, Z.; Fang, S. Bimetallic cerium and ferric oxides nanoparticles embedded within mesoporous carbon matrix: Electrochemical immunosensor for sensitive detection of carbohydrate antigen 19–9. Biosens. Bioelectron. 2019, 135, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; Nascimento, G.F.D.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N.; Gonçalves, D. Screen-printed electrodes modified with carbon black and polyelectrolyte films for determination of cancer marker carbohydrate antigen 19–9. Microchim. Acta 2020, 187, 417. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
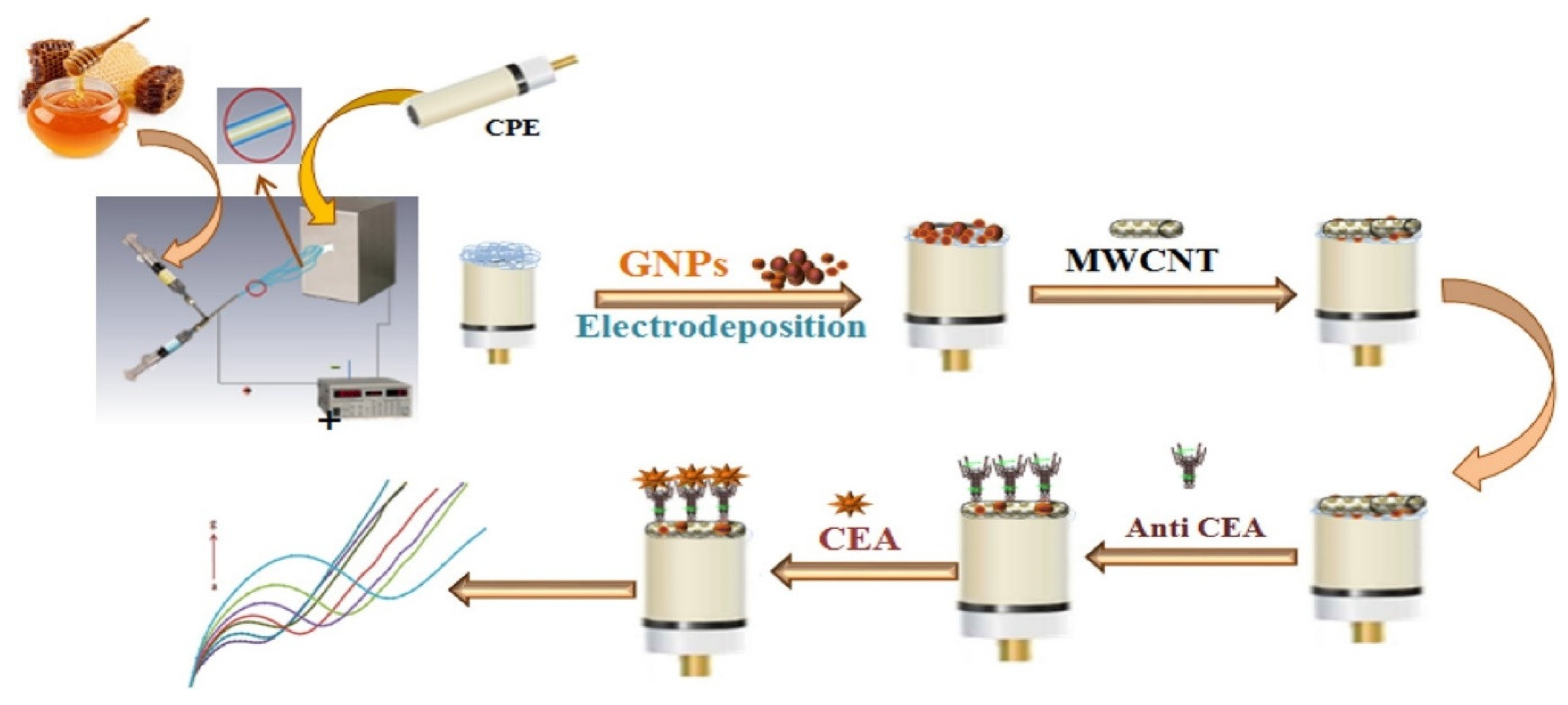
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Oliveira, O.N., Jr. Carbon nanotube matrix for highly sensitive biosensors to detect pancreatic cancer biomarker CA19–9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cai, X.; Cui, W.; Zhang, J. Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer. Molecules 2022, 27, 4578. [Google Scholar] [CrossRef]
- Zheng, X.; Hua, X.; Qiao, X.; Xia, F.; Tian, D.; Zhou, C. Simple and signal-off electrochemiluminescence immunosensor for alpha fetoprotein based on gold nanoparticle-modified graphite-like carbon nitride nanosheet nanohybrids. RSC Adv. 2016, 6, 21308–21316. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Wang, Z.; Xue, Y.; Dong, C.; Zeng, J.; Huang, Y.; Liang, J.; Zhou, Z. Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal. Biochem. 2018, 547, 37–44. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Guo, Y.-S.; Bi, S.; Zhang, S.-S. Ultrasensitive enhanced chemiluminescence enzyme immunoassay for the determination of α-fetoprotein amplified by double-codified gold nanoparticles labels. Biosens. Bioelectron. 2009, 24, 2707–2711. [Google Scholar] [CrossRef]
- Attia, M.; Youssef, A.; Khan, Z.A.; Abou-Omar, M. Alpha fetoprotein assessment by using a nano optical sensor thin film binuclear Pt-2-aminobenzimidazole-Bipyridine for early diagnosis of liver cancer. Talanta 2018, 186, 36–43. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhang, H.; Liu, S.; Jiang, J. An Electrochemical Sandwich Immunosensor Based on Signal Amplification Technique for the Determination of Alpha-Fetoprotein. Front. Chem. 2020, 8, 589560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, T.; Ye, Y.; Chen, P.; Ren, X.-N.; Rao, A.; Wan, Y.; Wang, B.; Luo, Z. A highly sensitive label-free electrochemical immunosensor based on an aligned GaN nanowires array/polydopamine heterointerface modified with Au nanoparticles. J. Mater. Chem. B 2019, 7, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wu, D.; Ma, H.; Pang, X.; Fan, D.; Wei, Q.; Du, B. Ultrasensitive Label-free Electrochemical Immunosensor based on Multifunctionalized Graphene Nanocomposites for the Detection of Alpha Fetoprotein. Sci. Rep. 2017, 7, 42361. [Google Scholar] [CrossRef]
- Aydın, M.; Aydın, E.B.; Sezgintürk, M.K. A disposable immunosensor using ITO based electrode modified by a star-shaped polymer for analysis of tumor suppressor protein p53 in human serum. Biosens. Bioelectron. 2018, 107, 1–9. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Electrochemical immunosensor based on chitosan/conductive carbon black composite modified disposable ITO electrode: An analytical platform for p53 detection. Biosens. Bioelectron. 2018, 121, 80–89. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Baghban, H.N.; Shadjou, N.; Mokhtarzadeh, A. Ultrasensitive electrochemical immunosensing of tumor suppressor protein p53 in unprocessed human plasma and cell lysates using a novel nanocomposite based on poly-cysteine/graphene quantum dots/gold nanoparticle. Int. J. Biol. Macromol. 2018, 107, 1348–1363. [Google Scholar] [CrossRef]
- Elshafey, R.; Siaj, M.; Tavares, A.C. Au nanoparticle decorated graphene nanosheets for electrochemical immunosensing of p53 antibodies for cancer prognosis. Analyst 2016, 141, 2733–2740. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Peng, Y.-R.; Lin, H.-Y.; Hsueh, T.-Y.; Lai, C.-S.; Hua, M.-Y. Preparation and characterization of Au/NiPc/Anti-p53/BSA electrode for application as a p53 antigen sensor. Chemosensors 2021, 9, 17. [Google Scholar] [CrossRef]
- Solhi, E.; Hasanzadeh, M. Critical role of biosensing on the efficient monitoring of cancer proteins/biomarkers using label-free aptamer based bioassay. Biomed. Pharmacother. 2020, 132, 110849. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Joshi, N.; Nascimento, G.F.D.; Wilson, D.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Gonçalves, D.; Oliveira, O.N. Determination of p53 biomarker using an electrochemical immunoassay based on layer-by-layer films with NiFe2O4 nanoparticles. Microchim. Acta 2020, 187, 619. [Google Scholar] [CrossRef] [PubMed]
- Deepa; Nohwal, B.; Chaudhary, R.; Pundir, C. Amperometric detection of tumor suppressor protein p53 via pencil graphite electrode for fast cancer diagnosis. Anal. Biochem. 2022, 639, 114528. [Google Scholar] [CrossRef]
- Amani, J.; Khoshroo, A.; Rahimi-Nasrabadi, M. Electrochemical immunosensor for the breast cancer marker CA 15–3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim. Acta 2018, 185, 79. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.; Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemical immunosensor for detection of CA 15–3 biomarker in point-of-care. Sens. Bio-Sens. Res. 2021, 33, 100445. [Google Scholar] [CrossRef]
- Dupont, J.; Tanwar, M.K.; Thaler, H.T.; Fleisher, M.; Kauff, N.; Hensley, M.L.; Sabbatini, P.; Anderson, S.; Aghajanian, C.; Holland, E.C.; et al. Early Detection and Prognosis of Ovarian Cancer Using Serum YKL-40. J. Clin. Oncol. 2004, 22, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Eftekhari, A.; Mahboob, S. An innovative immunosensor for ultrasensitive detection of breast cancer specific carbohydrate (CA 15–3) in unprocessed human plasma and MCF–7 breast cancer cell lysates using gold nanospear electrochemically assembled onto thiolated graphene quantum dots. Int. J. Biol. Macromol. 2018, 114, 1008–1017. [Google Scholar] [CrossRef]
- Khoshroo, A.; Mazloum-Ardakani, M.; Forat-Yazdi, M. Enhanced performance of label-free electrochemical immunosensor for carbohydrate antigen 15–3 based on catalytic activity of cobalt sulfide/graphene nanocomposite. Sens. Actuators B Chem. 2018, 255, 580–587. [Google Scholar] [CrossRef]
- Wang, A.-J.; Zhu, X.-Y.; Chen, Y.; Luo, X.; Xue, Y.; Feng, J.-J. Ultrasensitive label-free electrochemical immunoassay of carbohydrate antigen 15–3 using dendritic Au@Pt nanocrystals/ferrocene-grafted-chitosan for efficient signal amplification. Sens. Actuators B: Chem. 2019, 292, 164–170. [Google Scholar] [CrossRef]
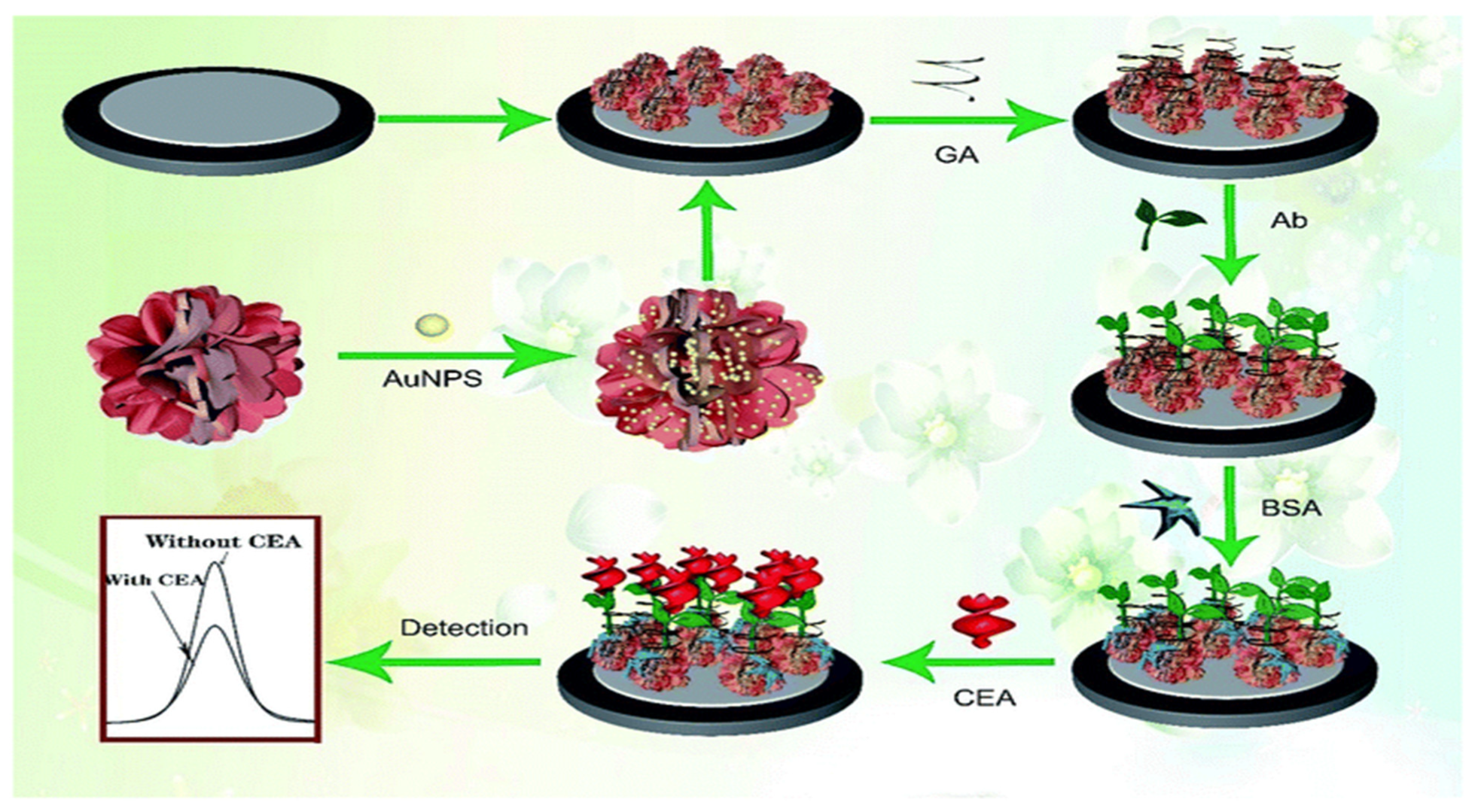
- Hasanzadeh, M.; Sahmani, R.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Mahboob, S. Ultrasensitive immunoassay of carcinoma antigen 125 in untreated human plasma samples using gold nanoparticles with flower like morphology: A new platform in early stage diagnosis of ovarian cancer and efficient management. Int. J. Biol. Macromol. 2018, 119, 913–925. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Gasparotto, G.; Costa, J.P.C.; Costa, P.I.; Zaghete, M.A.; Mazon, T. Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection. Mater. Sci. Eng. C 2017, 76, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.B.; Singh, V.; Vanjari, S.R.K.; Singh, S.G. One step biofunctionalized electrospun multiwalled carbon nanotubes embedded zinc oxide nanowire interface for highly sensitive detection of carcinoma antigen-125. Biosens. Bioelectron. 2017, 88, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gazze, A.; Ademefun, R.; Conlan, R.S.; Teixeira, S.R. Electrochemical impedence spectroscopy enabled CA125 detection; toward early ovarian cancer diagnosis using graphene biosensors. J. Interdiscip. Nanomed. 2018, 3, 82–88. [Google Scholar] [CrossRef]
- Sangili, A.; Kalyani, T.; Chen, S.-M.; Nanda, A.; Jana, S.K. Label-free electrochemical immunosensor based on one-step electrochemical deposition of AuNP-RGO nanocomposites for detection of endometriosis marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620–7630. [Google Scholar] [CrossRef]
- Gu, Y.; Gong, G.; Jiang, Y.; Qin, J.; Mei, Y.; Han, J. Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection. Chemosensors 2022, 10, 272. [Google Scholar] [CrossRef]
- Torati, S.R.; Kasturi, K.C.; Lim, B.; Kim, C. Hierarchical gold nanostructures modified electrode for electrochemical detection of cancer antigen CA125. Sens. Actuators B Chem. 2017, 243, 64–71. [Google Scholar] [CrossRef]
- de Castro, A.C.H.; Alves, L.M.; Siquieroli, A.C.S.; Madurro, J.M.; Brito-Madurro, A.G. Label-free electrochemical immunosensor for detection of oncomarker CA125 in serum. Microchem. J. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Wahyuni, H.Y.; Misonia, B.S.U.; Santhy, W.; Shabarni, G. A voltammetric immunosensor for detection of HER2 using gold modified-screen printed carbon electrode. Res. J. Chem. Environ. 2018, 22, 294–301. [Google Scholar]
- Lah, Z.M.A.N.H.; Ahmad, S.A.A.; Zaini, M.S.; Kamarudin, M.A. An Electrochemical Sandwich Immunosensor for the Detection of HER2 using Antibody-Conjugated PbS Quantum Dot as a label. J. Pharm. Biomed. Anal. 2019, 174, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahpour, H.; Naseri, A.; Rashidi, M.-R.; Khalilzadeh, B. Application of green synthesized WO3-poly glutamic acid nanobiocomposite for early stage biosensing of breast cancer using electrochemical approach. Sci. Rep. 2021, 11, 23994. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Kumar, P.; Malhotra, B.D. Amine-Functionalized MoO3@RGO Nanohybrid-Based Biosensor for Breast Cancer Detection. ACS Appl. Bio Mater. 2019, 2, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Ehzari, H.; Samimi, M.; Safari, M.; Gholivand, M.B. Label-free electrochemical immunosensor for sensitive HER2 biomarker detection using the core-shell magnetic metal-organic frameworks. J. Electroanal. Chem. 2020, 877, 114722. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Nurdjanah, D.; Wyantuti, S.; Anggraeni, A.; Gaffar, S. Gold nanoparticles modified screen-printed immunosensor for cancer biomarker HER2 determination based on anti HER2 bioconjugates. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar] [CrossRef]
- Chen, K.; Xue, J.; Zhou, Q.; Zhang, Y.; Zhang, M.; Zhang, Y.; Zhang, H.; Shen, Y. Coupling metal-organic framework nanosphere and nanobody for boosted photoelectrochemical immunoassay of Human Epididymis Protein 4. Anal. Chim. Acta 2020, 1107, 145–154. [Google Scholar] [CrossRef]
- Sarojini, S.; Tamir, A.; Lim, H.; Li, S.; Zhang, S.; Goy, A.; Pecora, A.; Suh, K.S. Early Detection Biomarkers for Ovarian Cancer. J. Oncol. 2012, 2012, 709049. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E.; Giudici, S.; Franchi, M.; Guidi, G.C.; Plebani, M.; Lippi, G. HE4 in ovarian cancer: From discovery to clinical application. Adv. Clin. Chem. 2011, 55, 2. [Google Scholar]
- Moore, R.G.; Miller, M.C.; DiSilvestro, P.; Landrum, L.M.; Gajewski, W.; Ball, J.J.; Skates, S.J. Evaluation of the Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Women with a Pelvic Mass. Obstet. Gynecol. 2011, 118, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cao, L.; Dong, H.; Tan, Z.; Hu, Y.; Liu, Q.; Liu, H.; Zhao, P.; Chen, L.; Liu, Y.; et al. Label-free immunosensors based on a novel multi-amplification signal strategy of TiO2-NGO/Au@Pd hetero-nanostructures. Biosens. Bioelectron. 2019, 127, 174–180. [Google Scholar] [CrossRef]
- Molina, R.; Escudero, J.M.; Augé, J.M.; Filella, X.; Foj, L.; Torné, A.; Lejarcegui, J.; Pahisa, J. HE4 a novel tumour marker for ovarian cancer: Comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases. Tumor Biol. 2011, 32, 1087–1095. [Google Scholar] [CrossRef]
- Li, J.; Dowdy, S.; Tipton, T.; Podratz, K.; Lu, W.-G.; Xie, X.; Jiang, S.-W. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev. Mol. Diagn. 2009, 9, 555–566. [Google Scholar] [CrossRef]
- Chang, X.; Ye, X.; Dong, L.; Cheng, H.; Cheng, Y.; Zhu, L.; Liao, Q.; Zhao, Y.; Tian, L.; Fu, T.; et al. Human Epididymis Protein 4 (HE4) as a Serum Tumor Biomarker in Patients with Ovarian Carcinoma. Int. J. Gynecol. Cancer 2011, 21, 852–858. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Li, N.; Zhang, Y.; Yan, T.; Ma, H.; Wei, Q. Sandwich-type electrochemical immunosensor for the detection of AFP based on Pd octahedral and APTES–M–CeO2–GS as signal labels. Biosens. Bioelectron. 2016, 79, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, N.; Ghaseminasab, K.; Hasanzadeh, M.; Kohansal, F.; Liu, Y.; Seidi, F. An Innovative Sandwich Type Biosensor towards Sensitive and Selective Monitoring of 2–Arachidonoylglycerol in Human Plasma Samples Using P (β–CD)–AuNPs–DDT as Amplificant Agent: A New Immuno-Platform for the Recognition of Endocannabinoids in Real Samples. Biosensors 2022, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Jia, Y.; Li, Y.; Wang, P.; Liu, Q.; Xu, Z.; Li, X.; Dong, Y. Sandwich-type electrochemical immunosensor for sensitive detection of CEA based on the enhanced effects of Ag NPs@CS spaced Hemin/rGO. Biosens. Bioelectron. 2018, 126, 785–791. [Google Scholar] [CrossRef]
- Xu, P.; Feng, W.; Wang, M.; Zhang, L.; Liang, G.; Jing, A. New Ultrasensitive Sandwich-Type Immunoassay of Dendritic Tri-Fan Blade-like PdAuCu Nanoparticles/Amine-Functionalized Graphene Oxide for Label-Free Detection of Carcinoembryonic Antigen. Micromachines 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, S.A.; Afsharan, H.; Khalilzadeh, B.; Ghahremani, M.H.; Carrara, S.; Omidi, Y. Gold and silver bio/nano-hybrids-based electrochemical immunosensor for ultrasensitive detection of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 141, 111439. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, H.; Xia, Z.; Li, S.; Deng, Y.; Liu, X. A sandwich-type electrochemical immunosensor using polythionine/AuNPs nanocomposites as label for ultrasensitive detection of carcinoembryonic antigen. Mater. Express 2019, 9, 444–450. [Google Scholar] [CrossRef]
- Tian, L.; Liu, L.; Li, Y.; Wei, Q.; Cao, W. Ultrasensitive sandwich-type electrochemical immunosensor based on trimetallic nanocomposite signal amplification strategy for the ultrasensitive detection of CEA. Sci. Rep. 2016, 6, 30849. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, M.; Mei, L.; Li, H. Ultra-Sensitive Electrochemical Determination of Carcinoembryonic Antigen by a Sandwich Immunosensor Graphene Oxide (GO)-Gold Substrate and a Silver-Coated Bovine Serum Albumin (BSA)-Platinum Nanocomposite on a Glassy Carbon Electrode (GCE). Anal. Lett. 2022, 55, 980–989. [Google Scholar] [CrossRef]
- Jing, A.; Xu, Q.; Feng, W.; Liang, G. An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene. Micromachines 2020, 11, 660. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Zhang, C.; Zhang, S.; Dong, Y. Enhanced peroxidase-like properties of Au@Pt DNs/NG/Cu2+ and application of sandwich-type electrochemical immunosensor for highly sensitive detection of CEA. Biosens. Bioelectron. 2018, 112, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, F.; Li, M.; Chen, L.; Dong, Y.; Wei, Q. Sandwich-type amperometric immunosensor using functionalized magnetic graphene loaded gold and silver core-shell nanocomposites for the detection of Carcinoembryonic antigen. J. Electroanal. Chem. 2017, 795, 1–9. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, D.; Chu, C.; Ma, Z. Label-assisted chemical adsorption triggered conversion of electroactivity of sensing interface to achieve the Ag/AgCl process for ultrasensitive detection of CA 19–9. Anal. Chim. Acta 2020, 1093, 43–51. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Carbohydrate antigen 19–9 electrochemical immunosensor based on 1D–MoS2 nanorods/LiNb3O8 and polyoxometalate-incorporated gold nanoparticles. Microchem. J. 2021, 170, 106643. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lv, H.; Feng, J.; Gao, Z.; Wang, P.; Dong, Y.; Liu, Q.; Zhao, Z. Sandwich-type electrochemical immunosensor based on Au@Ag supported on functionalized phenolic resin microporous carbon spheres for ultrasensitive analysis of α-fetoprotein. Biosens. Bioelectron. 2018, 106, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Z.; Wang, G. A novel signal amplification strategy electrochemical immunosensor for ultra-sensitive determination of p53 protein. Bioelectrochemistry 2021, 137, 107647. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N.; Machado, S.A. A sandwich-type electrochemical immunosensor based on Au-rGO composite for CA15-3 tumor marker detection. Microchim. Acta 2022, 189, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, S.A.; Khalilzadeh, B.; Pakchin, P.S.; Saber, R.; Ghahremani, M.H.; Omidi, Y. A highly sensitive and reliable detection of CA15-3 in patient plasma with electrochemical biosensor labeled with magnetic beads. Biosens. Bioelectron. 2018, 122, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Nara, S. Dual gold nanostructure-based electrochemical immunosensor for CA125 detection. Appl. Nanosci. 2018, 8, 1843–1853. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Ghanbari, H.; Saber, R.; Omidi, Y. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68–74. [Google Scholar] [CrossRef]
- Iyer, M.S.; Fu-Ming, W.; Rajangam, I. Electrochemical detection of CA125 using thionine and gold nanoparticles supported on heteroatom-doped graphene nanocomposites. Appl. Nanosci. 2021, 11, 2167–2180. [Google Scholar] [CrossRef]
- Shamsipur, M.; Emami, M.; Farzin, L.; Saber, R. A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens. Bioelectron. 2018, 103, 54–61. [Google Scholar] [CrossRef]
- Ma, Q. Dual-Mode Electrochemical Immunosensor Based on Au@Ag NRs as Double Signal Indicator for Sensitive Detection of HER2. J. Electrochem. Soc. 2021, 168, 027515. [Google Scholar] [CrossRef]
- Yola, M.L. Sensitive sandwich-type voltammetric immunosensor for breast cancer biomarker HER2 detection based on gold nanoparticles decorated Cu-MOF and Cu2ZnSnS4 NPs/Pt/g-C3N4 composite. Microchim. Acta 2021, 188, 78. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M. A Sandwich-Type Electrochemical Immunosensor Using Antibody-Conjugated Pt-Doped CdTe QDs as Enzyme-Free Labels for Sensitive HER2 Detection Based on a Magnetic Framework. Front. Chem. 2022, 2022, 501. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cao, L.; Dong, H.; Tan, Z.; Liu, Q.; Zhang, W.; Zhao, P.; Li, Y.; Liu, Y.; Dong, Y. Sensitive amperometric immunosensor with improved electrocatalytic Au@Pd urchin-shaped nanostructures for human epididymis specific protein 4 antigen detection. Anal. Chim. Acta 2019, 1069, 117–125. [Google Scholar] [CrossRef]
- Fan, L.; Yan, Y.; Guo, B.; Zhao, M.; Li, J.; Bian, X.; Wu, H.; Cheng, W.; Ding, S. Trimetallic hybrid nanodendrites and magnetic nanocomposites-based electrochemical immunosensor for ultrasensitive detection of serum human epididymis protein 4. Sens. Actuators B Chem. 2019, 296, 126697. [Google Scholar] [CrossRef]
- Cadkova, M.; Kovarova, A.; Dvorakova, V.; Metelka, R.; Bilkova, Z.; Korecka, L. Electrochemical quantum dots-based magneto-immunoassay for detection of HE4 protein on metal film-modified screen-printed carbon electrodes. Talanta 2018, 182, 111–115. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Wei, D.; Wang, X.; Yan, T.; Du, B.; Wei, Q. Label-free photoelectrochemical immunosensor for carcinoembryonic antigen detection based on g-C3N4 nanosheets hybridized with Zn0.1Cd0.9S nanocrystals. Sens. Actuators B Chem. 2018, 256, 812–819. [Google Scholar] [CrossRef]
- Fan, G.-C.; Zhu, H.; Du, D.; Zhang, J.-R.; Zhu, J.-J.; Lin, Y. Enhanced photoelectrochemical immunosensing platform based on CdSeTe@ CdS: Mn core-shell quantum dots-sensitized TiO2 amplified by CuS nanocrystals conjugated signal antibodies. Anal. Chem. 2016, 88, 3392–3399. [Google Scholar] [CrossRef]
- Nie, G.; Tang, Y.; Zhang, B.; Wang, Y.; Guo, Q. Label-free photoelectrochemical immunosensing platform for detection of carcinoembryonic antigen through photoactive conducting poly(5-formylindole) nanocomposite. Biosens. Bioelectron. 2018, 116, 60–66. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Zhao, F.; Zeng, B. LED visible-light driven label-free photoelectrochemical immunosensor based on WO3/Au/CdS photocatalyst for the sensitive detection of carcinoembryonic antigen. Electrochim. Acta 2019, 297, 372–380. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Wang, Z.; Wang, Y.; Ye, X.; Wong, W.; Li, C.; Sun, D. Gold/WS2 nanocomposites fabricated by in-situ ultrasonication and assembling for photoelectrochemical immunosensing of carcinoembryonic antigen. Microchim. Acta 2018, 185, 570. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Kuang, X.; Wang, Z.; Wei, Q. A network signal amplification strategy of ultrasensitive photoelectrochemical immunosensing carcinoembryonic antigen based on CdSe/melamine network as label. Biosens. Bioelectron. 2016, 85, 764–770. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, J.; Li, W.; Ma, C.; Chen, S.; Li, H.; Hong, C. Bimetallic PtCu nanoparticles supported on molybdenum disulfide-functionalized graphitic carbon nitride for the detection of carcinoembryonic antigen. Microchim. Acta 2020, 187, 538. [Google Scholar] [CrossRef]
- Chen, G.; Qin, Y.; Jiao, L.; Huang, J.; Wu, Y.; Hu, L.; Gu, W.; Xu, D.; Zhu, C. Nanozyme-Activated Synergistic Amplification for Ultrasensitive Photoelectrochemical Immunoassay. Anal. Chem. 2021, 93, 6881–6888. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhang, Y.; Wang, Q.; Ren, X.; Wu, D.; Wei, Q. Photoelectrochemical Immunosensor for Detection of Carcinoembryonic Antigen Based on 2D TiO2 Nanosheets and Carboxylated Graphitic Carbon Nitride. Sci. Rep. 2016, 6, 27385. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Xing, B.; Zhang, T.; Khan, M.S.; Wu, D.; Wei, Q. Label-free photoelectrochemical immunoassay for CEA detection based on CdS sensitized WO3@BiOI heterostructure nanocomposite. Biosens. Bioelectron. 2018, 99, 493–499. [Google Scholar] [CrossRef]
- Liu, K.; Deng, H.; Wang, Y.; Cheng, S.; Xiong, X.; Li, C. A sandwich-type photoelectrochemical immunosensor based on ReS2 nanosheets for high-performance determination of carcinoembryonic antigen. Sens. Actuators B Chem. 2020, 320, 128341. [Google Scholar] [CrossRef]
- Liu, X.-P.; Chen, J.-S.; Mao, C.-J.; Jin, B.-K. A label-free photoelectrochemical immunosensor for carcinoembryonic antigen detection based on a gC 3 N 4/CdSe nanocomposite. Analyst 2021, 146, 146–155. [Google Scholar] [CrossRef]
- Zang, Y.; Ju, Y.; Hu, X.; Zhou, H.; Yang, Z.; Jiang, J.; Xue, H. WS2 nanosheets-sensitized CdS quantum dots heterostructure for photoelectrochemical immunoassay of alpha-fetoprotein coupled with enzyme-mediated biocatalytic precipitation. Analyst 2018, 143, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, F.; Li, H.; Li, Z.; Kang, Q.; Shen, D. A ratiometric photoelectrochemical immunosensor based on gC 3 N 4@ TiO 2 NTs amplified by signal antibodies-Co 3 O 4 nanoparticle conjugates. Analyst 2018, 143, 5030–5037. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Luo, M.; Chen, L.; Chen, J.; Li, C. A photoelectrochemical immunosensor for detection of α-fetoprotein based on Au-ZnO flower-rod heterostructures. Appl. Surf. Sci. 2017, 402, 429–435. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.-C. A novel signal-on photoelectrochemical immunosensor for detection of alpha-fetoprotein by in situ releasing electron donor. Biosens. Bioelectron. 2017, 98, 155–160. [Google Scholar] [CrossRef] [PubMed]
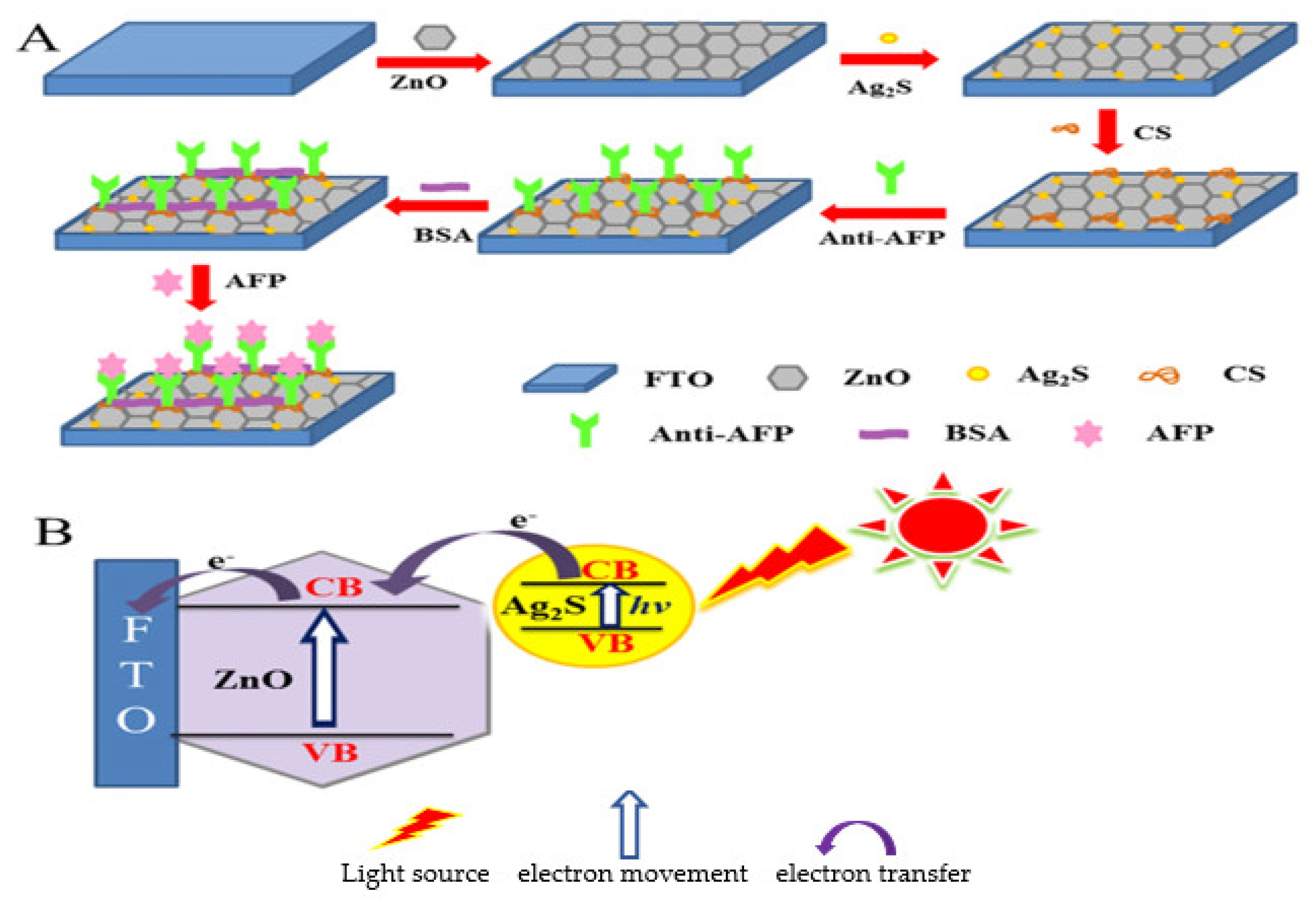
- Jiang, Y.; Liu, D.; Yang, Y.; Xu, R.; Zhang, T.; Sheng, K.; Song, H. Photoelectrochemical detection of alpha-fetoprotein based on ZnO inverse opals structure electrodes modified by Ag2S nanoparticles. Sci. Rep. 2016, 6, 38400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Zhou, D.; Shao, L.; Chen, X.; Song, H. Label-free photoelectrochemical biosensor for alpha-fetoprotein detection based on Au/CsxWO3 heterogeneous films. Talanta 2021, 225, 122074. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, H.; Zhang, Y.; Lv, Y.; Li, H.; Liu, S.; Shen, Y.; Zhang, Y. Metal-Free All-Carbon Nanohybrid for Ultrasensitive Photoelectrochemical Immunosensing of alpha-Fetoprotein. ACS Sens. 2018, 3, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhao, J.; Zhou, Q.; Pan, D.; Zhang, Y.; Zhang, Y.; Shen, Y. Boosting the Sensitivity of a Photoelectrochemical Immunoassay by Using SiO2@polydopamine Core-Shell Nanoparticles as a Highly Efficient Quencher. ACS Appl. Nano Mater. 2019, 2, 1579–1588. [Google Scholar] [CrossRef]
- Ge, S.; Liang, L.; Lan, F.; Zhang, Y.; Wang, Y.; Yan, M.; Yu, J. Photoelectrochemical immunoassay based on chemiluminescence as internal excited light source. Sens. Actuators B Chem. 2016, 234, 324–331. [Google Scholar] [CrossRef]
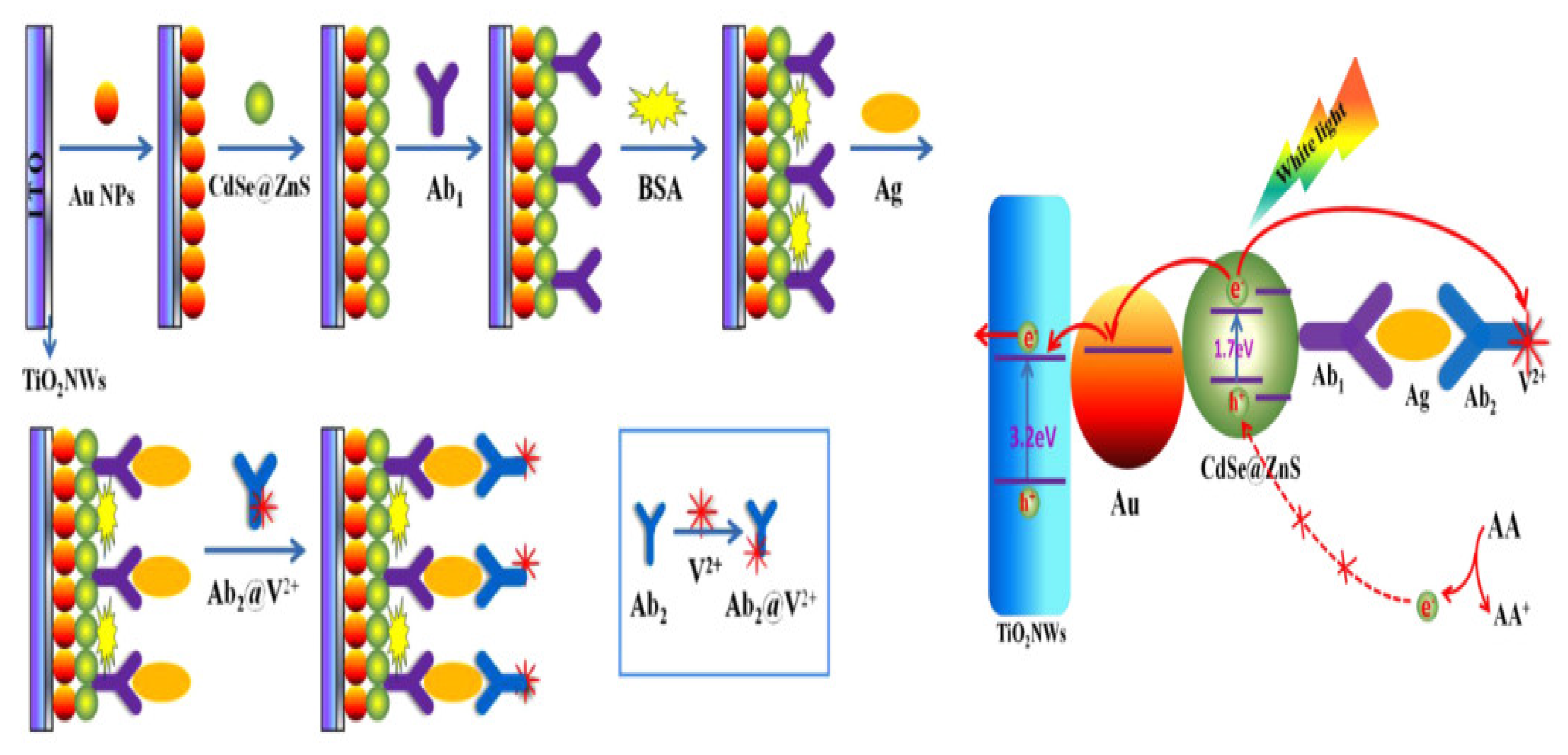
- Zhu, H.; Fan, G.-C.; Abdel-Halim, E.; Zhang, J.-R.; Zhu, J.-J. Ultrasensitive photoelectrochemical immunoassay for CA19-9 detection based on CdSe@ ZnS quantum dots sensitized TiO2NWs/Au hybrid structure amplified by quenching effect of Ab2@ V2+ conjugates. Biosens. Bioelectron. 2016, 77, 339–346. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Xi, J.; Zhao, F.; Zeng, B. In situ formation of inorganic/organic heterojunction photocatalyst of WO3/Au/polydopamine for immunoassay of human epididymal protein 4. Electrochim. Acta 2020, 331, 135350. [Google Scholar] [CrossRef]
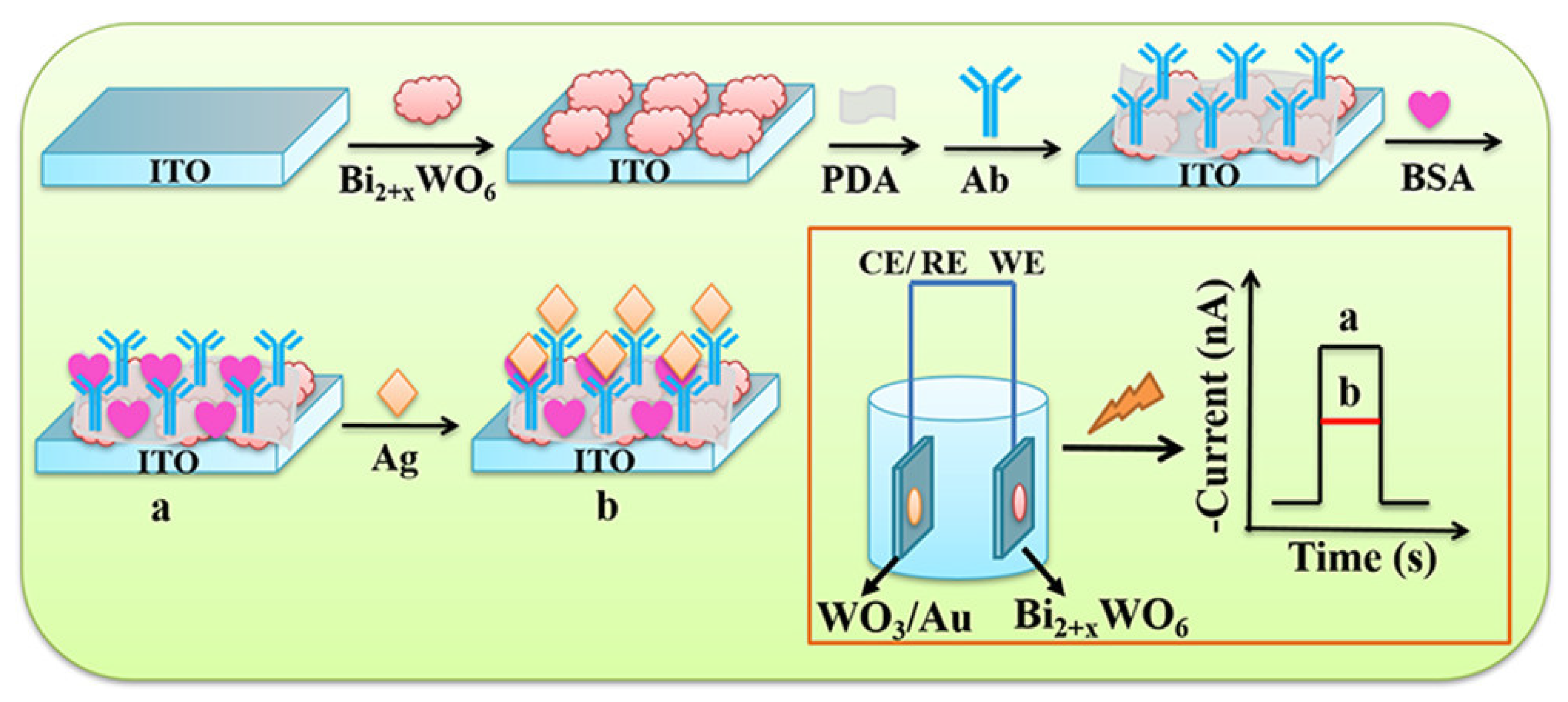
- Zhang, B.; Wang, H.; Xi, J.; Zhao, F.; Zeng, B. Novel Bi2+xWO6 p–n Homojunction Nanostructure: Preparation, Characterization, and Application for a Self-Powered Cathodic Photoelectrochemical Immunosensor. ACS Sens. 2020, 5, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Bery, N.; Tardy, C.; Ligat, L.; Favre, G.; Rabbitts, T.H.; Olichon, A. Selection and Characterization of a Nanobody Biosensor of GTP-Bound RHO Activities. Antibodies 2019, 8, 8. [Google Scholar] [CrossRef]
- Simões, B.; Guedens, W.J.; Keene, C.; Kubiak-Ossowska, K.; Mulheran, P.; Kotowska, A.M.; Scurr, D.J.; Alexander, M.R.; Broisat, A.; Johnson, S.; et al. Direct Immobilization of Engineered Nanobodies on Gold Sensors. ACS Appl. Mater. Interfaces 2021, 13, 17353–17360. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-J.; Wang, G.-Q.; Zheng, J.-Y.; Wang, A.-J.; Mei, L.-P.; Feng, J.-J. Dual-Mode Photoelectrochemical and Electrochemical Immunosensors for Human Epididymis Protein 4 Based on SPR-Promoted Au Nanoparticle/CdS Nanosheet Heterostructures. ACS Appl. Nano Mater. 2022, 2022, 14934–14941. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Multiplexed electrochemical immunosensors for clinical biomarkers. Sensors 2017, 17, 965. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, H.; Siaj, M. Label-free multiplex electrochemical immunosensor for early diagnosis of lysosomal storage disorders. Sci. Rep. 2022, 12, 9334. [Google Scholar] [CrossRef]
- Kuntamung, K.; Jakmunee, J.; Ounnunkad, K. A label-free multiplex electrochemical biosensor for the detection of three breast cancer biomarker proteins employing dye/metal ion-loaded and antibody-conjugated polyethyleneimine-gold nanoparticles. J. Mater. Chem. B 2021, 9, 6576–6585. [Google Scholar] [CrossRef]
- Cotchim, S.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Multiplexed label-free electrochemical immunosensor for breast cancer precision medicine. Anal. Chim. Acta 2020, 1130, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Salimi, A.; Hallaj, R. Magnetoimmunosensor for simultaneous electrochemical detection of carcinoembryonic antigen and α-fetoprotein using multifunctionalized Au nanotags. J. Electroanal. Chem. 2018, 811, 8–15. [Google Scholar] [CrossRef]
- Rong, Q.; Feng, F.; Ma, Z. Metal ions doped chitosan-poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef] [PubMed]
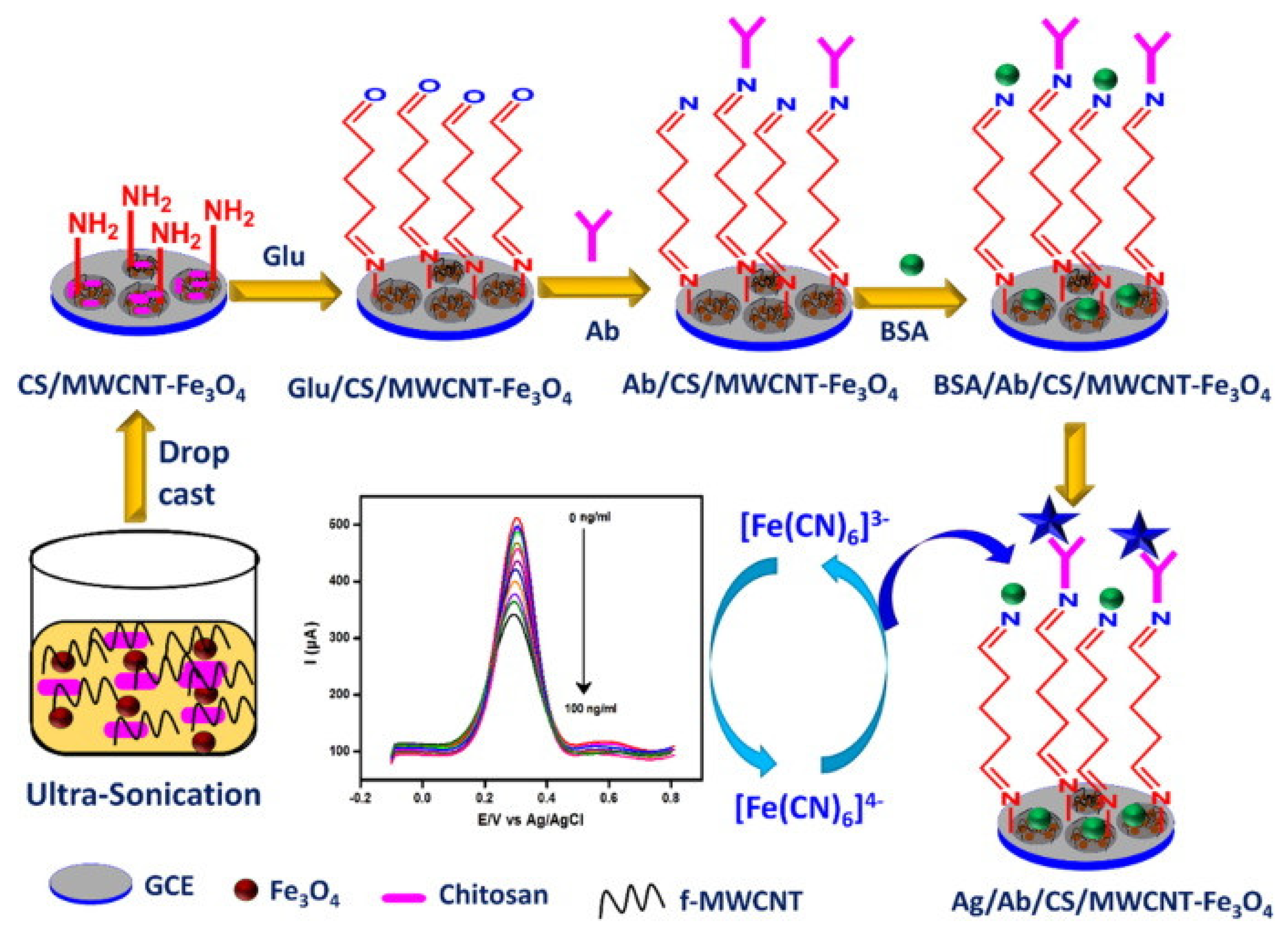
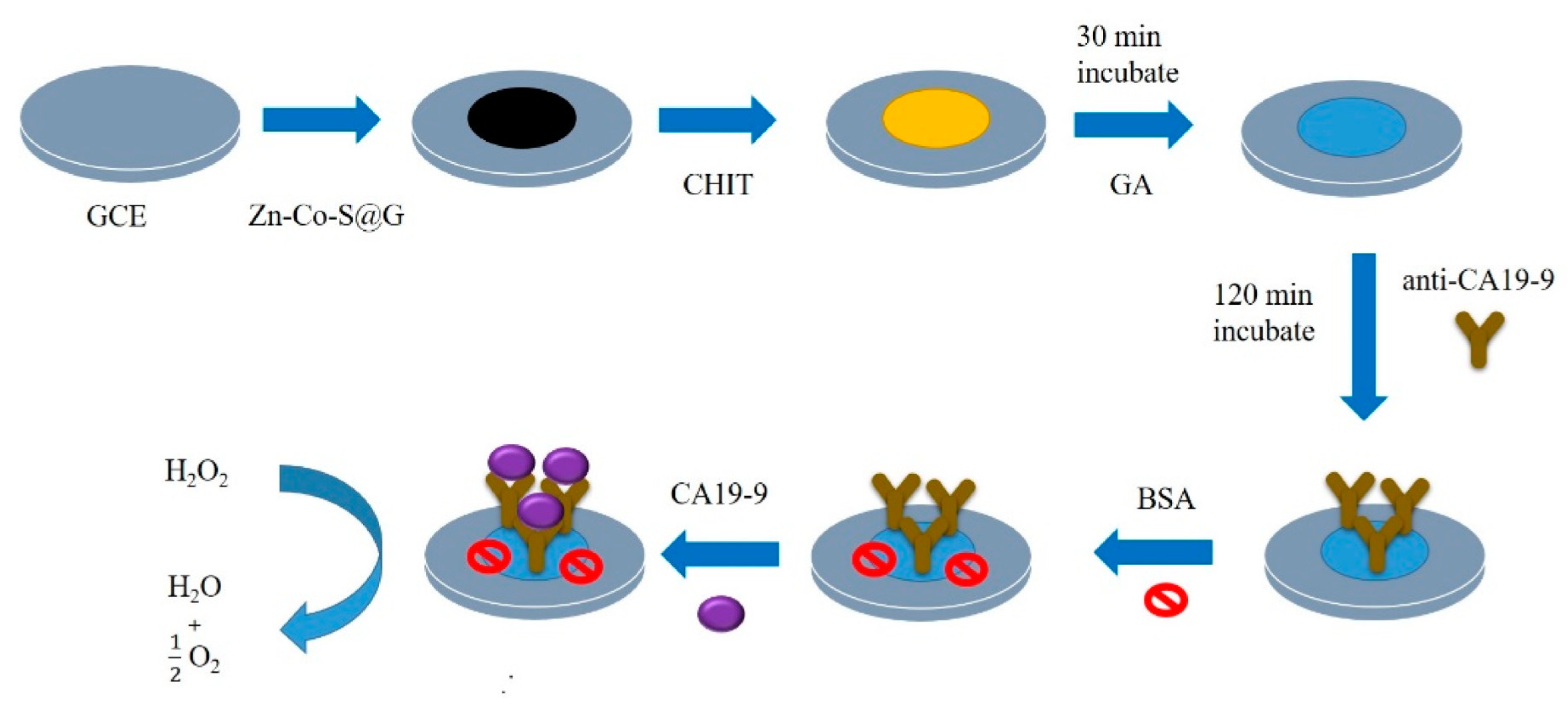

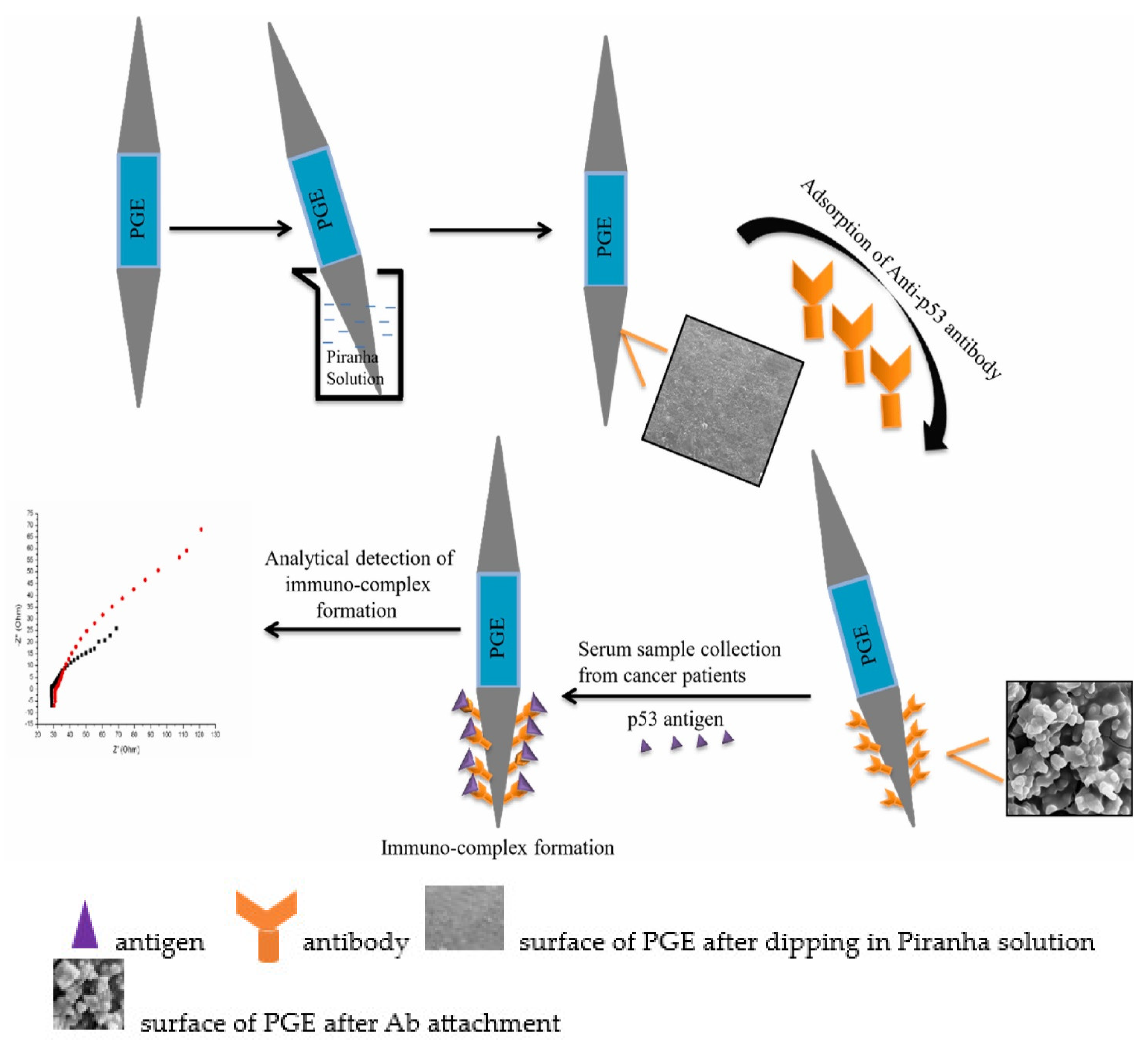
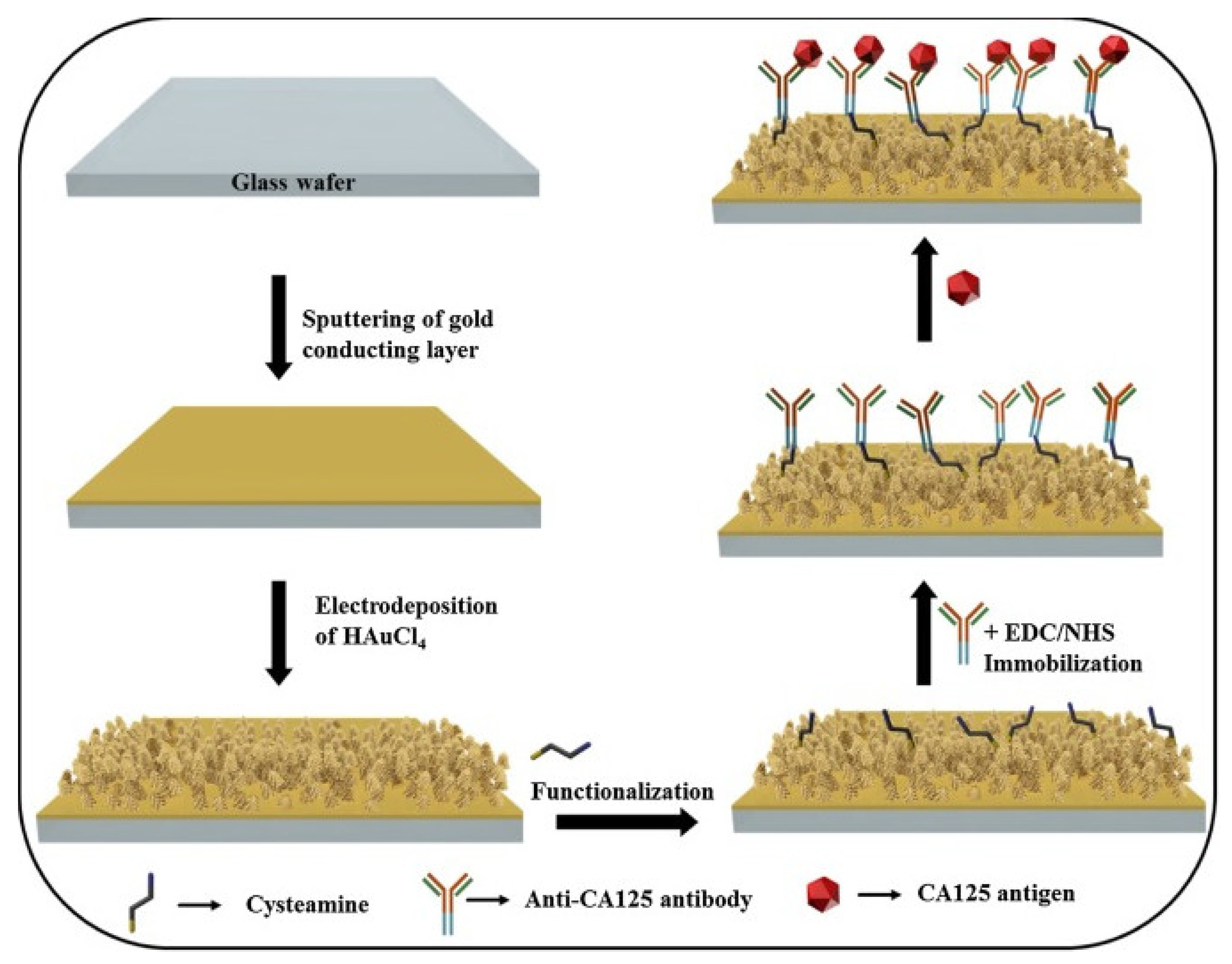

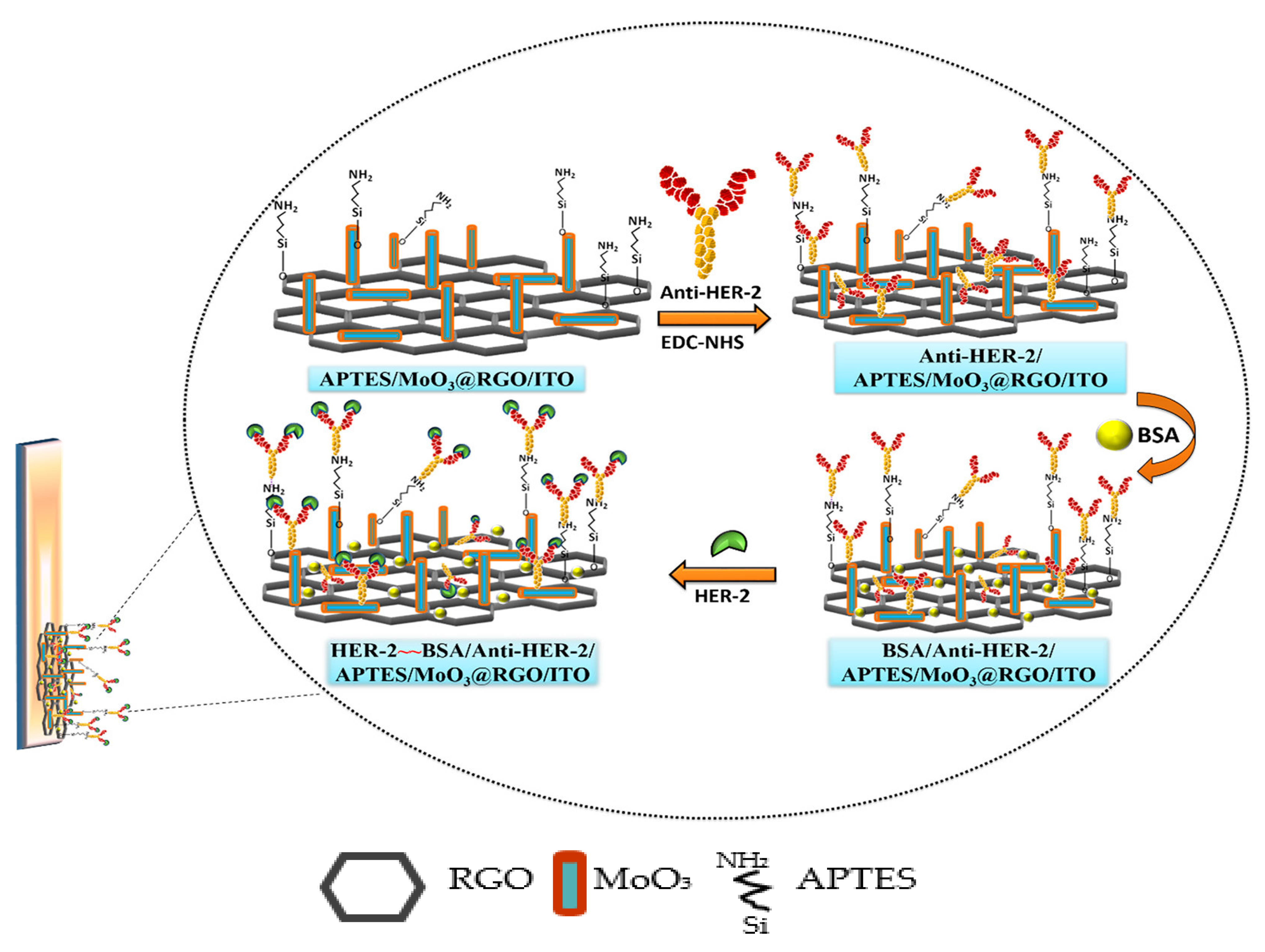
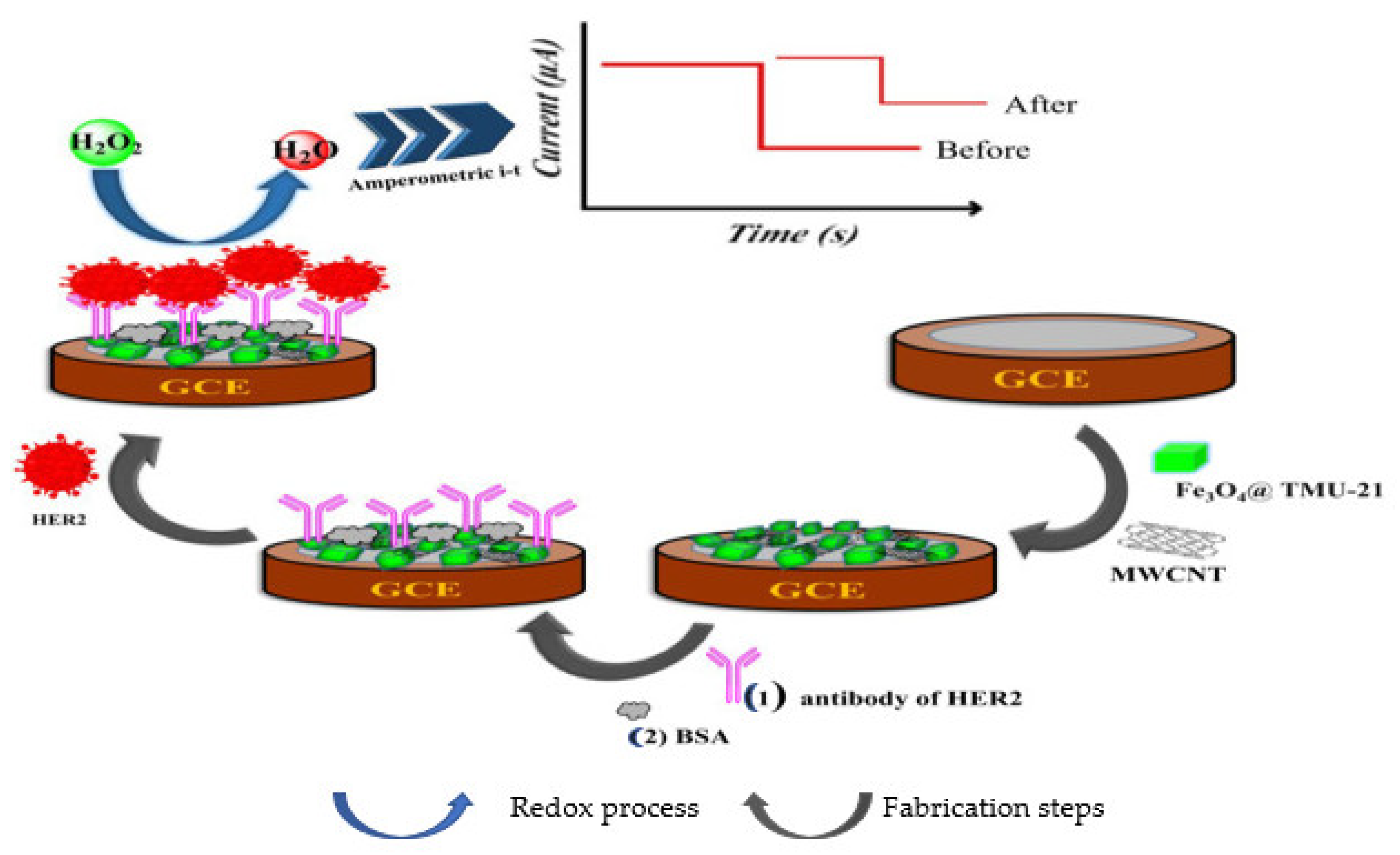


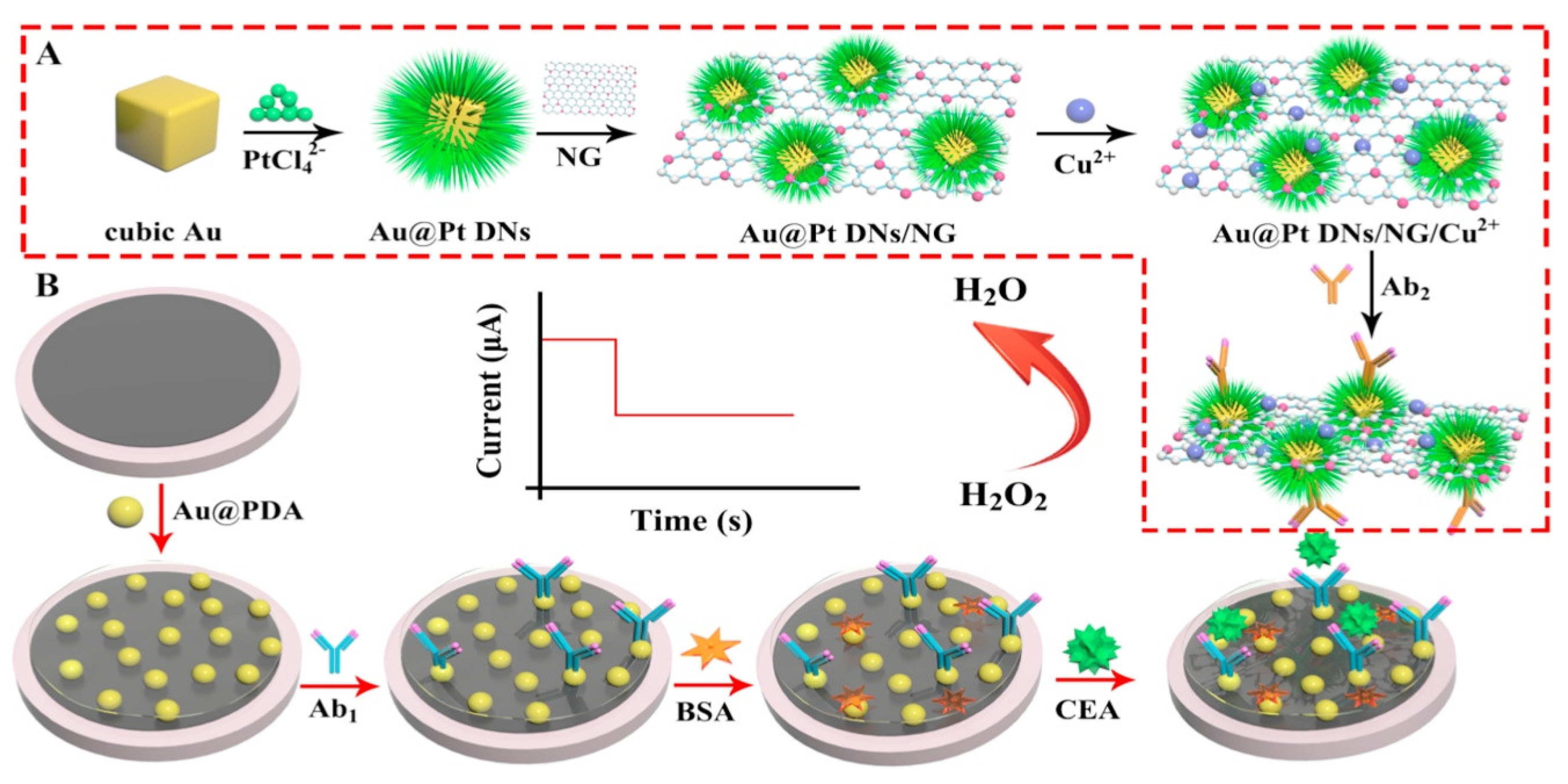

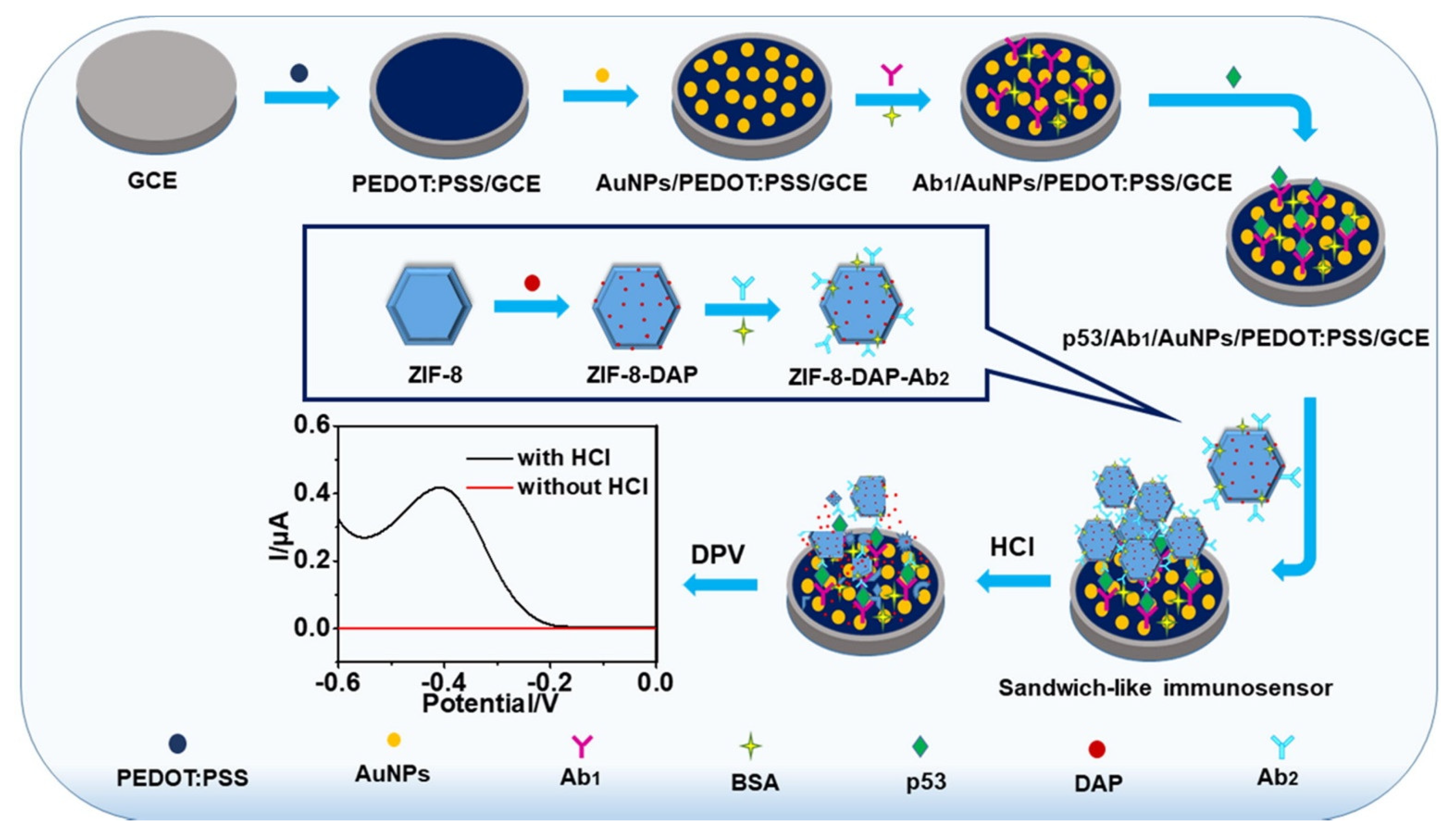
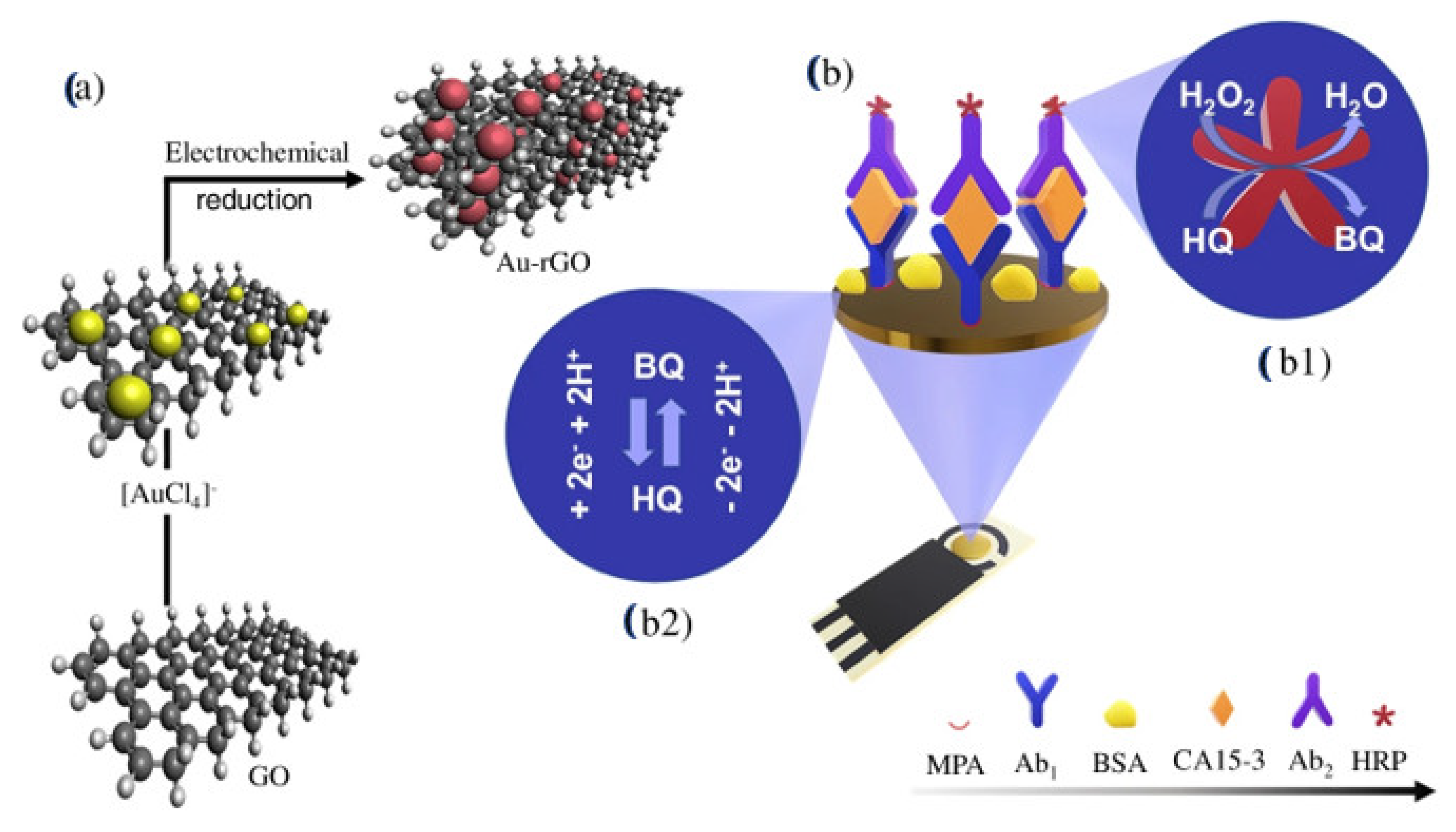
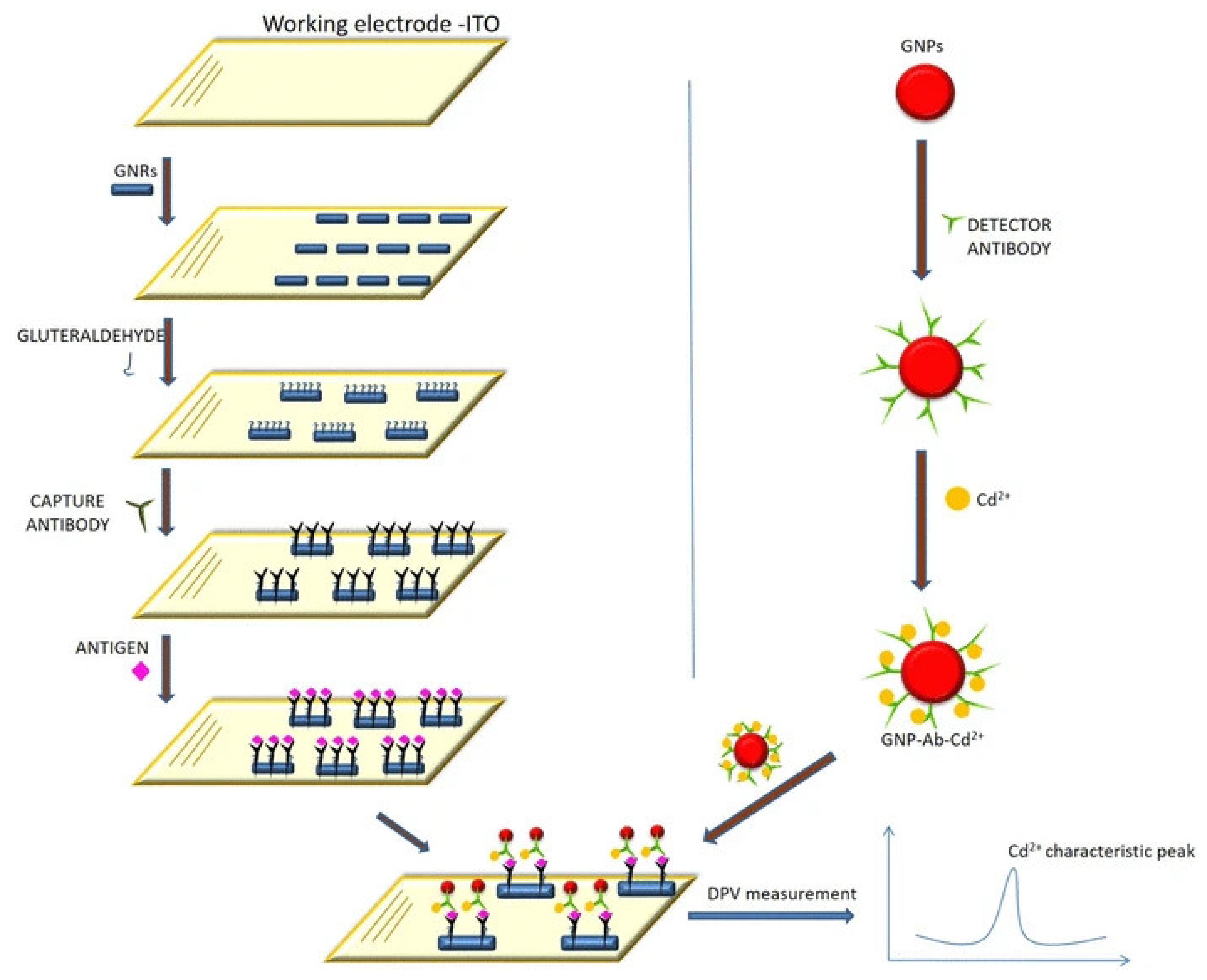
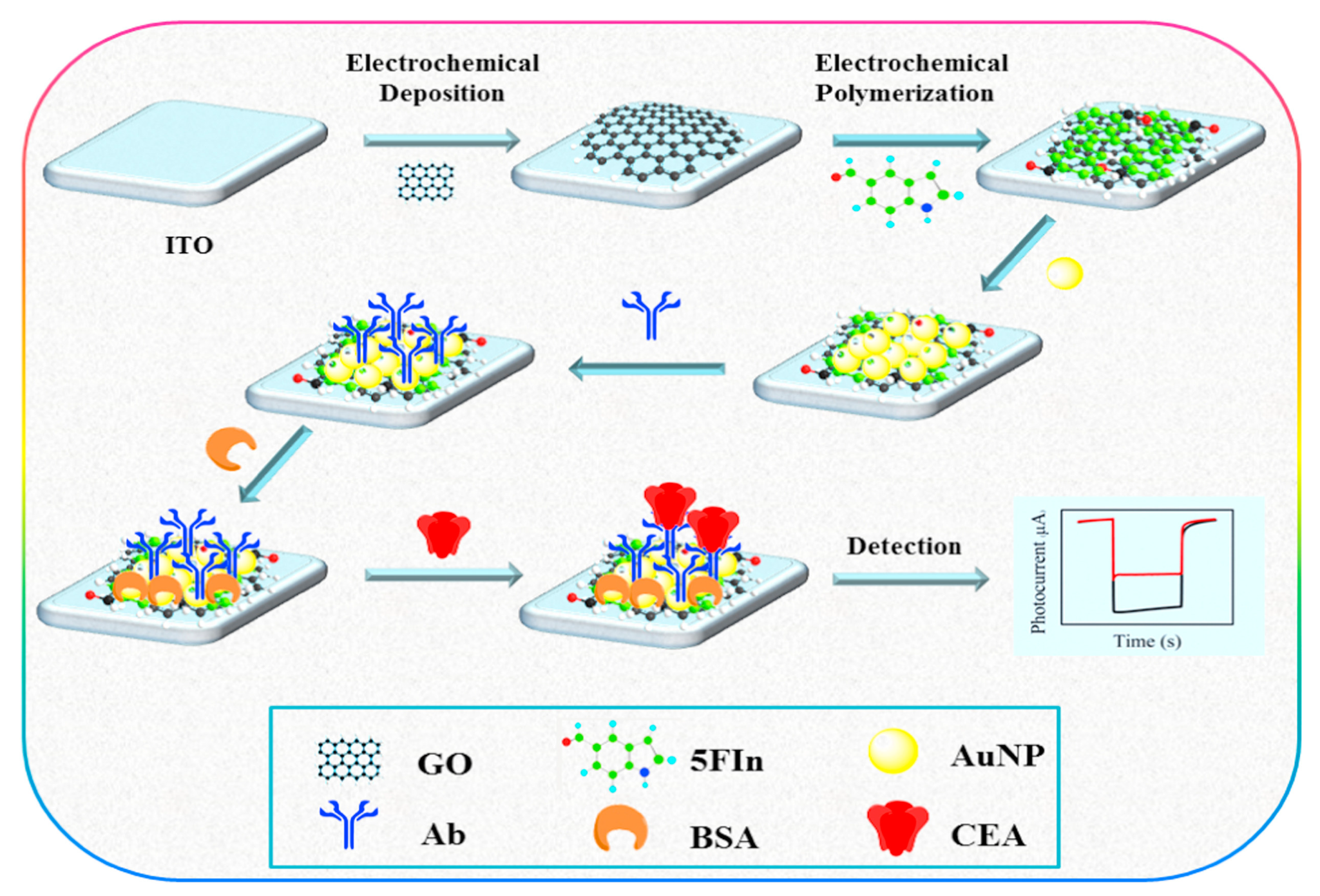
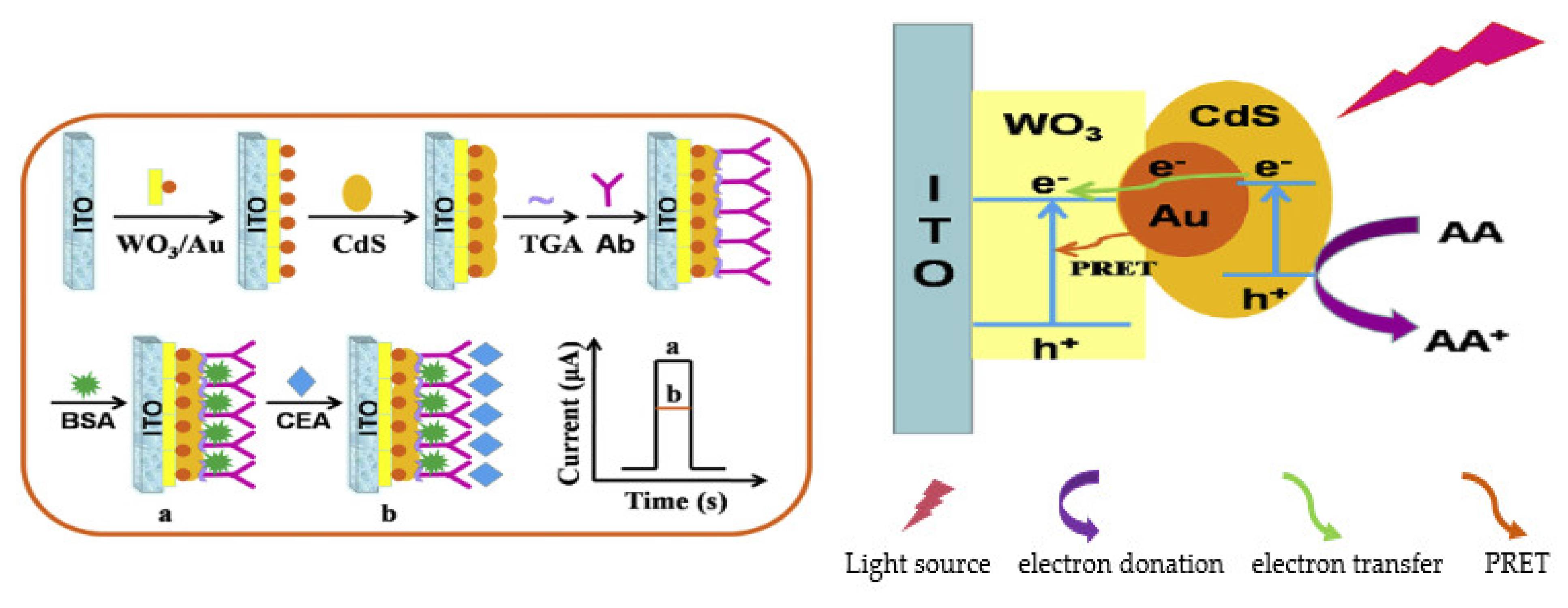



| Biomarkers | Type of Sensor | Electroactive/ Photoactive Materials/Substrate | Label | LOD | Linear Concentration | Ref. |
|---|---|---|---|---|---|---|
| CEA | EC | AuNPs | AgNPs@CS–hemin/rGO–Ab2 | 6.7 × 10−6 ng/mL | 2 × 10−5 ng/mL to 200 ng/mL | [107] |
| EC | Au/PDA | Ab2/PdAuCu NPs/Fc–NH2–GO | 7 × 10−5 ng/mL | 1 × 10−4 to 200 ng/mL | [108] | |
| EC | T–GO/AuNPs | HRP–(strp–AgNPs)–mAb | 7.5 × 10−5 ng/mL | 1 × 10−4 to 5 × 10−3 ng/mL | [109] | |
| EC | rGO/CS/Au NPs | PTh–Au | 14.71 × 10−5 ng/mL. | 0.3 to 30 ng/mL | [110] | |
| EC | CD–NGs | NiAuPt–NGs | 27 × 10−5 ng/mL | 0.001 to 100 ng/mL | [111] | |
| EC | GO–AuNPs | Ag@BSA–Pt/Ab2 | 76 × 10−5 ng/mL | 0.005 to 100 ng/mL | [112] | |
| EC | 3DPt/HGO | Au–HRP–Ab2 | 0.0006 ng/mL | 0.001–150 ng/mL | [113] | |
| EC | Au@PDA | Au@PtDNs/NG/Cu2+–Ab2 | 16.7 × 10−5 ng/mL | 5 × 10−4 to 50 ng/mL | [114] | |
| EC | Au NPs | Au@Ag/Fe3O4–GS/Ni2+ | 69.7 × 10−6 ng/mL | 1 × 10−4 to 100 ng/mL | [115] | |
| EC | AuNPs/NB–ERGO | Label–free | 0.00045 ng/mL | 0.001 to 40 ng/mL | [39] | |
| EC | Ag/MoS2/rGO | Label–free | 1.6 × 10−6 ng/mL | 1 × 10−5 to 100 ng/mL | [40] | |
| EC | Gz–PYSE/PE | Label–free | 42.5 × 10−4 ng/mL | – | [41] | |
| EC | AuNPs/CNOs/SWCTNs/CS | Label–free | 1 × 10−4 ng/mL | 1 × 10−4 ng/mL to 400 ng/mL | [42] | |
| EC | NH2–G/Thi/AuNPs | Label–free | 1 × 10−2 ng/mL | 5 × 10−2 to 500 ng/mL | [37] | |
| EC | Au@Bi2MoO6 | Label–free | 3 × 10−4 ng/mL | 1 × 10−3 to 1 × 103 ng/mL | [43] | |
| EC | Cu2S/Pd/CuO | Label–free | 33.1 × 10−6 ng/mL | 1 × 10−4 to 100 ng/mL | [44] | |
| EC | EG/CNDTs@PPI | Label–free | 14.5 × 10−4 ng/mL | 0.005 to 300 ng/mL | [45] | |
| EC | MWCNTs/GNPs/HNF | Label–free | 0.09 ng/mL | 0.4–125 ng/mL | [46] | |
| EC | mAb/POct pAb/POct | Label–free | 10.8 fM 9.08 fM | – – | [38] | |
| EC | PtPd/N–GQDs@Au | Label–free | 2 × 10−6 ng/mL | 5 × 10−6 to 50 ng/mL | [48] | |
| EC | Au–Ag/rGO@PDA | Label–free | 28.6 × 10−5 ng/mL | 0.001 to 80 ng/mL | [50] | |
| EC | Au@SiO2NPs | Label–free | 0.01 ng/mL | 0.5 to 10 ng/mL | [47] | |
| EC | rGO/MoS2@PANI | Label–free | 3 × 10−4 ng/mL | 0.001–80 ng/mL | [51] | |
| PEC | Zn0.1 Cd0.9S/g–C3N4 | Label–free | 14 × 10−4 ng/mL | 5 × 10−3 to 20 ng/mL | [133] | |
| PEC | TiO2/CdSeTe@CdS:Mn | Ab2–CuS | 16 × 10−5 ng/mL | 5 × 10−4 to 100 ng/mL | [134] | |
| PEC | P5FLn/erGO | Label–free | 14 × 10−5 ng/mL | 5 × 10−4 to 50 ng/mL | [135] | |
| PEC | WO3/Au/CdS | Label–free | 1 × 10−3 ng/mL | 0.01 to 10 ng/mL | [136] | |
| PEC | Au/WS2 | Label–free | 5 × 10−4 ng/mL | 1 × 10−3 to 40 ng/mL | [137] | |
| PEC | Au–TiO2 | CdSe/melamine | 5 × 10−3 ng/mL | 5 × 10−3 to 1000 ng/mL | [138] | |
| PEC | AuNPs | MoS2/g–C3N4–PtCu–Ab2 | 33 × 10−6 ng/mL | 1 × 10−4 to 80 ng/mL | [139] | |
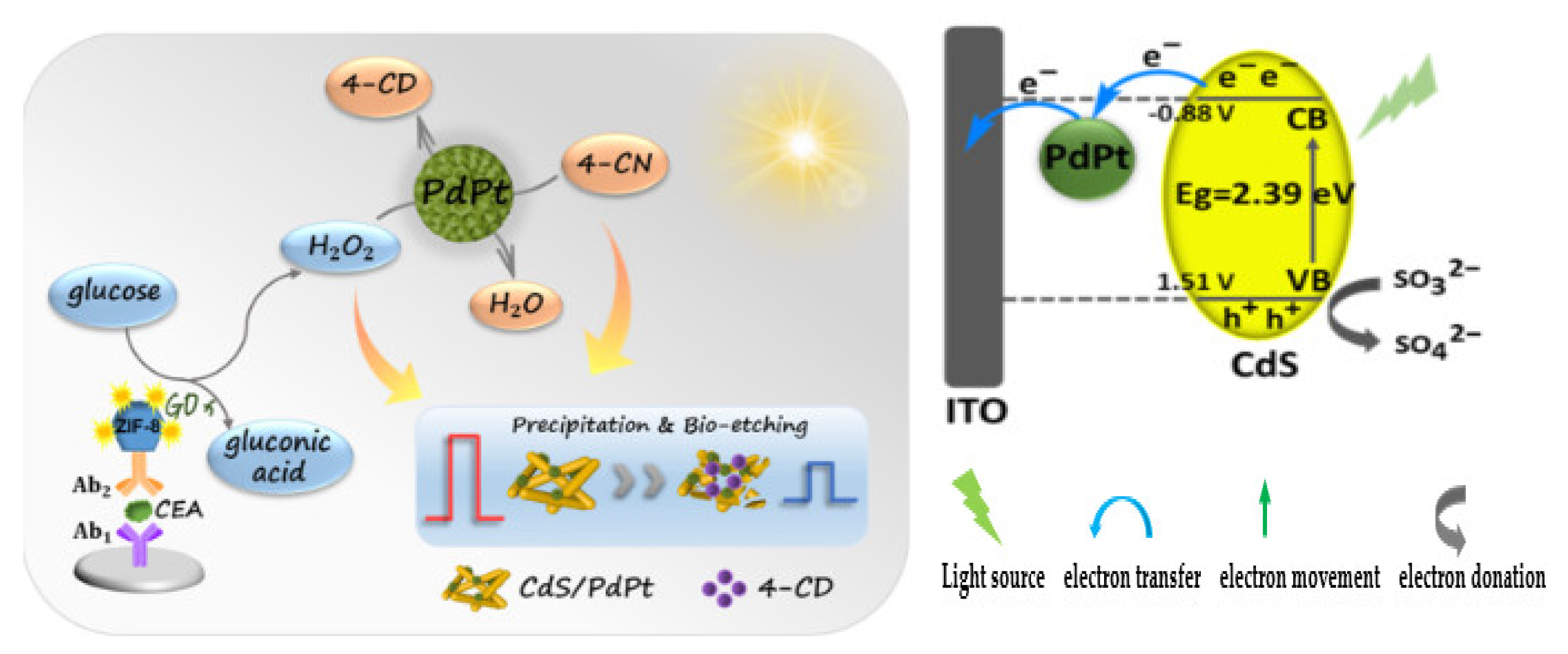
| PEC | CdS/PdPt | ZIF–8@GOx–Ab2 | 21 × 10−5 ng/mL | 1 × 10−3 to 5 ng/mL | [140] | |
| PEC | C3N4–BiOCl/PDDA | CuO–Ab2 | 1 × 10−4 ng/mL | 1 × 10−4 to 10 ng/mL | [27] | |
| PEC | g–C3N4/TiO2 | Label–free | 21 × 10−4 ng/mL | 0.01 to 10 ng/mL | [141] | |
| PEC | WO3@BiOI@CdS | Label–free | 32 × 10−4 ng/mL | 0.01 to 50 ng/mL | [142] | |
| PEC | Cu:TiO2/g–C3N4 | ALP–Au–Ab2 | 1 × 10−3 ng/mL | 0.5 × 10−3 –1000 ng/mL | [28] | |
| PEC | 2D–ReS2 | ALP–Ab2 | 46.8 × 10−5 ng/mL | 5 × 10−4 to 10.0 ng/mL | [143] | |
| PEC | Au@TiO2 | CdSe@BiVO4–Ab2 | 5 × 10−4 ng/mL | 0.01 to 50 ng/mL | [32] | |
| PEC | g–C3N4/CdSe –PDDA | Label–free | 0.21 ng/mL | 10 to 1 × 105 ng/ml | [144] | |
| CA 19–9 | EC | CS–MWCNT–Fe3O4 | Label–free | 16.3 × 10−5 ng/ml | 1 × 10−3 to 100 ng/mL | [55] |
| EC | CeO2/FeOx@mC | Label–free | 1 × 10−5 U/mL | 1 × 10−4 to 10 U/mL | [56] | |
| EC | GA/CHIT/Zn–Co–S@G | Label–free | 0.82 U/mL | 6.3 to 300 U/mL | [53] | |
| EC | Au NPs/Au NPs@PThi | Label–free | 0.26 U/mL | 6.5 to 520 U/mL | [52] | |
| EC | CB–PEL | Label–free | 0.07 U/mL | 0.01 to 40 U/mL | [57] | |
| EC | CNO/GO | Label–free | 0.12 U/mL | 0.3–100 U/mL | [58] | |
| EC | GO–MA | PDA–Ag NPs | 3.2 × 10−5 U/mL | 1 × 10−4 to 100 U/mL | [116] | |
| EC | MPA/ME/Au | Label–free | 0.01 U/mL | 0.05–500 U/mL | [60] | |
| EC | PEI/MWCNTs/NHS–EDC | Label–free | 0.35 U/mL | – | [59] | |
| EC | AuNPs@POM | 1D MoS2 NRs/LNO | 0.030 µU/mL | – | [117] | |
| PEC | TiO2 NWs/Au/CdSe@ZnS | Ab2@V2+ | 0.0039 U/mL | 0.01 to 200 U/mL | [154] | |
| AFP | EC | PDA–N–MWCNT | NH2 –GS/Au@Pt | 5 × 10−5 ng/mL | 1 × 10−4 to 10 ng/mL | [14] |
| EC | GaN–PDA/Au NPs | Label–free | 3 × 10−3 ng/mL | 0.01 to 100 ng/mL | [66] | |
| EC | TB–Au–Fe3O4–rGO | Label–free | 27 × 10−7 ng/mL | 1.0 × 10−5 to 10.0 ng/mL | [67] | |
| EC | Au NPs | Au@Ag/PDA–PR–MCS–Ab2 | 6.7 × 10−6 ng/mL | 2 × 10−5 to 100 ng/mL | [118] | |
| EC | Au NPs | Pd/APTES–M–CeO2 –GS | 33 × 10−6 ng/mL | 1 × 10−4 to 50 ng/mL | [105] | |
| EC | GO–MB–Au NPs | AuC–HRP–anti–AFP | 15 × 10−4 ng/mL | 5 × 10−3 to 20 ng/mL | [65] | |
| PEC | WS2/CdS | HRP–Ab2 | 43 × 10−5 ng/mL | 1 × 10−3 to 20 ng/mL | [145] | |
| PEC | TiO2 NTs/g–C3N4 | Co3O4 NPs–Ab2 | 2 × 10−4 ng/mL | 4 × 10−4 to 40 ng/mL | [146] | |
| PEC | Au–ZnO | Label–free | 56 × 10−5 ng/mL | 5 × 10−3 to 50 ng/mL | [147] | |
| PEC | CdSe QDs | Bio–anti–AFP/SA/Bio–APOAA | 31 × 10−5 ng/mL | 1 × 10−3 to 1 × 103 ng/mL | [148] | |
| PEC | ZnO/Ag2S/CS | Label–free | 8 × 10−3 ng/mL | 0.05 to 200 ng/mL | [149] | |
| PEC | AuCsxWO3/CS | Label–free | 7 × 10−3 ng/mL | 0.01 to 500 ng/mL | [150] | |
| PEC | GO | Ab2@AC60–Gr–GO | 54 × 10−5 ng/mL | 1 × 10−3 ng/mL to 100 ng/mL | [151] | |
| p53 | EC | P–Cys/GQDs/GNPs–streptavidin/HRP | Label–free | 6.5 × 10−8 ng/mL | 5.92 × 10−7 to 12.96 × 10−4 ng/mL | [70] |
| EC | AuNPs/ERGO | Label–free | 88 × 10−6 ng/ml | 1 × 10−4 to 10 ng/mL | [71] | |
| EC | Chitosan–CB | Label–free | 3 × 10−6 ng/mL | 1 × 10−5 to 2 × 10−3 ng/mL | [69] | |
| EC | StarPGMA | Label–free | 7 × 10−6 ng/mL | 2 × 10−5 to 4 × 10−3 ng/mL | [68] | |
| EC | PEI/NiFe2O4 NPs | Label–free | 5 × 10−6 ng/mL | 1 × 10−3 to 10 ng/mL. | [74] | |
| EC | NiPc | Label–free | – | 1 × 10−4 to 0.5 ng/mL | [72] | |
| EC | AuNPs/PEDOT:PSS | ZIF–8–DAP–Ab2 | 0.09 ng/mL | 1–120 ng/mL | [119] | |
| EC | PGE/piranha | Label–free | 0.01 ng/mL | 0.01 to 10 ng/mL | [75] | |
| CA 15–3 | EC | Au–rGO | HRP–Ab2 | 8 × 10−8 ng/mL | 1 × 10−7 to 1 × 103 ng/mL | [120] |
| EC | Strp/biotinylated mAb | biotinylated mAb/Strp–MB/biotinylated HRP | 15 × 10−6 U/mL | 50 to 15 × 10−6 U/mL | [121] | |
| EC | CysA/AuNSs/GQDs | Label–free | 0.1 U/mL | 0.16–125 U/mL | [79] | |
| EC | CoS2–GR–AuNPs | Label–free | 0.03 U/mL | 0.1–150 U/mL | [80] | |
| EC | MSA/AuSPEs | Label–free | 0.95 U/mL. | 1.0 to 1000 U/mL | [77] | |
| EC | CuS–RGO | Label–free | 0.3 U/mL | 1.0 to 150 U/mL | [76] | |
| EC | Au@Pt NCs/Fc–g–CS | Label–free | 0.17 U/mL | 0.5–200 U/mL | [81] | |
| CA 125 | EC | ZnO NRs–Au NPs | Label–free | 2.5 ng/µL | – | [84] |
| EC | rGO/Thi/AuNPs | Label–free | 0.01 U/mL | 0.1 to 200 U/mL | [83] | |
| EC | AuNPs/Cys A/ERGO/PDA | Label–free | 0.1 U/mL | 0.1 to 400 U/mL | [82] | |
| EC | AuNPs–PB–PtNP–PANI | Label–free | 4.4 × 10−3 U/mL | 0.01–5000 U/mL | [49] | |
| EC | PANI/Gr | Label–free | 0.923 ng/µL | 0.92 × 10−3 to 15.2 ng/µL | [86] | |
| EC | N–rGO@CMWCNTs/CS@AuNPs | Label–free | 4 × 10−5 ng/mL | 1 × 10−5 to 100 ng/mL | [88] | |
| EC | AuNRs | AuNP–Ab–Cd2+) | 3.4 U/mL | 20–100 U/mL | [122] | |
| EC | 3DrGO–MWCNTs–PAMAM/AuNPs | Ab–Suc–Cs@MNPs–TB | 6× 10–6 U/mL | 0.0005–75 U/mL | [123] | |
| EC | Poly(3–HPA)) | Label–free | 1.45 U/mL | 5 to 80 U/mL | [90] | |
| EC | CS–AuNPs/MWCNT/GO | AuNP–LOx | 0.002 U/mL | 0.01 to 100 U/mL | [124] | |
| EC | GNs/Au/Cysteamine | Label–free | 5.5 U/mL | 10 to 100 U/mL | [89] | |
| EC | MZnONF | Label–free | 0.00113 U/mL | 0.001 to 1000 U/mL | [85] | |
| PEC | CdTe–CS | Ab2–SiO2@PDA | 3×10 –7 U/mL | 1× 10–6 to 100 U/mL | [152] | |
| EC | NrGO/CNT and NrGO/CNF | Label–free | – | 10 to 32 × 10−4 U/mL | [125] | |
| PEC | CdS/ZNRs/RGO | ABEI–GO@HRP | 2.0 × 10−4 U/mL | 5.0 × 10−4 to 500 U/mL | [153] | |
| EC | AuNPs/RGO | Label–free | 4.2 × 10−5 U/mL | 1 × 10−4 to 300 U/mL | [87] | |
| HER2 | EC | WO3/P–Glu | Label–free | 1 × 10−6 ng/mL | 1 × 10−6 to 1 ng/mL | [93] |
| EC | APTES/MoO3@RGO | Label–free | 0.001 ng/mL | 0.001–500 ng/mL | [94] | |
| EC | APTMS–Fe3O4 | Hyd@AuNPs–APTMS–Fe3O4 | 2.0 × 10−5 ng/mL | 5.0 × 10−4 to 50.0 ng/mL | [126] | |
| EC | SPCE/Ab1 | Ab2–PbS QDs | 0.28 ng/mL | 1 to 100 ng/mL | [92] | |
| EC | MnO2 NSs/AuNPs | Au@Ag NRs | 16.7 × 10−6 ng/mL (DPV) and 33.3 × 10−6 ng/mL (chronoamperometry) | 50 × 10−6 to 100 ng/mL (DPV) and 1 × 10−4 to 100 ng/mL (chronoamperometry) | [127] | |
| EC | Fe3O4@TMU–24 | Pt:CdTe QDs | 17.5 × 10−5 ng/mL | 1 × 10−3 ng/mL to 100 ng/mL | [129] | |
| EC | AuNPs/Cu–MOF | CZTS NPs/Pt/g–C3N4/anti–HER2–Ab2 | 3 × 10−6 ng/mL | 1 × 10−5 to 1 × 10−3 ng/mL | [128] | |
| EC | Fe3O4@TMU–21/MWCNTs | Label–free | 3 × 10−4 ng/mL. | 1 × 10−3 to 100 ng/mL | [95] | |
| EC | AuNPs/MPA | Label–free | 2.9 ng/mL. | 0 to 10 ng/mL | [91] | |
| EC | GNPs/APTMS/PEG–NHS–Mal | Label–free | 1.20 × 10−2 ng/mL | – | [96] | |
| HE4 | EC | TiO2–rGO/Au@Pd HSs | Label–free | 13.33 fM/mL | 40 to 60 × 106 fM/mL | [101] |
| EC | Fe3O4@SiO2@Au MNCs | AgPtCo NDs–Ab2 | 48.7 × 10−5 ng/mL | 0.001–50 ng/mL | [131] | |
| EC | AuNPs/N–doped GNs/FAO | Label–free | 1210 ng/mL | 1 × 104 to 6.5 × 104 ng/mL | [6] | |
| EC | AuNRs/NH2–GS | Au@PdUSs–Ab2 | 0.33 pmol/L | 1 to 50 × 103 pmol/L | [130] | |
| EC | Hg–SPCE and Bi–SPCE | CdSe–ZnS QDs–Ab2 | 12 pM and 89 pM | 20 to 40 × 103 pM and 100 to 2 × 103 pM | [132] | |
| PEC | MWCNTs–PDA–AuNPs | Nb3@nPCN–224 | 5.6 × 10−4 ng/mL | 1 × 10−3 to10.0 ng/mL | [97] | |
| PEC | Bi2+xWO6 | Label–free | 77.8 × 10−6 ng/mL | 0.001 to 100 ng/mL | [156] | |
| PEC | WO3/Au/PDA | Label–free | 15.6 × 10−4 ng/mL | 0.01 to 200 ng/mL | [155] | |
| PEC | AuNPs/CdS NSs | Label–free | 10.84 × 10−4 ng/mL | 0.01 to 200 ng/mL | [159] | |
| MUC 1, CA 153 and HER2 | Multiplexed EC | PEI–AuNPs + AQ (MUC 1) TH (CA 153) Ag+ (HER2) | Label–free | 0.10 to 100 ng/mL (MUC1) 0.10 to 100 U/mL (CA 153) 0.10 to 100 ng/mL (HER2) | 0.53 ng/mL (MUC1), 0.21 U/mL (CA 153) and 0.50 ng/mL (HER2) | [162] |
| CEA, CA153 and CA 125 | Multiplexed EC | MB–Chi/GR | Label–free | 4 × 10−5 ng/mL (CEA) 0.04 mU/mL (CA 153) 0.04 mU/mL (CA 125) | 1 × 10−4 to 0.1 ng/mL (CEA) 0.10 to 100.00 mU/mL (CA 153) 0.10 to 100.00 mU/mL (CA 125) | [163] |
| CEA and AFP | Multiplexed EC | Fe3O4 | thionine–4ATP–Au NP (CEA) ferrocene–4ATP–Au NPs (AFP) | 0.05–120 ng/mL (CEA), 0.018 ng/mL (AFP) | 0.05–100 ng/mL (CEA), 0.012 ng/mL (AFP) | [164] |
| CEA, CA 199, CA125 and CA 242 | Multiplexed EC | CHIT–AuNPs | Cu–CP–anti–CEA; Pb–CP–anti–CA199; Cd–CP–anti–CA125; Zn–CP–anti–CA242 | 0.02 ng/mL(CEA); 0.4 U/mL (CA 199); 0.3 U/mL (CA 125) and 0.4 U/mL(CA 242) | 0.1 to 100 ng/mL (CEA); 1 to 150 U/mL (CA 199); 1 to 150 U/mL (CA 125); 1 to 150 U/mL (CA 242); | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekwujuru, E.U.; Olatunde, A.M.; Klink, M.J.; Ssemakalu, C.C.; Chili, M.M.; Peleyeju, M.G. Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers. Sensors 2023, 23, 4106. https://doi.org/10.3390/s23084106
Ekwujuru EU, Olatunde AM, Klink MJ, Ssemakalu CC, Chili MM, Peleyeju MG. Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers. Sensors. 2023; 23(8):4106. https://doi.org/10.3390/s23084106
Chicago/Turabian StyleEkwujuru, Ezinne U., Abimbola M. Olatunde, Michael J. Klink, Cornelius C. Ssemakalu, Muntuwenkosi M. Chili, and Moses G. Peleyeju. 2023. "Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers" Sensors 23, no. 8: 4106. https://doi.org/10.3390/s23084106
APA StyleEkwujuru, E. U., Olatunde, A. M., Klink, M. J., Ssemakalu, C. C., Chili, M. M., & Peleyeju, M. G. (2023). Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers. Sensors, 23(8), 4106. https://doi.org/10.3390/s23084106






