Plasmonic Resonance Coupling of Nanodisk Array/Thin Film on the Optical Fiber Tip for Integrated and Miniaturized Sensing Detection
Abstract
1. Introduction
2. Material and Method
2.1. Material
2.2. Plasmonic Nanostructure-Based Fiber-Optic Sensing Probe
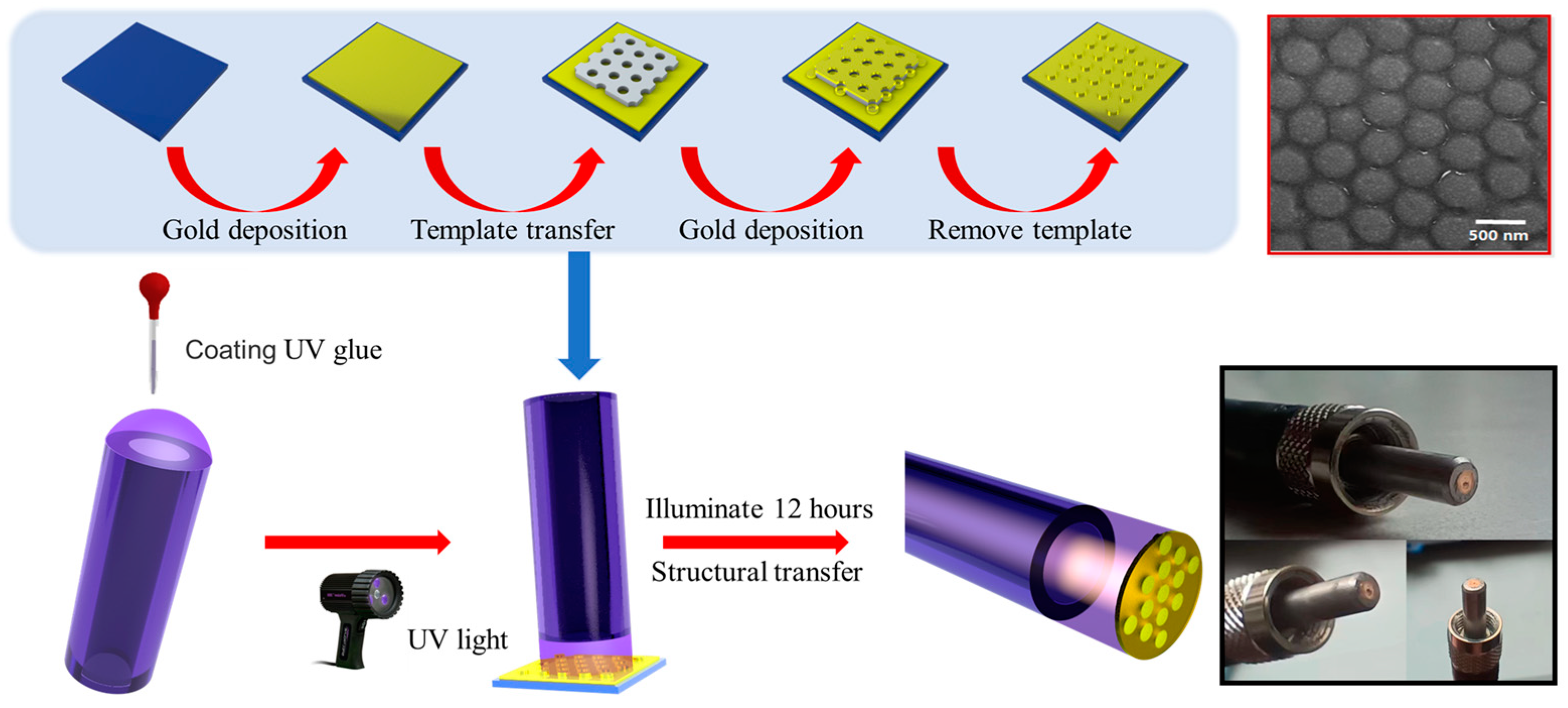
2.3. Experimental Fabrication and Characterization of the Nanodisk Array/Thin Film-Based Fiber Probe
3. Results and Discussion
3.1. Theoretical Explanation and the Superiority of the Designed Nanostructure
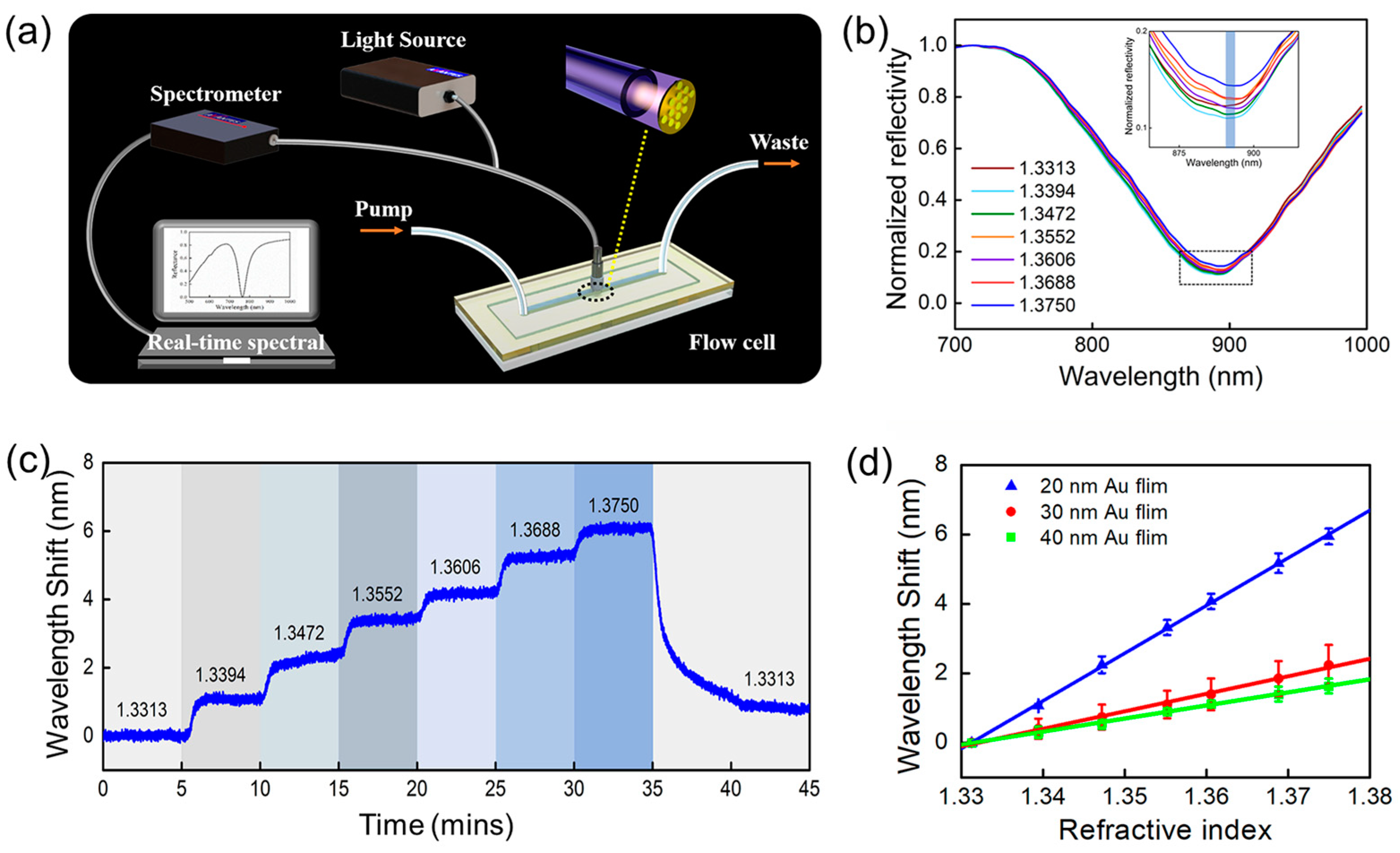
3.2. The Evaluation of Bulk RI Sensitivity
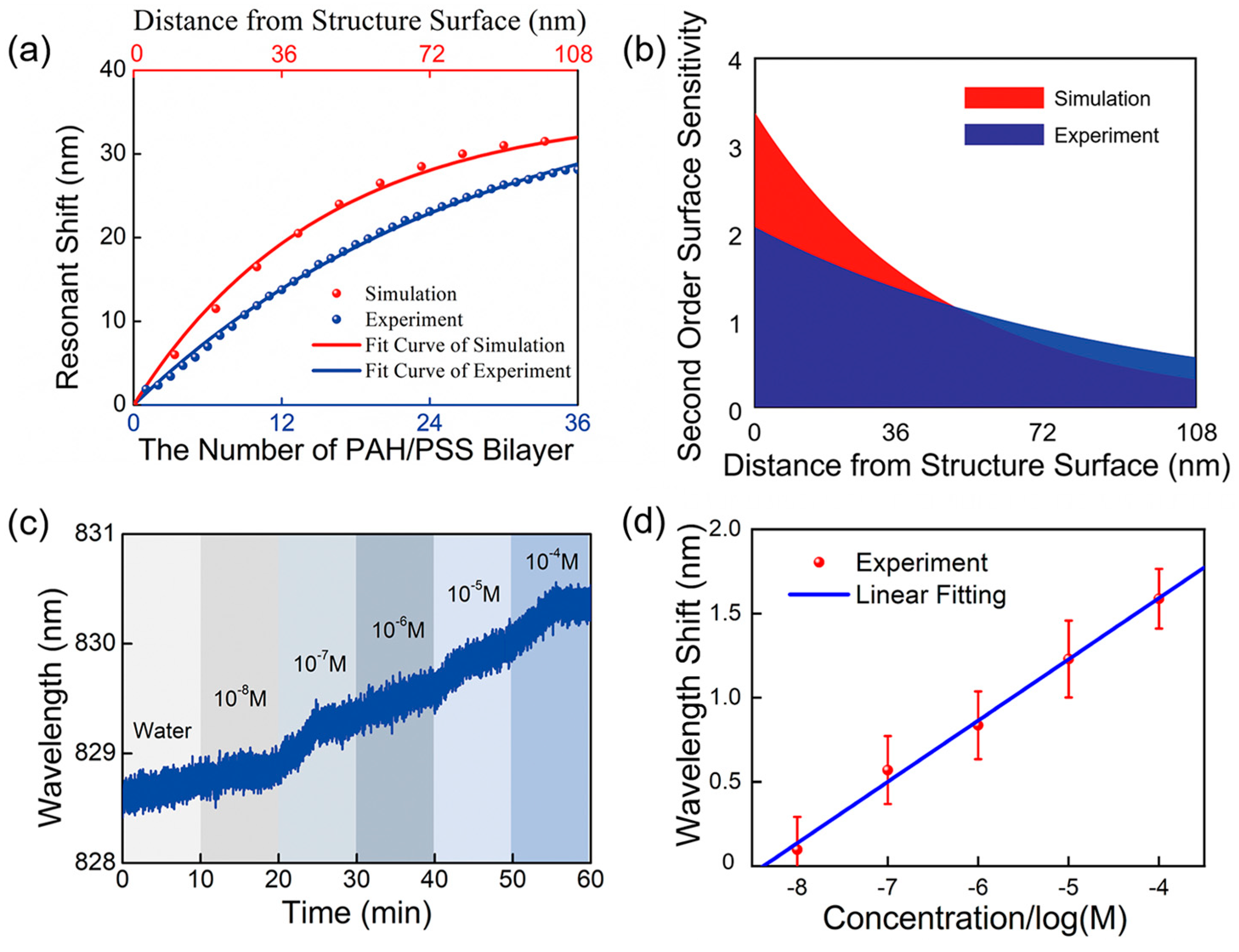
3.3. Surface Sensitivity and Biomolecular Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Crescitelli, A.; Vaiano, P.; Quero, G.; Consales, M.; Pisco, M.; Esposito, E.; Cusano, A. Lab-on-fiber technology: A new vision for chemical and biological sensing. Analyst 2015, 140, 8068–8079. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A. Lab on Fiber Technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Liu, F.; Guan, B.O.; Albert, J. Ultrasensitive plasmonic sensing in air using optical fibre spectral combs. Nat. Commun. 2016, 7, 13371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.; Wang, J.S. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Consales, M.; Del Villar, I.; Matias, I.R.; Cusano, A. Lab on Fiber Technology towards Advanced and Multifunctional Point-of-Care Platforms for Precision Medicine. In Encyclopedia of Sensors and Biosensors, 1st ed.; Roger, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 4, pp. 504–527. [Google Scholar]
- Qian, S.Y.; Liang, Y.Z.; Ma, J.; Zhang, Y.; Zhao, J.Z.; Peng, W. Boronic acid modified fiber optic SPR sensor and its application in saccharide detection. Sens. Actuators B-Chem. 2015, 220, 1217–1223. [Google Scholar] [CrossRef]
- Li, L.X.; Liang, Y.Z.; Guang, J.Y.; Cui, W.L.; Zhang, X.P.; Masson, J.F.; Peng, W. Dual Kretschmann and Otto configuration fiber surface plasmon resonance biosensor. Opt. Express 2017, 25, 26949–26956. [Google Scholar] [CrossRef]
- Li, L.X.; Liang, Y.Z.; Liu, Q.; Peng, W. Dual-Channel Fiber-Optic Biosensor for Self-Compensated Refractive Index Measurement. IEEE Photon. Technol. Lett. 2016, 28, 2110–2113. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Lee, S.K. Fabrication and measurement of a fiber optic localized surface plasmon resonance sensor chip for molecular diagnostics. Sens. Actuators A 2021, 331, 112982. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B-Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Hu, S.Q.; Liang, J.H.; Chen, J.Y.; Cheng, H.D.; Lin, Q.Y.; Shi, W.C.; Yuan, J.M.; Liu, G.S.; Chen, L.; Chen, Z.; et al. Highly sensitive vector magnetic fiber sensor based on hyperbolic metamaterials. Sci. China Phys. Mech. Astron. 2022, 65, 114211. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.J.; Shafi, M.; Liu, R.C.; Zha, Z.P.; Feng, D.J.; Liu, M.; Du, X.J.; Yue, W.W.; Jiang, S.Z. Optical fiber SPR biosensor complying with a 3D composite hyperbolic metamaterial and a graphene film. Photonics Res. 2021, 9, 379–388. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.G.; Zhou, X.; Zhang, Y.N. Review on the graphene based optical fiber chemical and biological sensors. Sens. Actuators B-Chem. 2016, 231, 324–340. [Google Scholar] [CrossRef]
- Fu, H.Y.; Zhang, S.W.; Chen, H.; Weng, J. Graphene Enhances the Sensitivity of Fiber-Optic Surface Plasmon Resonance Biosensor. IEEE Sens. J. 2015, 15, 5478–5482. [Google Scholar] [CrossRef]
- Li, D.C.; Wu, J.W.; Wu, P.; Lin, Y.; Sun, Y.J.; Zhu, R.; Yang, J.; Xu, K.X. Affinity based glucose measurement using fiber optic surface plasmon resonance sensor with surface modification by borate polymer. Sens. Actuators B-Chem. 2015, 213, 295–304. [Google Scholar] [CrossRef]
- Wang, R.L.; Zhang, H.Z.; Liu, Q.Y.; Liu, F.; Han, X.L.; Liu, X.Q.; Li, K.W.; Xiao, G.Z.; Albert, J.; Lu, X.H.; et al. Operando monitoring of ion activities in aqueous batteries with plasmonic fiber-optic sensors. Nat. Commun. 2022, 13, 547. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.Y.; Caucheteur, C. Tilted fiber Bragg grating sensors. Laser Photonics Rev. 2013, 7, 83–108. [Google Scholar] [CrossRef]
- Shevchenko, Y.Y.; Albert, J. Plasmon resonances in gold-coated tilted fiber Bragg gratings. Opt. Lett. 2007, 32, 211–213. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Tong, R.J.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, M.S.; Jiang, S.Z.; Du, L.T.; Cheng, Y.Y.; Li, P.L.; Wang, C.A.X. Design and fabrication of Gr/Ag-coated tilted grating sensor for ultra-sensitive detection of DNA hybridization. Sens. Actuators B-Chem. 2022, 359, 131587. [Google Scholar] [CrossRef]
- Kong, L.X.; Chi, M.J.; Ren, C.; Ni, L.F.; Li, Z.; Zhang, Y.S. Micro-Lab on Tip: High-Performance Dual-Channel Surface Plasmon Resonance Sensor Integrated on Fiber-Optic End Facet. Sens. Actuators B-Chem. 2022, 351, 130978. [Google Scholar] [CrossRef]
- Pisco, M.; Cusano, A. Lab-On-Fiber Technology: A Roadmap toward Multifunctional Plug and Play Platforms. Sensors 2020, 20, 4705. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.R.; Li, R.; Liang, Y.Z.; Chu, S.W.; Zhang, X.; Fang, Y.R.; Peng, W. Theoretical Design of Broadband Tunable Nanograting-Coupled Fiber-Optic Surface Plasmon Resonance For Advanced Sensing. J. Light. Technol. 2023, 41, 761–767. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L. Lab-on-fiber: Plasmonic nano-arrays for sensing. Nanoscale 2020, 12, 7485–7499. [Google Scholar] [CrossRef] [PubMed]
- Consoles, M.; Quero, G.; Spazioni, S.; Principe, M.; Micco, A.; Galdi, V.; Cutolo, A.; Cusano, A. Metasurface-Enhanced Lab-on-Fiber Biosensors. Laser Photonics Rev. 2020, 14, 2000180. [Google Scholar] [CrossRef]
- Xiong, Y.F.; Xu, F. Multifunctional integration on optical fiber tips: Challenges and opportunities. Adv. Photonics 2020, 2, 064001. [Google Scholar] [CrossRef]
- Polley, N.; Sardar, S.; Werner, P.; Gersonde, I.; Kanehira, Y.; Bald, I.; Repp, D.; Pertsch, T.; Pacholski, C. Photothermomechanical Nanopump: A Flow-Through Plasmonic Sensor at the Fiber Tip. ACS Nano 2023, 17, 1403–1413. [Google Scholar] [CrossRef]
- Kim, H.-M.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Fiber Optic Plasmonic Sensors Based on Nanodome Arrays with Nanogaps. ACS Sens. 2022, 7, 1451–1457. [Google Scholar] [CrossRef]
- Li, L.X.; Zhao, L.L.; Zong, X.Y.; Li, P.L.; Yu, K.; Liu, Y.F. A Near-Infrared Plasmonic Sensor Based on Wedge Fiber-Optic for Ultra-Sensitive Hg2+ Detection. J. Light. Technol. 2022, 40, 7946–7951. [Google Scholar] [CrossRef]
- Sun, X.Q.; Lei, Z.Y.; Zhong, H.; He, C.J.; Liu, S.H.; Meng, Q.F.; Liu, Q.W.; Chen, S.F.; Kong, X.Y.; Yang, T. A quasi-3D Fano resonance cavity on optical fiber end-facet for high signal-to-noise ratio dip-and-read surface plasmon sensing. Light Adv. Manuf. 2022, 3, 46. [Google Scholar] [CrossRef]
- He, X.L.; Yi, H.; Long, J.; Zhou, X.; Yang, J.; Yang, T. Plasmonic crystal cavity on single-mode optical fiber end facet for label-free biosensing. Appl. Phys. Lett. 2016, 108, 231105. [Google Scholar] [CrossRef][Green Version]
- Liang, Y.Z.; Cui, W.L.; Li, L.X.; Yu, Z.Y.; Peng, W.; Xu, T. Large-Scale Plasmonic Nanodisk Structures for a High Sensitivity Biosensing Platform Fabricated by Transfer Nanoprinting. Adv. Opt. Mater. 2019, 7, 1801269. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Lin, Y.B.; Zou, Y.; Mo, Y.Y.; Guo, J.P.; Lindquist, R.G. E-Beam Patterned Gold Nanodot Arrays on Optical Fiber Tips for Localized Surface Plasmon Resonance Biochemical Sensing. Sensors 2010, 10, 9397–9406. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, D.; Wang, J.Y.; Lu, J.S.; Yao, G.N.; Liu, D.L.; Luo, H.; Li, Q.; Qiu, M. Solvent-Free Nanofabrication Based on Ice-Assisted Electron-Beam Lithography. Nano Lett. 2020, 20, 8841–8846. [Google Scholar] [CrossRef] [PubMed]


| Reference | Resonant Structure | Sensing huichePerformance | Manufacturing Technology | Cost | Advantages |
|---|---|---|---|---|---|
| [26] | Au nanohole array with phase gradient | S ≈ 20 × 10−12 m/(ng mL−1) LOD ≈ 50 × 10−12 M | Focused ion beam (FIB) | High | High sensitivity |
| [35] | Au nanodot arrays | S = 196 nm/RIU LOD ≈ 6 pM | Electron beam lithography (EBL) | High | High LOD |
| [36] | Au nanodisk array | S = 187.4 nm/RIU | Ice-assisted electron beam lithography (iEBL) | High | Streamlined and ecofriendly |
| This work | Au nanodisk array/thin film | S = 137.28 nm/RIU LOD ≈ 19.35 μM | UV curing glue transfer technology | Low | Batch fabrication and convenience for molecular modification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wei, X.; He, Y.; Liang, Y.; Fang, Y.; Peng, W. Plasmonic Resonance Coupling of Nanodisk Array/Thin Film on the Optical Fiber Tip for Integrated and Miniaturized Sensing Detection. Sensors 2023, 23, 4163. https://doi.org/10.3390/s23084163
He H, Wei X, He Y, Liang Y, Fang Y, Peng W. Plasmonic Resonance Coupling of Nanodisk Array/Thin Film on the Optical Fiber Tip for Integrated and Miniaturized Sensing Detection. Sensors. 2023; 23(8):4163. https://doi.org/10.3390/s23084163
Chicago/Turabian StyleHe, Hao, Xinran Wei, Yijin He, Yuzhang Liang, Yurui Fang, and Wei Peng. 2023. "Plasmonic Resonance Coupling of Nanodisk Array/Thin Film on the Optical Fiber Tip for Integrated and Miniaturized Sensing Detection" Sensors 23, no. 8: 4163. https://doi.org/10.3390/s23084163
APA StyleHe, H., Wei, X., He, Y., Liang, Y., Fang, Y., & Peng, W. (2023). Plasmonic Resonance Coupling of Nanodisk Array/Thin Film on the Optical Fiber Tip for Integrated and Miniaturized Sensing Detection. Sensors, 23(8), 4163. https://doi.org/10.3390/s23084163








