Home-Based Measurements of Nocturnal Cardiac Parasympathetic Activity in Athletes during Return to Sport after Sport-Related Concussion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol and Devices
2.3. Data Analysis
3. Results
3.1. Subjects and Concussion Symptoms
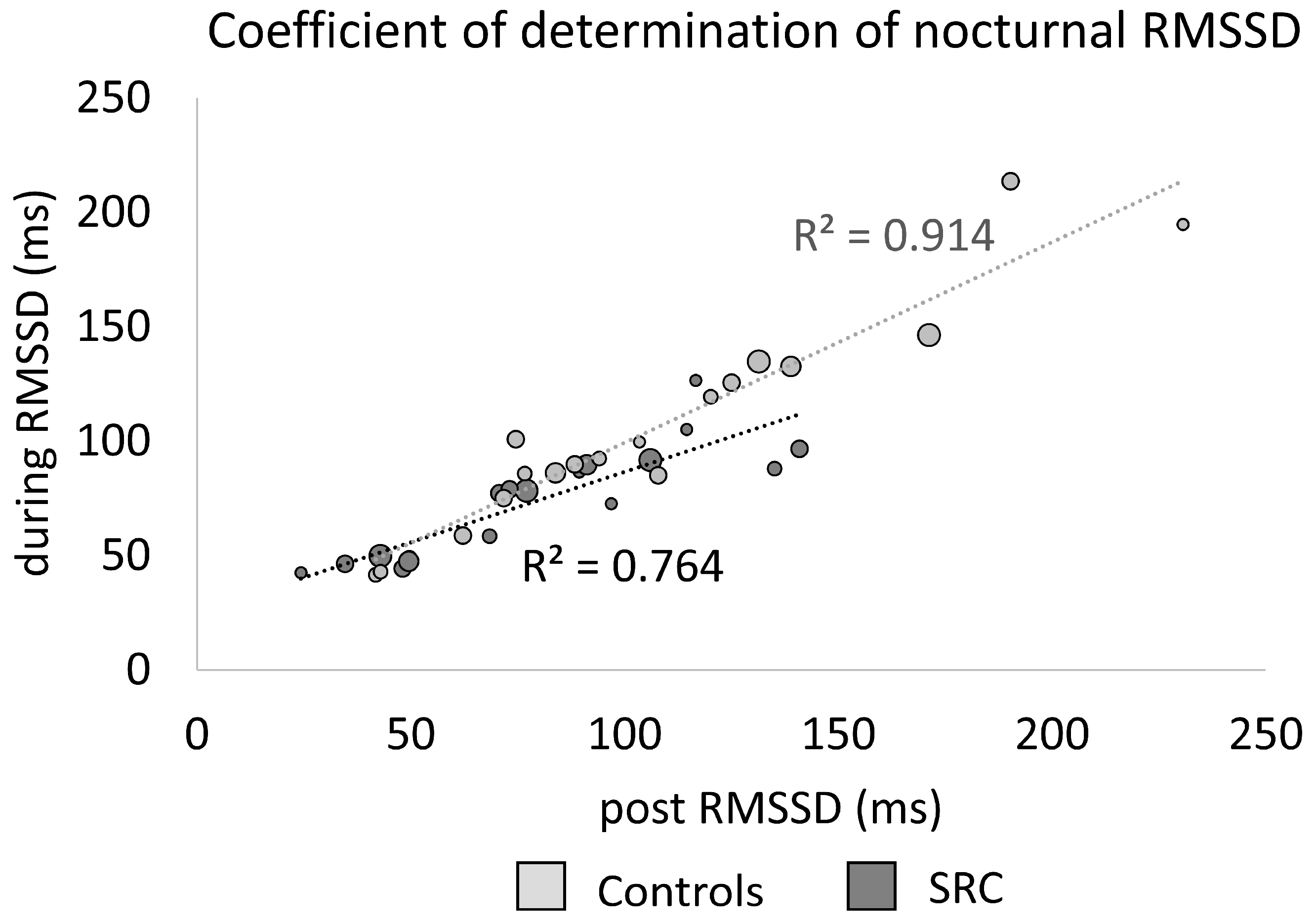
3.2. Nocturnal Recordings
3.3. Nocturnal Heart Rate and Cardiac Parasympathetic Activity
3.4. Correlations of Sleep-Associated Symptoms and Nocturnal Cardiac Parasympathetic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCrory, P.; Meeuwisse, W.; Dvořák, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.J.; Batycky, J.; Campbell, C.; Schneider, K.; Smirl, J.; Debert, C.T. Autonomic dysfunction in adults following mild traumatic brain injury: A systematic review. NeuroRehabilitation 2022, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, M.G.; Mainwaring, L.; Senthinathan, A.; Churchill, N.; Thomas, S.; Richards, D. Psychological and Physiological Markers of Stress in Concussed Athletes Across Recovery Milestones. J. Head Trauma Rehabil. 2017, 32, E38–E48. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.A.; Dech, R.T.; Guzik, P.; Neary, J.P. Heart rate variability and implication for sport concussion. Clin. Physiol. Funct. Imaging 2018, 38, 733–742. [Google Scholar] [CrossRef]
- Mishica, C.; Kyröläinen, H.; Hynynen, E.; Nummela, A.; Holmberg, H.-C.; Linnamo, V. Evaluation of nocturnal vs. morning measures of heart rate indices in young athletes. PLoS ONE 2022, 17, e0262333. [Google Scholar] [CrossRef]
- Costa, J.A.; Brito, J.; Nakamura, F.Y.; Figueiredo, P.; Oliveira, E.; Rebelo, A. Sleep patterns and nocturnal cardiac autonomic activity in female athletes are affected by the timing of exercise and match location. Chronobiol. Int. 2019, 36, 360–373. [Google Scholar] [CrossRef]
- Hauglund, N.L.; Pavan, C.; Nedergaard, M. Cleaning the sleeping brain—The potential restorative function of the glymphatic system. Curr. Opin. Physiol. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- de Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef]
- Stevens, D.J.; Alghwiri, A.; Appleton, S.L.; Rogers, J.M.; Plummer, S.L.; Grant, C.; Bickley, K.; Alvaro, P.K.; Kennett, S.; Adams, R.; et al. Should We Lose Sleep Over Sleep Disturbances After Sports-Related Concussion? A Scoping Review of the Literature. J. Head Trauma Rehabil. 2022, 37, E206–E219. [Google Scholar] [CrossRef]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. The Risk of Sleep Disorder Among Persons with Mild Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 55. [Google Scholar] [CrossRef]
- de Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef]
- Howell, D.R.; Oldham, J.R.; Brilliant, A.N.; Meehan, W.P. Trouble Falling Asleep After Concussion Is Associated With Higher Symptom Burden Among Children and Adolescents. J. Child Neurol. 2019, 34, 256–261. [Google Scholar] [CrossRef]
- Hoffman, N.L.; O’Connor, P.J.; Schmidt, M.D.; Lynall, R.C.; Schmidt, J.D. Relationships between Post-Concussion Sleep and Symptom Recovery: A Preliminary Study. J. Neurotrauma 2020, 37, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Stokes, M.; Bell, K.R. Autonomic nervous system dysfunction in mild traumatic brain injury: A review of related pathophysiology and symptoms. Brain Inj. 2019, 33, 1129–1136. [Google Scholar] [CrossRef]
- Tkachenko, N.; Singh, K.; Hasanaj, L.; Serrano, L.; Kothare, S.V. Sleep Disorders Associated With Mild Traumatic Brain Injury Using Sport Concussion Assessment Tool 3. Pediatr. Neurol. 2016, 57, 46–50.e1. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Symptom correlates of cerebral blood flow following acute concussion. NeuroImage Clin. 2017, 16, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Empatica. E4 Wristband|Real-Time Physiological Signals|Wearable PPG, EDA, Temperature, Motion Sensors. Available online: https://www.empatica.com/research/e4/ (accessed on 3 December 2022).
- McCarthy, C.; Pradhan, N.; Redpath, C.; Adler, A. Validation of the Empatica E4 wristband. In Proceedings of the 2016 IEEE EMBS International Student Conference (ISC), Ottawa, ON, Canada, 29–31 May 2016. [Google Scholar]
- Kapella, M.C.; Vispute, S.; Zhu, B.; Herdegen, J.J. Actigraphy scoring for sleep outcome measures in chronic obstructive pulmonary disease. Sleep Med. 2017, 37, 124–129. [Google Scholar] [CrossRef]
- Kubios HRV (Ver. 3.5) User’s Guide; Kubios Oy: Kuopio, Finland, 2021.
- van Lier, H.G.; Pieterse, M.E.; Garde, A.; Postel, M.G.; de Haan, H.A.; Vollenbroek-Hutten, M.M.R.; Schraagen, J.M.; Noordzij, M.L. A standardized validity assessment protocol for physiological signals from wearable technology: Methodological underpinnings and an application to the E4 biosensor. Behav. Res. 2020, 52, 607–629. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Herzig, D.; Testorelli, M.; Olstad, D.S.; Erlacher, D.; Achermann, P.; Eser, P.; Wilhelm, M. Heart-Rate Variability During Deep Sleep in World-Class Alpine Skiers: A Time-Efficient Alternative to Morning Supine Measurements. Int. J. Sports Physiol. Perform. 2017, 12, 648–654. [Google Scholar] [CrossRef]
- Abaji, J.P.; Curnier, D.; Moore, R.D.; Ellemberg, D. Persisting Effects of Concussion on Heart Rate Variability during Physical Exertion. J. Neurotrauma 2016, 33, 811–817. [Google Scholar] [CrossRef]
- Gall, B.; Parkhouse, W.; Goodman, D. Heart Rate Variability of Recently Concussed Athletes at Rest and Exercise. Med. Sci. Sport. Exerc. 2004, 36, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, M.; Verweel, L.; Thomas, S.G.; Taha, T.; Keightley, M.; Wilson, K.E.; Reed, N. Heart rate variability following youth concussion: How do autonomic regulation and concussion symptoms differ over time postinjury? BMJ Open Sport Exerc. Med. 2018, 4, e000355. [Google Scholar] [CrossRef] [PubMed]
- Senthinathan, A.; Mainwaring, L.; Hutchison, M.G. Heart Rate Variability of Athletes Across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, E.M.; Schnyer, D.M.; Germain, A.; Williams, S.G.; Lettieri, C.J.; McKeon, A.B.; Scharf, S.M.; Stocker, R.; Albrecht, J.; Badjatia, N.; et al. Sleep, Sleep Disorders, and Circadian Health following Mild Traumatic Brain Injury in Adults: Review and Research Agenda. J. Neurotrauma 2018, 35, 2615–2631. [Google Scholar] [CrossRef]
- Kleiman, E.; Millner, A.J.; Joyce, V.W.; Nash, C.C.; Buonopane, R.J.; Nock, M.K. Using Wearable Physiological Monitors With Suicidal Adolescent Inpatients: Feasibility and Acceptability Study. JMIR Mhealth Uhealth 2019, 7, e13725. [Google Scholar] [CrossRef]
- Cella, M.; Okruszek, Ł.; Lawrence, M.; Zarlenga, V.; He, Z.; Wykes, T. Using wearable technology to detect the autonomic signature of illness severity in schizophrenia. Schizophr. Res. 2018, 195, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef]

| Concussed (n = 18) | Controls (n = 18) | p | |
|---|---|---|---|
| Sex | m = 15, f = 3 | m = 15, f = 3 | |
| Age (yrs.) | 23 (±5) | 23 (±5) | 0.919 |
| Height (m) | 1.84 (±0.10) | 1.84 (±0.10) | 0.994 |
| Weight (kg) | 81 (±13) | 81 (±14) | 0.981 |
| BMI (kg/m2) | 24 (±2) | 24 (±2) | 0.833 |
| Previous concussions | 1 (0, 4) | 1 (0, 4) | 0.552 |
| Sport | Soccer (8) | Soccer (7) | |
| Basketball (3) | Basketball (4) | ||
| Handball (2) | Handball (1) | ||
| Am. Football (2) | Am. Football (4) | ||
| Ice Hockey (2) | Ice Hockey (1) | ||
| Modern Pentathlon (1) | Modern Pentathlon (1) |
| Concussed (n = 18) | Controls (n = 18) | p * | p (adj.) | |
|---|---|---|---|---|
| Number of symptoms | 7.00 (± 4.88) | 0.50 (±4.20) | <0.001 | <0.01 |
| Symptom severity | 15.50 (±15.54) | 0.50 (±6.04) | <0.001 | <0.01 |
| Fatigue or low energy | 2.00 (±1.77) | 0.00 (±0.70) | <0.001 | <0.01 |
| Drowsiness | 0.00 (±1.55) | 0.00 (±0.51) | 0.025 | 0.055 |
| Headache | 1.50 (±1.68) | 0.00 (±0.00) | <0.001 | <0.01 |
| “Pressure in head” | 2.00 (±1.41) | 0.00 (±0.24) | <0.001 | <0.01 |
| Neck pain | 1.00 (±1.42) | 0.00 (±0.38) | 0.002 | 0.006 |
| Dizziness | 0.00 (±0.86) | 0.00 (±0.47) | 0.036 | 0.072 |
| Balance problems | 0.00 (±1.04) | 0.00 (±0.00) | 0.045 | 0.083 |
| Feeling slowed down | 0.50 (±1.52) | 0.00 (±0.24) | 0.003 | 0.008 |
| “Don’t feel right” | 1.50 (±1.71) | 0.00 (±0.24) | <0.001 | <0.01 |
| Difficulty concentrating | 2.00 (±1.57) | 0.00 (±0.24) | <0.001 | <0.01 |
| Difficulty remembering | 0.50 (±1.26) | 0.00 (±0.51) | 0.011 | 0.026 |
| SRC During RTS | SRC Post RTS | Controls | Group Differences (p) | Group Differences (p adj.) | |||
|---|---|---|---|---|---|---|---|
| During RTS | Post RTS | During RTS | Post RTS | ||||
| HR (bpm) | 56 ± 6 [CI: 51–57] | 55 ± 7 [CI: 50–57] | 55 ± 8 [CI: 48–56] | 0.515 | 0.567 | 0.567 | 0.567 |
| RMSSD (ms) | 77.74 ± 24.25 [CI: 61.78–85.90] | 75.12 ± 34.41 [CI: 62.29–96.51] | 95.68 ± 46.86 [CI: 83.87–130.48] | 0.021 * | 0.085 | 0.126 | 0.255 |
| CV HR (in %) | 5.50 ± 4.55 | / | 5.00 ± 2.55 | 0.491 | / | 0.567 | / |
| CV RMSSD (in %) | 17.50 ± 6.33 | / | 13.00 ± 7.63 | 0.199 | / | 0.398 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delling, A.C.; Jakobsmeyer, R.; Coenen, J.; Christiansen, N.; Reinsberger, C. Home-Based Measurements of Nocturnal Cardiac Parasympathetic Activity in Athletes during Return to Sport after Sport-Related Concussion. Sensors 2023, 23, 4190. https://doi.org/10.3390/s23094190
Delling AC, Jakobsmeyer R, Coenen J, Christiansen N, Reinsberger C. Home-Based Measurements of Nocturnal Cardiac Parasympathetic Activity in Athletes during Return to Sport after Sport-Related Concussion. Sensors. 2023; 23(9):4190. https://doi.org/10.3390/s23094190
Chicago/Turabian StyleDelling, Anne Carina, Rasmus Jakobsmeyer, Jessica Coenen, Nele Christiansen, and Claus Reinsberger. 2023. "Home-Based Measurements of Nocturnal Cardiac Parasympathetic Activity in Athletes during Return to Sport after Sport-Related Concussion" Sensors 23, no. 9: 4190. https://doi.org/10.3390/s23094190





