Biomagnetism: The First Sixty Years
Abstract
:1. Introduction
2. The Magnetocardiogram
2.1. The First Measurement of the Magnetocardiogram
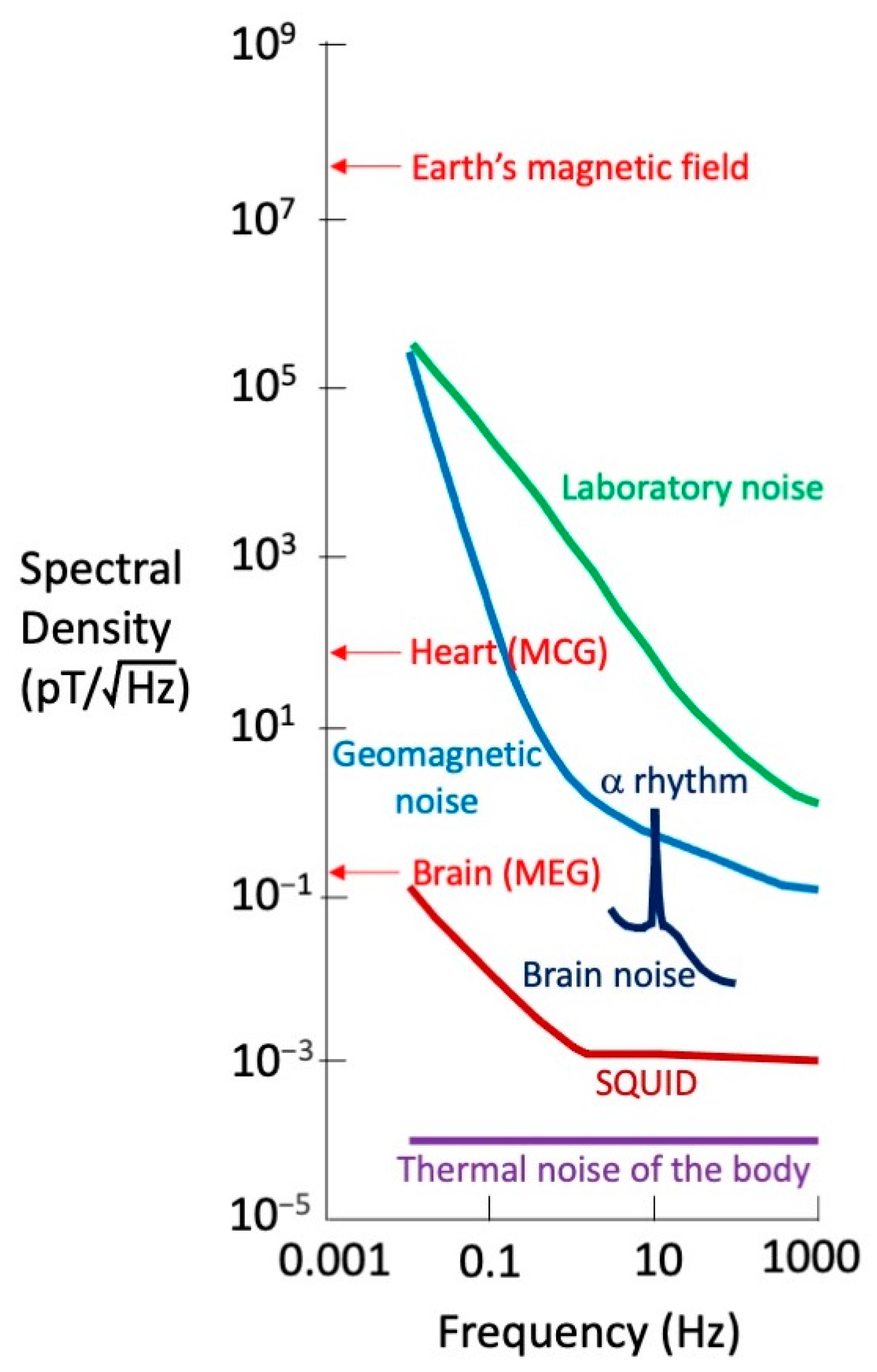
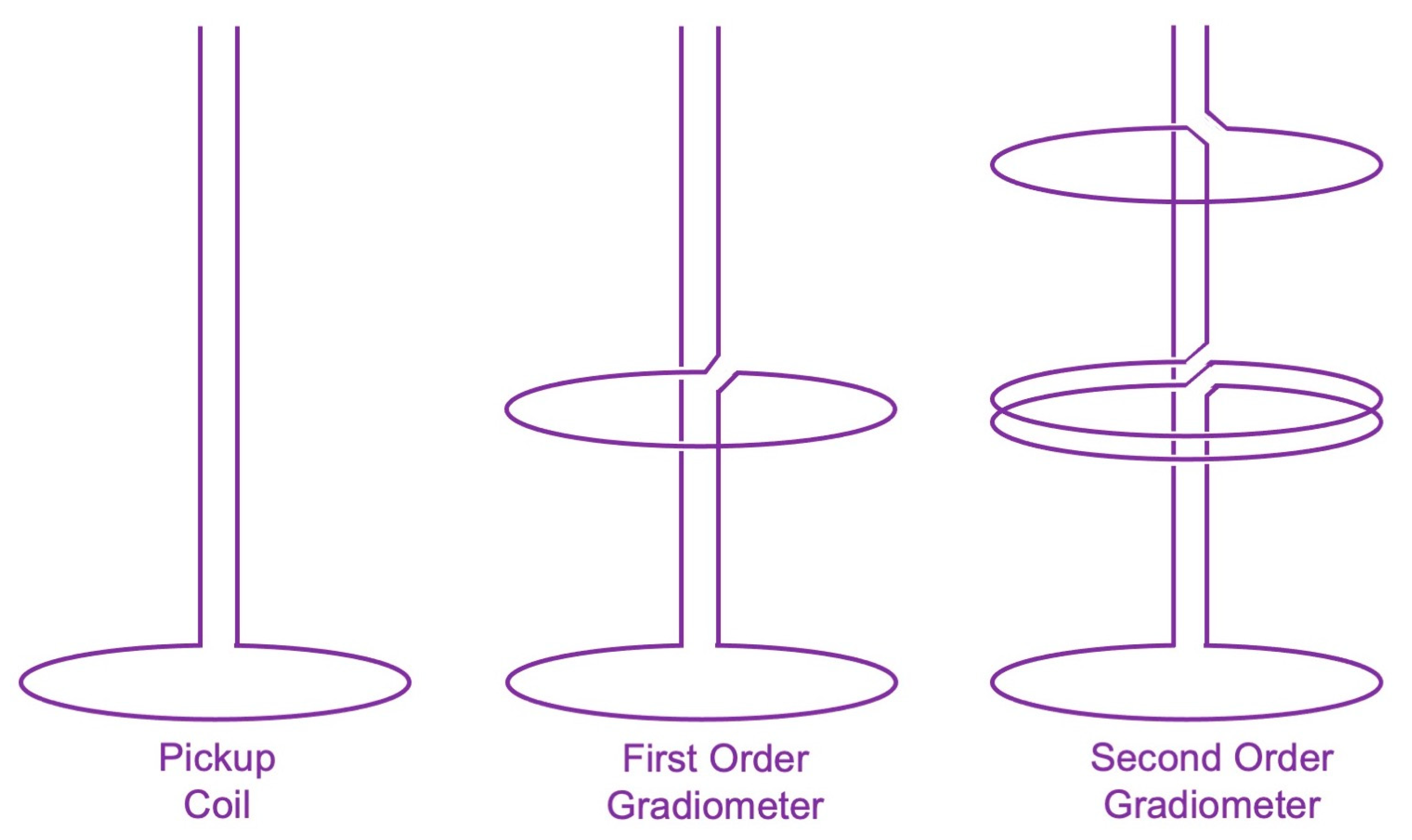
2.2. The Shielded Room and the SQUID
2.3. Growth of Magnetocardiology in the 1970s
2.4. Theoretical Calculations of the Magnetocardiogram
2.5. Information Content of the Magnetocardiogram
2.6. Instrumentation and Clinical Applications
3. Isolated Tissue
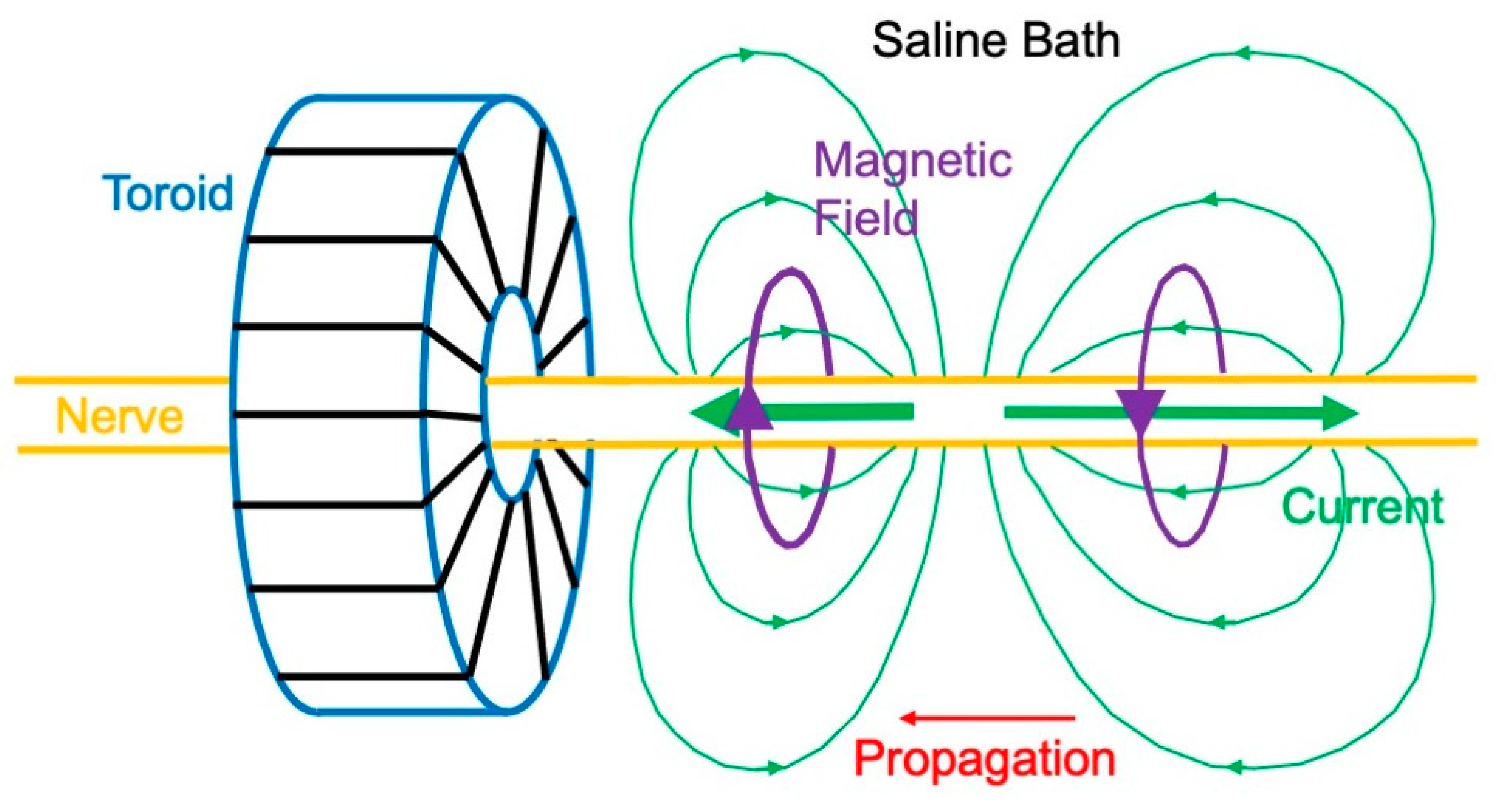
3.1. Magnetic Field of a Nerve
3.2. Ampere’s Law Versus the Law of Biot and Savart
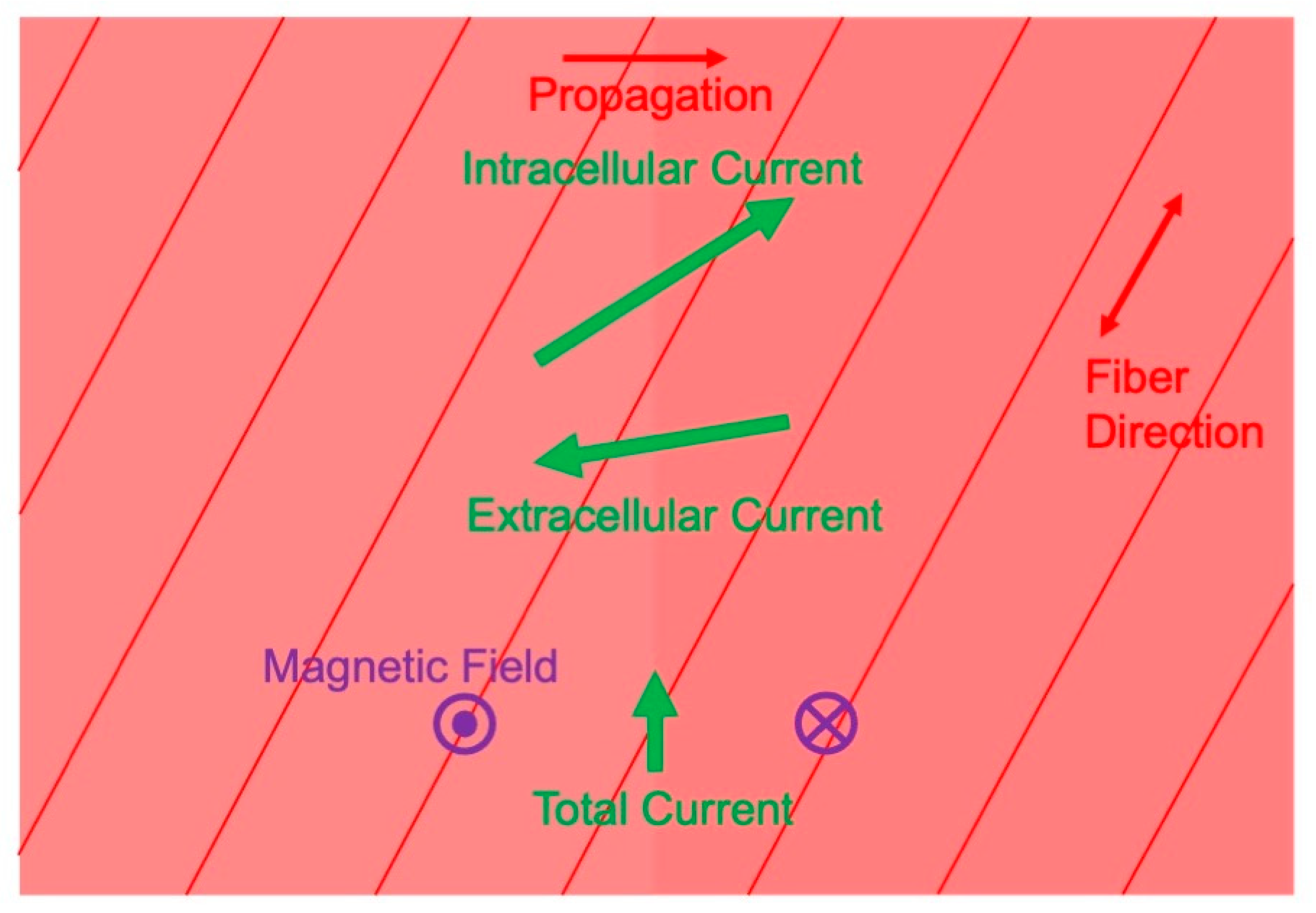
3.3. Magnetic Field Produced by Cardiac Muscle
3.4. MicroSQUID
4. The Magnetoencephalogram
4.1. First Measurements of the Magnetoencephalogram
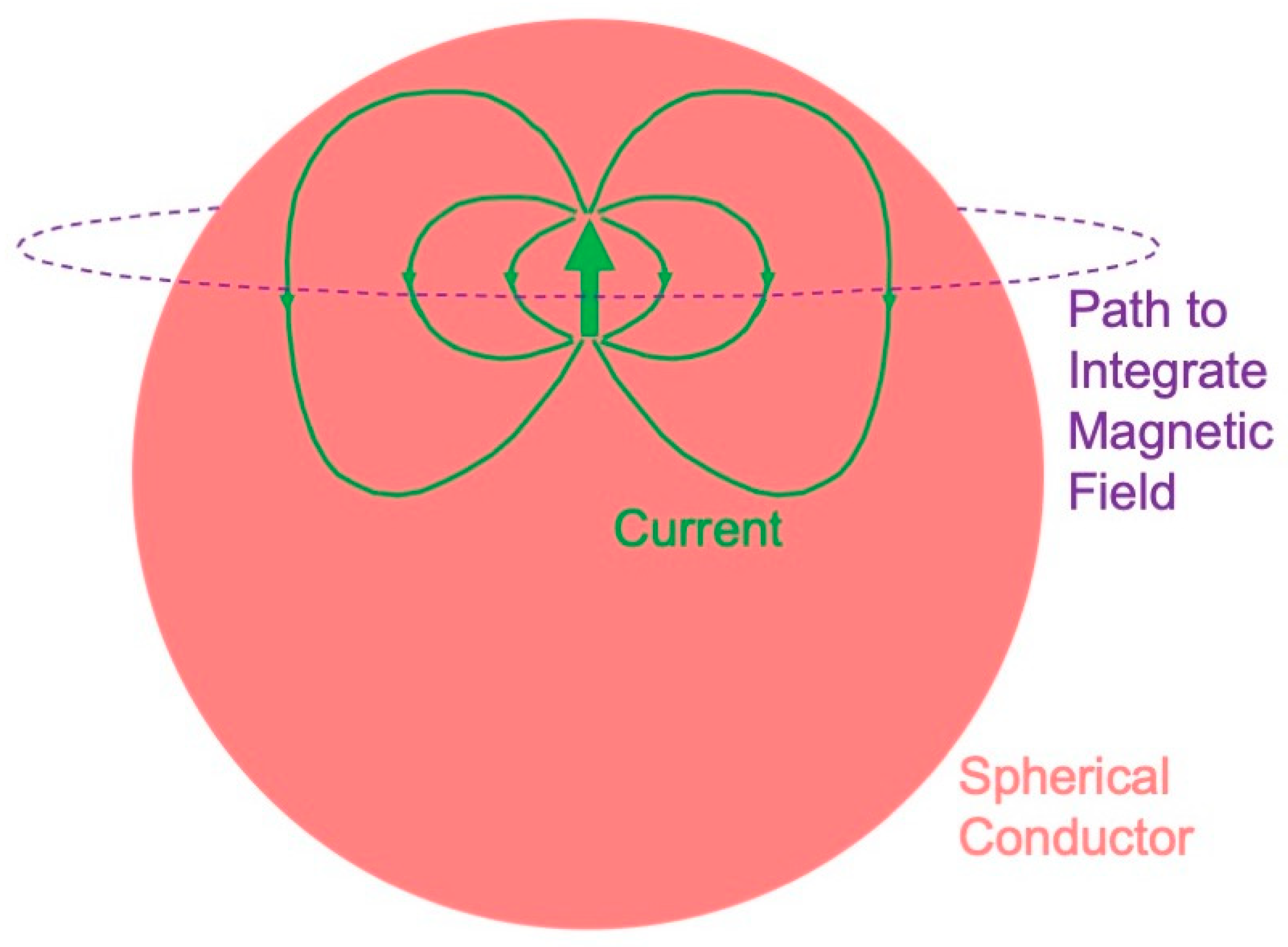
4.2. Magnetic Field of a Dipole in a Spherical Conductor
- A radial dipole produces no magnetic field (Figure 8). This result is best proven using Ampere’s law: the magnetic field integrated along a closed loop is proportional to the net current threading the loop. The symmetry is sufficient that the integral over the path (dashed circle in Figure 8) equals the path length times the magnetic field. The current produced by a dipole, including the return current, must be contained within the sphere because the region outside is not conducting. Hence, the net current threading the loop (dipole plus return current) is zero, so the magnetic field of a radial dipole vanishes.
- The radial component of the magnetic field is the same as for a dipole in a homogenous, unbounded conductor. In other words, the conductor–insulator interface at the surface of the sphere does not affect the radial component of the magnetic field. Furthermore, this result holds for a heterogeneous sphere, as long as the conductivity varies only with depth. For these reasons, investigators prefer measuring the radial component of the magnetic field when studying the MEG.
- The tangential component of the magnetic field is affected by the conductor–insulator boundary, but it does not depend on a conductivity that radially varies.
4.3. Relative Merits of the Magnetoencephalogram and the Electroencephalogram
4.4. Solving the Inverse Problem
4.5. Clinical Applications
5. New Technologies
5.1. Optically Pumped Alkali Vapor Magnetometers
5.2. Other Magnetometer Technologies
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baule, G.M.; McFee, R. Detection of the magnetic field of the heart. Am. Heart J. 1963, 66, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography: Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413–497. [Google Scholar] [CrossRef]
- Baule, G.; McFee, R. Theory of magnetic detection of the heart’s electrical activity. J. Appl. Phys. 1965, 36, 2066–2077. [Google Scholar] [CrossRef]
- Cohen, D. Magnetic fields around the torso: Production by electrical activity of the human heart. Science 1967, 156, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.E. SQUID instruments and shielding for low-level magnetic measurements. J. Appl. Phys. 1977, 48, 702–710. [Google Scholar] [CrossRef]
- Stroink, G.; Blackford, B.; Brown, B.; Horacek, M. Aluminum shielded room for biomagnetic measurements. Rev. Sci. Instrum. 1981, 52, 463–468. [Google Scholar] [CrossRef]
- Ma, Y.P.; Wikswo, J.P. Magnetic shield for wide-bandwidth magnetic measurements for nondestructive testing and biomagnetism. Rev. Sci. Instrum. 1991, 62, 2654–2661. [Google Scholar] [CrossRef]
- Cohen, D.; Edelsack, E.A.; Zimmerman, J.E. Magnetocardiograms taken inside a shielded room with a superconducting point-contact magnetometer. Appl. Phys. Lett. 1970, 16, 278–280. [Google Scholar] [CrossRef]
- Romani, G.L.; Williamson, S.J.; Kaufman, L. Biomagnetic instrumentation. Rev. Sci. Instrum. 1982, 53, 1815–1845. [Google Scholar] [CrossRef]
- Fagaly, R.L. Superconducting quantum interference device instruments and applications. Rev. Sci. Instrum. 2006, 77, 101101. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Frederick, N.V. Miniature ultrasensitive superconducting magnetic gradiometer and its use in cardiography and other applications. Appl. Phys. Lett. 1971, 19, 16–19. [Google Scholar] [CrossRef]
- Cohen, D.; Chandler, L. Measurements and a simplified interpretation of magnetocardiograms from humans. Circulation 1969, 39, 395–402. [Google Scholar] [CrossRef]
- Cohen, D.; Lepeschkin, E.; Hosaka, H.; Massell, B.F.; Myers, G. Abnormal patterns and physiological variations in magnetocardiograms. J. Electrocardiol. 1976, 9, 398–409. [Google Scholar] [CrossRef]
- Kariniemi, V.; Ahopelto, J.; Karp, P.J.; Katila, T.E. The fetal magnetocardogram. J. Perinat. Med. 1974, 2, 214–216. [Google Scholar]
- Saarinen, M.; Karp, P.J.; Katila, T.E.; Siltanen, P. The magnetocardiogram in cardiac disorders. Cardiovasc. Res. 1974, 8, 820–834. [Google Scholar]
- Rosen, A.; Inouye, G.T.; Morse, A.L.; Judge, D.L. Magnetic recordings of the heart’s electrical activity with a cryogenic magnetometer. J. Appl. Phys. 1971, 42, 3682–3684. [Google Scholar] [CrossRef]
- Farrell, D.E.; Tripp, J.H.; Norgren, R. Magnetic study of the His-Purkinje conduction system in man. IEEE Trans. Biomed. Eng. 1980, 27, 345–350. [Google Scholar] [CrossRef]
- Barry, W.H.; Fairbank, W.M.; Harrison, D.C.; Lehrman, K.L.; Malmivuo, J.; Wikswo, J.P. Measurement of the human magnetic heart vector. Science 1977, 198, 1159–1162. [Google Scholar] [CrossRef]
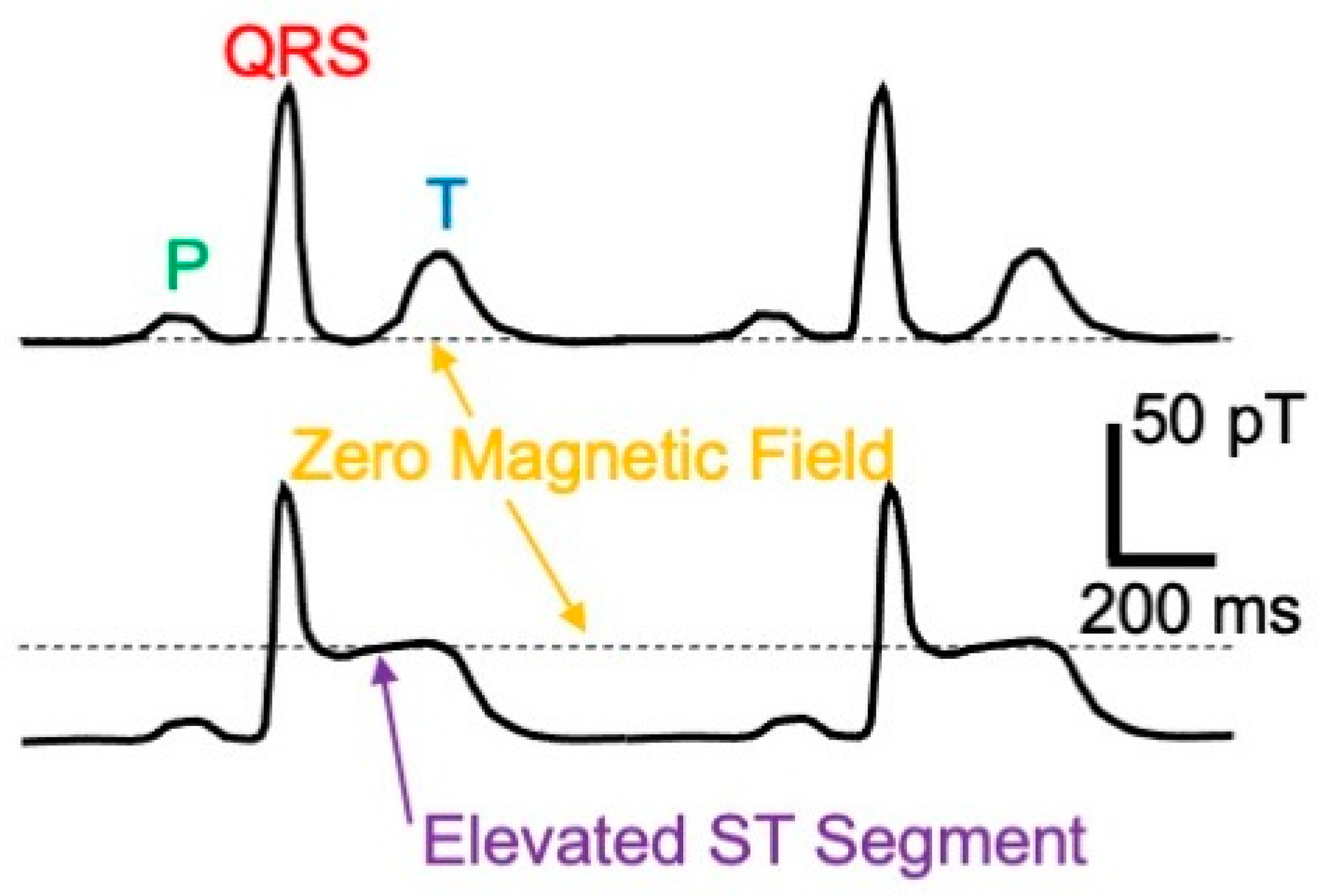
- Cohen, D.; Kaufman, L.A. Magnetic determination of the relationship between the S-T segment shift and the injury current produced by coronary artery occlusion. Circ. Res. 1975, 36, 414–424. [Google Scholar] [CrossRef]
- Stroink, G.; Meeder, R.J.J.; Elliott, P.; Lant, J.; Gardner, M.J. Arrhythmia vulnerability assessment using magnetic field maps and body surface potential maps. Pacing Clin. Electrophysiol. 1999, 22, 1718–1728. [Google Scholar] [CrossRef]
- Hänninen, H.; Takala, P.; Korhonen, P.; Oikarinen, L.; Mäkijärvi, M.; Nenonen, J.; Katila, T.; Toivonen, L. Features of ST segment and T-wave in exercise-induced myocardial ischemia evaluated with multichannel magnetocardiography. Ann. Med. 2002, 34, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Goernig, M.; Liehr, M.; Tute, C.; Schlosser, M.; Haueisen, J.; Figulla, H.R.; Leder, U. Magnetocardiography based spatiotemporal correlation analysis is superior to conventional ECG analysis for identifying myocardial injury. Ann. Biomed. Eng. 2009, 37, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.S.W.; Leithäuser, B.; Park, J.-W.; Yu, C.-M. Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: A review of clinical data. Int. J. Cardiol. 2013, 167, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Geselowitz, D.B. On the magnetic field generated outside an inhomogeneous volume conductor by internal current sources. IEEE Trans. Magn. 1970, 6, 346–347. [Google Scholar] [CrossRef]
- Plonsey, R. The nature of sources of bioelectric and biomagnetic fields. Biophys. J. 1982, 39, 309–312. [Google Scholar] [CrossRef]
- Horáček, B. Digital model for studies in magnetocardiography. IEEE Trans. Magn. 1973, 9, 440–444. [Google Scholar] [CrossRef]
- Hren, R.; Stroink, G.; Horáček, B.M. Spatial resolution of body surface potential maps and magnetic field maps: A model study applied to the identification of ventricular preexcitation sites. Med. Biol. Eng. Comput. 1998, 36, 145–157. [Google Scholar] [CrossRef]
- Purcell, C.J.; Stroink, G.; Horacek, B.M. Effect of torso boundaries on electric potential and magnetic field of a dipole. IEEE Trans. Biomed. Eng. 1988, 35, 671–678. [Google Scholar] [CrossRef]
- Nenonen, J.; Purcell, C.J.; Horacek, B.M.; Stroink, G.; Katila, T. Magnetocardiographic functional localization using a current dipole in a realistic torso. IEEE Trans. Biomed. Eng. 1991, 38, 658–664. [Google Scholar] [CrossRef]
- Plonsey, R. Capability and limitations of electrocardiography and magnetocardiography. IEEE Trans. Biomed. Eng. 1972, 19, 239–244. [Google Scholar] [CrossRef]
- Rush, S. On the independence of magnetic and electric body surface recordings. IEEE Trans. Biomed. Eng. 1975, 22, 157–167. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Barach, J.P. Possible sources of new information in the magnetocardiogram. J. Theor. Biol. 1982, 95, 721–729. [Google Scholar] [CrossRef]
- Knuutila, J.; Ahlfors, S.; Ahonen, A.; Hällström, J.; Kajola, M.; Lounasmaa, O.V.; Vilkman, V.; Tesche, C. Large-area low-noise seven-channel dc SQUID magnetometer for brain research. Rev. Sci. Instrum. 1987, 58, 2145–2156. [Google Scholar] [CrossRef]
- Schneider, S.; Hoenig, E.; Reichenberger, H.; Abraham-Fuchs, K.; Moshage, W.; Oppelt, A.; Stefan, H.; Weikl, A.; Wirth, A. Multichannel biomagnetic system for study of the electrical activity in the brain and heart. Radiology 1990, 176, 825–830. [Google Scholar] [CrossRef]
- Ahonen, A.I.; Hämäläinen, M.S.; Kajola, M.J.; Knuutila, J.E.T.; Laine, P.P.; Lounasmaa, O.V.; Parkkonen, L.T.; Simola, J.T.; Tesche, C.D. 122-channel SQUID instrument for investigating the magnetic signals from the human brain. Phys. Scr. 1993, 49, 198. [Google Scholar] [CrossRef]
- Fenici, R.; Brisinda, D.; Meloni, A.M. Clinical application of magnetocardiography. Expert Rev. Mol. Diagn. 2005, 5, 291–313. [Google Scholar] [CrossRef]
- Korhonen, P.; Husa, T.; Tierala, I.; Väänänen, H.; Mäkijärvi, M.; Katila, T.; Toivonen, L. Increased intra-QRS fragmentation in magnetocardiography as a predictor of arrhythmic events and mortality in patients with cardiac dysfunction after myocardial infarction. J. Cardiovasc. Electrophysiol. 2006, 17, 396–401. [Google Scholar] [CrossRef]
- van Leeuwen, P.; Halier, B.; Bader, W.; Geissler, J.; Trowitzsch, E.; Grönemeyer, D.H.W. Magnetocardiography in the diagnosis of fetal arrhythmia. Br. J. Obstet. Gynaecol. 1999, 106, 1200–1208. [Google Scholar] [CrossRef]
- Tantimongcolwat, T.; Naenna, T.; Isarankura-Na-Ayudhya, C.; Embrechts, M.J.; Prachayasittikul, V. Identification of ischemic heart disease via machine learning analysis on magnetocardiograms. Comput. Biol. Med. 2008, 38, 817–825. [Google Scholar] [CrossRef]
- Kangwanariyakul, Y.; Nantasenamat, C.; Tantimongcolwat, T.; Naenna, T. Data mining of magnetocardiograms for prediction of ischemic heart disease. EXCLI J. 2010, 9, 82–95. [Google Scholar]
- Tao, R.; Zhang, S.; Huang, X.; Tao, M.; Ma, J.; Ma, S.; Zhang, C.; Zhang, T.; Tang, F.; Lu, J.; et al. Magnetocardiography-based ischemic heart disease detection and localization using machine learning methods. IEEE Trans. Biomed. Eng. 2019, 66, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Barach, J.P.; Freeman, J.A. Magnetic field of a nerve impulse: First measurements. Science 1980, 208, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Samson, P.; Giffard, R.P. A low-noise low input impedance amplifier for magnetic measurements of nerve action currents. IEEE Trans. Biomed. Eng. 1983, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Wikswo, J.P. The magnetic field of a single axon: A comparison of theory and experiment. Biophys. J. 1985, 48, 93–109. [Google Scholar] [CrossRef]
- Gielen, F.L.H.; Roth, B.J.; Wikswo, J.P. Capabilities of a toroid-amplifier system for magnetic measurement of current in biological tissue. IEEE Trans. Biomed. Eng. 1986, 33, 910–921. [Google Scholar] [CrossRef]
- Wijesinghe, R.S.; Gielen, F.L.H.; Wikswo, J.P. A model for compound action potentials and currents in a nerve bundle. I: The forward calculation. Ann. Biomed. Eng. 1991, 19, 43–72. [Google Scholar] [CrossRef]
- Wijesinghe, R.S.; Wikswo, J.P. A model for compound action potentials and currents in a nerve bundle. II: A sensitivity analysis of model parameters for the forward and inverse calculations. Ann. Biomed. Eng. 1991, 19, 73–96. [Google Scholar] [CrossRef]
- Wijesinghe, R.S.; Gielen, F.L.H.; Wikswo, J.P. A model for compound action potentials and currents in a nerve bundle. III: A comparison of the conduction velocity distributions calculated from compound action currents and potentials. Ann. Biomed. Eng. 1991, 19, 97–121. [Google Scholar] [CrossRef]
- van Egeraat, J.M.; Friedman, R.N.; Wikswo, J.P. Magnetic field of a single muscle fiber: First measurements and a core conductor model. Biophys. J. 1990, 57, 663–667. [Google Scholar] [CrossRef]
- van Egeraat, J.M.; Stasaski, R.; Barach, J.P.; Friedman, R.N.; Wikswo, J.P. The biomagnetic signature of a crushed axon: A comparison of theory and experiment. Biophys. J. 1993, 64, 1299–1305. [Google Scholar] [CrossRef]
- Leifer, M.C.; Wikswo, J.P. Optimization of a clip-on SQUID current probe. Rev. Sci. Instrum. 1983, 54, 1017–1022. [Google Scholar] [CrossRef]
- Hentz, V.R.; Wikswo, J.P.; Abraham, G.S. Magnetic measurement of nerve action currents: A new intraoperative recording technique. Peripher. Nerve Regen. 1986, 1, 27–36. [Google Scholar]
- Walbeehm, E.T.; van Heel, E.B.M.D.; Kuypers, P.D.L.; Terenghi, G.; Hovius, S.E.R. Nerve compound action current (NCAC) measurements and morphometric analysis in the proximal segment after nerve transection and repair in a rabbit model. J. Peripher. Nerv. Syst. 2003, 8, 108–115. [Google Scholar] [CrossRef]
- Swinney, K.R.; Wikswo, J.P. A calculation of the magnetic field of a nerve action potential. Biophys. J. 1980, 32, 719–731. [Google Scholar] [CrossRef]
- Woosley, J.K.; Roth, B.J.; Wikswo, J.P. The magnetic field of a single axon: A volume conductor model. Math. Biosci. 1985, 76, 1–36. [Google Scholar] [CrossRef]
- Barach, J.P. The effect of ohmic return currents on biomagnetic fields. J. Theor. Biol. 1987, 125, 187–191. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Swinney, K.R. A comparison of scalar multipole expansions. J. Appl. Phys. 1984, 56, 3039–3049. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Swinney, K.R. Scalar multipole expansions and their dipole equivalents. J. Appl. Phys. 1985, 57, 4301–4308. [Google Scholar] [CrossRef]
- Roth, B.J.; Woods, M.C. The magnetic field associated with a plane wave front propagating through cardiac tissue. IEEE Trans. Biomed. Eng. 1999, 46, 1288–1292. [Google Scholar] [CrossRef]
- Weber dos Santos, R.; Koch, H. Interpreting biomagnetic fields of planar wave fronts in cardiac muscle. Biophys. J. 2005, 88, 3731–3733. [Google Scholar] [CrossRef]
- Barbosa, C.R.H. Simulation of a plane wavefront propagating in cardiac tissue using a cellular automata model. Phys. Med. Biol. 2003, 48, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Holzer, J.R.; Fong, L.E.; Sidorov, V.Y.; Wikswo, J.P.; Baudenbacher, F. High resolution magnetic images of planar wave fronts reveal bidomain properties of cardiac tissue. Biophys. J. 2004, 87, 4326–4332. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Guo, W.-Q.; Wikswo, J.P. The effects of spiral anisotropy on the electric potential and the magnetic field at the apex of the heart. Math. Biosci. 1988, 88, 191–221. [Google Scholar] [CrossRef]
- Murdick, R.A.; Roth, B.J. A comparative model of two mechanisms from which a magnetic field arises in the heart. J. Appl. Phys. 2004, 95, 5116–5122. [Google Scholar] [CrossRef]
- McBride, K.K.; Roth, B.J.; Sidorov, V.Y.; Wikswo, J.P.; Baudenbacher, F.J. Measurements of transmembrane potential and magnetic field at the apex of the heart. Biophys. J. 2010, 99, 3113–3118. [Google Scholar] [CrossRef]
- Fong, L.E.; Holzer, J.R.; McBride, K.K.; Lima, E.A.; Baudenbacher, F. High-resolution room-temperature sample scanning superconducting quantum interference device microscope configurable for geological and biomagnetic applications. Rev. Sci. Instrum. 2005, 76, 053703. [Google Scholar] [CrossRef]
- Sepulveda, N.G.; Wikswo, J.P. Electric and magnetic fields from two-dimensional anisotropic bisyncytia. Biophys. J. 1987, 51, 557–568. [Google Scholar] [CrossRef]
- Baudenbacher, F.; Peters, N.T.; Baudenbacher, P.; Wikswo, J.P. High resolution imaging of biomagnetic fields generated by action currents in cardiac tissue using a LTS-SQUID microscope. Physica C 2002, 368, 24–31. [Google Scholar] [CrossRef]
- Roth, B.J.; Sepulveda, N.G.; Wikswo, J.P. Using a magnetometer to image a two-dimensional current distribution. J. Appl. Phys. 1989, 65, 361–372. [Google Scholar] [CrossRef]
- Dallas, W.J. Fourier space solution to the magnetostatic imaging problem. Appl. Opt. 1985, 24, 4543–4546. [Google Scholar] [CrossRef]
- Cohen, D. Magnetoencephalography: Evidence of magnetic fields produced by alpha-rhythm currents. Science 1968, 161, 784–786. [Google Scholar] [CrossRef]
- Cohen, D. Magnetoencephalography: Detection of the brain’s electrical activity with a superconducting magnetometer. Science 1972, 175, 664–666. [Google Scholar] [CrossRef]
- Okada, Y.C.; Wu, J.; Kyuhou, S. Genesis of MEG signals in a mammalian CNS structure. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 474–485. [Google Scholar] [CrossRef]
- Murakami, S.; Okada, Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J. Physiol. 2006, 575, 925–936. [Google Scholar] [CrossRef]
- Brenner, D.; Williamson, S.J.; Kaufman, L. Visually evoked magnetic fields of the human brain. Science 1975, 190, 480–482. [Google Scholar] [CrossRef]
- Teyler, T.J.; Cuffin, B.N.; Cohen, D. The visual evoked magnetoencephalogram. Life Sci. 1975, 17, 683–691. [Google Scholar] [CrossRef]
- Reite, M.; Zimmerman, J.E.; Edrich, J.; Zimmerman, J. The human magnetoencephalogram: Some EEG and related correlations. Electroencephalogr. Clin. Neurophysiol. 1976, 40, 59–66. [Google Scholar] [CrossRef]
- Brenner, D.; Lipton, J.; Kaufman, L.; Williamson, S.J. Somatically evoked magnetic fields of the human brain. Science 1978, 199, 81–83. [Google Scholar] [CrossRef]
- Okada, Y.C.; Williamson, S.J.; Kaufman, L. Magnetic field of the human sensorimotor cortex. Int. J. Neurosci. 1982, 17, 33–38. [Google Scholar] [CrossRef]
- Cheyne, D.; Weinberg, H. Neuromagnetic fields accompanying unilateral finger movements: Pre-movement and movement-evoked fields. Exp. Brain Res. 1989, 78, 604–612. [Google Scholar] [CrossRef]
- Reite, M.; Edrich, J.; Zimmerman, J.T.; Zimmerman, J.E. Human magnetic auditory evoked fields. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Aittoniemi, K.; Järvinen, M.-L.; Katila, T.; Varpula, T. Auditory evoked transient and sustained magnetic fields of the human brain localization of neural generators. Exp. Brain Res. 1980, 40, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Kaila, K.; Katila, T.; Tuomisto, T.; Varpula, T. Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: Implications for their neural generation. Electroencephalogr. Clin. Neurophysiol. 1982, 54, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Reinikainen, K.; Kaukoranta, E.; Hämäläinen, M.; Ilmoniemi, R.; Penttinen, A.; Salminen, J.; Teszner, D. Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalogr. Clin Neurophysiol. 1984, 57, 254–263. [Google Scholar] [CrossRef]
- Hari, R.; Hämäläinen, M.; Ilmoniemi, R.; Kaukoranta, E.; Reinikainen, K.; Salminen, J.; Alho, K.; Näätänen, R.; Sams, M. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: Neuromagnetic recordings in man. Neurosci. Lett. 1984, 50, 127–132. [Google Scholar] [CrossRef]
- Pantev, C.; Makeig, S.; Hoke, M.; Galambos, R.; Hampson, S.; Gallen, C. Human auditory evoked gamma-band magnetic fields. Proc. Natl. Acad. Sci. USA 1991, 88, 8996–9000. [Google Scholar] [CrossRef]
- Romani, G.L.; Williamson, S.J.; Kaufman, L. Tonotopic organization of the human auditory cortex. Science 1982, 216, 1339–1340. [Google Scholar] [CrossRef]
- Grynszpan, F.; Geselowitz, D.B. Model studies of the magnetocardiogram. Biophys. J. 1973, 13, 911–925. [Google Scholar] [CrossRef]
- Cuffin, B.N.; Cohen, D. Magnetic fields of a dipole in special volume conductor shapes. IEEE Trans. Biomed. Eng. 1977, 24, 372–381. [Google Scholar] [CrossRef]
- Cohen, D.; Cuffin, B.N. Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 1983, 56, 38–51. [Google Scholar] [CrossRef]
- Sarvas, J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys. Med. Biol. 1987, 32, 11–22. [Google Scholar] [CrossRef]
- Kaufman, L.; Kaufman, J.H.; Wang, J.-Z. On cortical folds and neuromagnetic fields. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 211–226. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Sarvas, J. Feasibility of the homogeneous head model in the interpretation of neuromagnetic fields. Phys. Med. Biol. 1987, 32, 91–97. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Sarvas, J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989, 36, 165–171. [Google Scholar] [CrossRef]
- Meijs, J.W.H.; Bosch, F.G.C.; Peters, M.J.; Lopes da Silva, F.H. On the magnetic field distribution generated by a dipolar current source situated in a realistically shaped compartment model of the head. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 286–298. [Google Scholar] [CrossRef]
- Weinberg, H.; Brickett, P.; Coolsma, F.; Baff, M. Magnetic localisation of intracranial dipoles: Simulation with a physical model. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 159–170. [Google Scholar] [CrossRef]
- Barth, D.S.; Sutherling, W.; Broffman, J.; Beatty, J. Magnetic localization of a dipolar current source implanted in a sphere and a human cranium. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 260–273. [Google Scholar] [CrossRef]
- Rose, D.F.; Ducla-Soares, E.; Sato, S. Improved accuracy of MEG localization in the temporal region with inclusion of volume current effects. Brain Topogr. 1989, 1, 175–181. [Google Scholar] [CrossRef]
- Leahy, R.M.; Mosher, J.C.; Spencer, M.E.; Huang, M.X.; Lewine, J.D. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 159–173. [Google Scholar] [CrossRef]
- Okada, Y.C.; Lahteenmäki, A.; Xu, C. Experimental analysis of distortion of magnetoencephalography signals by the skull. Clin. Neurophysiol. 1999, 110, 230–238. [Google Scholar] [CrossRef]
- Cuffin, B.N.; Cohen, D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 1979, 47, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Cuffin, B.N.; Yunokuchi, K.; Maniewski, R.; Purcell, C.; Cosgrove, G.R.; Ives, J.; Kennedy, J.G.; Schomer, D.L. MEG versus EEG localization test using implanted sources in the human brain. Ann. Neurol. 1990, 28, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.J. MEG versus EEG localization test. Ann. Neurol. 1991, 30, 222. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Hämäläinen, M.; Ilmoniemi, R.; Lounasmaa, O.V. Comment on “MEG versus EEG localization test using implanted sources in the human brain”. Ann. Neurol. 1991, 30, 222–223. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Gevins, A.; Williamson, S.J. The future of EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 1–9. [Google Scholar] [CrossRef]
- Baillet, S.; Garnero, L. A Bayesian approach to introducing anatomo-functional priors in the EEG/MEG inverse problem. IEEE Trans. Biomed. Eng. 1997, 44, 374–385. [Google Scholar] [CrossRef]
- Ueno, S.; Iramina, K. Modeling and source localization of MEG activities. Brain Topogr. 1990, 3, 151–165. [Google Scholar] [CrossRef]
- Iramina, K.; Ueno, S. Source estimation of spontaneous MEG activity and auditory evoked responses in normal subjects during sleep. Brain Topogr. 1996, 8, 297–301. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Williamson, S.J.; Kaufman, L. Magnetic source images determined by a lead-field analysis: The unique minimum-norm least-squares estimation. IEEE Trans. Biomed. Eng. 1992, 39, 665–675. [Google Scholar] [CrossRef]
- Jeffs, B.; Leahy, R.; Singh, M. An evaluation of methods for neuromagnetic image reconstruction. IEEE Trans. Biomed. Eng. 1987, 34, 713–723. [Google Scholar] [CrossRef]
- Mosher, J.C.; Lewis, P.S.; Leahy, R.M. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans. Biomed. Eng. 1992, 39, 541–557. [Google Scholar] [CrossRef]
- Mosher, J.C.; Leahy, R.M. Recursive MUSIC: A framework for EEG and MEG source localization. IEEE Trans. Biomed. Eng. 1998, 45, 1342–1354. [Google Scholar] [CrossRef]
- Mosher, J.C.; Leahy, R.M. Source localization using recursively applied and projected (RAP) MUSIC. IEEE Trans. Signal Process. 1999, 47, 332–340. [Google Scholar] [CrossRef]
- van Veen, B.D.; van Drongelen, W.; Yuchtman, M.; Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997, 44, 867–880. [Google Scholar] [CrossRef]
- Hillebrand, A.; Singh, K.D.; Holliday, I.E.; Furlong, P.L.; Barnes, G.R. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 2005, 25, 199–211. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar]
- Pascual-Marqui, R.D.; Esslen, M.; Lehmann, D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 91–95. [Google Scholar]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Ioannides, A.A.; Bolton, J.P.R.; Clarke, C.J.S. Continuous probabilistic solutions to the biomagnetic inverse problem. Inverse Probl. 1990, 6, 523–542. [Google Scholar] [CrossRef]
- Ribary, U.; Ioannides, A.A.; Singh, K.D.; Hasson, R.; Bolton, J.P.; Lado, F.; Mogilner, A.; Llinás, R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Natl. Acad. Sci. USA 1991, 88, 11037–11041. [Google Scholar] [CrossRef]
- Gorodnitsky, I.F.; George, J.S.; Rao, B.D. Neuromagnetic source imaging with FOCUSS: A recursive weighted minimum norm algorithm. Electroencephalogr. Clin. Neurophysiol. 1995, 95, 231–251. [Google Scholar] [CrossRef]
- Bowyer, S.M.; Moran, J.E.; Mason, K.M.; Constantinou, J.E.; Smith, B.J.; Barkley, G.L.; Tepley, N. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology 2004, 62, 2247–2255. [Google Scholar] [CrossRef]
- Moran, J.E.; Bowyer, S.M.; Tepley, N. Multi-resolution FOCUSS: A source imaging technique applied to MEG data. Brain Topogr. 2005, 18, 1–17. [Google Scholar] [CrossRef]
- Bowyer, S.M. Coherence a measure of brain networks: Past and present. Neuropsychiat. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Schoffelen, J.-M.; Gross, J. Source connectivity analysis with MEG and EEG. Hum. Brain Mapp. 2009, 30, 1857–1865. [Google Scholar] [CrossRef]
- Hillebrand, A.; Barnes, G.R.; Bosboom, J.L.; Berendse, H.W.; Stam, C.J. Frequency-dependent functional connectivity within resting-state networks: An atlas-based MEG beamformer solution. NeuroImage 2012, 59, 3909–3921. [Google Scholar] [CrossRef]
- Barth, D.S.; Sutherling, W.; Engel, J.; Beatty, J. Neuromagnetic localization of epileptiform spike activity in the human brain. Science 1982, 218, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.F.; Smith, P.D.; Sato, S. Magnetoencephalography and epilepsy research. Science 1987, 238, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Rampp, S.; Stefan, H.; Wu, X.; Kaltenhäuser, M.; Maess, B.; Schmitt, F.C.; Wolters, C.H.; Hamer, H.; Kasper, B.S.; Schwab, S.; et al. Magnetoencephalography for epileptic focus localization in a series of 1000 cases. Brain 2019, 142, 3059–3071. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Magnetoencephalograhy: From SQUIDs to neuroscience: Neuroimage 20th anniversary special edition. NeuroImage 2012, 61, 386–396. [Google Scholar] [CrossRef]
- Salmelin, R.; Kiesilä, P.; Uutela, K.; Service, E.; Solonen, O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann. Neurol. 1996, 40, 157–162. [Google Scholar] [CrossRef]
- Volkmann, J.; Joliot, M.; Mogilner, A.; Ioannides, A.A.; Lado, F.; Fazzini, E.; Ribary, U.; Llinás, R. Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology 1996, 46, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.M.; Aurora, S.K.; Moran, J.E.; Tepley, N.; Welch, K.M.A. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann. Neurol. 2001, 50, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, F.; Roehri, N.; Villalon, S.M.; Trébuchon, A.; Chen, S.; Lagarde, S.; Carron, R.; Gavaret, M.; Giusiano, B.; McGonigal, A.; et al. Deep brain activities can be detected with magnetoencephalography. Nat. Commun. 2019, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Koelle, D.; Kleiner, R.; Ludwig, F.; Dantsker, E.; Clarke, J. High-transition-temperature superconducting quantum interference devices. Rev. Mod. Phys. 1999, 71, 631–686. [Google Scholar] [CrossRef]
- Faley, M.I.; Dammers, J.; Maslennikov, Y.V.; Schneiderman, J.F.; Winkler, D.; Koshelets, V.P.; Shah, N.J.; Dunin-Borkowki, R.E. High-Tc SQUID biomagnetometers. Supercond. Sci. Technol. 2017, 30, 083001. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. NeuroImage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Pantazis, D.; Adler, A. MEG source localization via deep learning. Sensors 2021, 21, 4278. [Google Scholar] [CrossRef]
- Bison, G.; Wynands, R.; Weis, A. A laser-pumped magnetometer for the mapping of human cardiomagnetic fields. Appl. Phys. B 2003, 76, 325–328. [Google Scholar] [CrossRef]
- Xia, H.; Baranga, B.-A.; Hoffman, D.; Romalis, M.V. Magnetoencephalography with an atomic magnetometer. Appl. Phys. Lett. 2006, 89, 211104. [Google Scholar] [CrossRef]
- Bison, G.; Castagna, N.; Hofer, A.; Knowles, P.; Schenker, J.-L.; Kasprzak, M.; Saudan, H.; Weis, A. A room temperature 19-channel magnetic field mapping device for cardiac signals. Appl. Phys. Lett. 2009, 95, 173701. [Google Scholar] [CrossRef]
- Shah, V.K.; Wakai, R.T. A compact, high performance atomic magnetometer for biomedical applications. Phys. Med. Biol. 2013, 58, 8153–8161. [Google Scholar] [CrossRef]
- Sander, T.H.; Preusser, J.; Mhaskar, R.; Kitching, J.; Trahms, L.; Knappe, S. Magnetoencephalography with a chip-scale atomic magnetometer. Biomed. Opt. Express 2012, 3, 981–990. [Google Scholar] [CrossRef]
- Boto, E.; Meyer, S.S.; Shah, V.; Alem, O.; Knappe, S.; Kruger, P.; Fromhold, T.M.; Lim, M.; Glover, P.M.; Morris, P.G.; et al. A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers. NeuroImage 2017, 149, 404–414. [Google Scholar] [CrossRef]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef]
- Brookes, M.J.; Leggett, J.; Rea, M.; Hill, R.M.; Holmes, N.; Boto, E.; Bowtell, R. Magnetoencephalography with optically pumped magnetometers (OPM-MEG): The next generation of functional neuroimaging. Trends Neurosci. 2022, 45, 621–634. [Google Scholar] [CrossRef]
- Borna, A.; Carter, T.R.; Colombo, A.P.; Jau, Y.-Y.; McKay, J.; Weisend, M.; Taulu, S.; Stephen, J.M.; Schwindt, P.D.D. Non-invasive functional-brain-imaging with an OPM-based magnetoencephalography system. PLoS ONE 2020, 15, e0227684. [Google Scholar] [CrossRef]
- Feys, O.; Corvilain, P.; Aeby, A.; Sculier, C.; Holmes, N.; Brookes, M.; Goldman, S.; Wens, V.; De Tiège, X. On-scalp optically pumped magnetometers versus cryogenic magnetoencephalography for diagnostic evaluation of epilepsy in school-aged children. Radiology 2022, 304, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.F.; Turner, M.J.; Schloss, J.M.; Glenn, D.R.; Song, Y.; Lukin, M.D.; Park, H.; Walsworth, R.L. Optical magnetic detection of single-neuron action potentials using quantum defects in diamond. Proc. Natl. Acad. Sci. USA 2016, 113, 14133–14138. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.L.; Troise, L.; Hansen, N.W.; Olsson, C.; Wojciechowski, A.M.; Achard, J.; Brinza, O.; Staacke, R.; Kieschnick, M.; Meijer, J.; et al. Detection of biological signals from a live mammalian muscle using an early stage diamond quantum sensor. Sci. Rep. 2021, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-M.; Hu, L.; Fu, X. Integrated giant magnetoresistance technology for approachable weak biomagnetic signal detections. Sensors 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Tonini, D.; Liang, S.; Saha, R.; Chugh, V.K.; Wang, J.-P. Giant magnetoresistance biosensors in biomedical applications. ACS Appl. Mater. Interfaces 2022, 14, 9945–9969. [Google Scholar] [CrossRef]
- Caruso, L.; Wunderle, T.; Lewis, C.M.; Valadeiro, J.; Trauchessec, V.; Rosillo, J.T.; Amaral, J.P.; Ni, J.; Jendritza, P.; Fermon, C.; et al. In vivo magnetic recording of neuronal activity. Neuron 2017, 95, 1283–1291. [Google Scholar] [CrossRef]
- Chopin, C.; Torrejon, J.; Solignac, A.; Fermon, C.; Jendritza, P.; Fries, P.; Pannetier-Lecoeur, M. Magnetoresistive sensor in two-dimension on a 25 μm thick silicon substrate for in vivo neuronal measurements. ACS Sens. 2020, 5, 3493–3500. [Google Scholar] [CrossRef]
- Kanno, A.; Nakasato, N.; Oogane, M.; Fujiwara, K.; Nakano, T.; Arimoto, T.; Matsuzaki, H.; Ando, Y. Scalp attached tangential magnetoencephalography using tunnel magneto-resistive sensors. Sci. Rep. 2022, 12, 6106. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhang, Q.; Filippov, D.A.; Li, K.; Wu, J.; Tao, J.; Jiang, L.; Cao, L.; Srinivasan, G. High-resolution magnetic sensors in ferrite/piezoelectric heterostructure with giant magnetodielectric effect at zero bias field. Rev. Sci. Instum. 2021, 92, 045006. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, B.J. Biomagnetism: The First Sixty Years. Sensors 2023, 23, 4218. https://doi.org/10.3390/s23094218
Roth BJ. Biomagnetism: The First Sixty Years. Sensors. 2023; 23(9):4218. https://doi.org/10.3390/s23094218
Chicago/Turabian StyleRoth, Bradley J. 2023. "Biomagnetism: The First Sixty Years" Sensors 23, no. 9: 4218. https://doi.org/10.3390/s23094218
APA StyleRoth, B. J. (2023). Biomagnetism: The First Sixty Years. Sensors, 23(9), 4218. https://doi.org/10.3390/s23094218





