Design and Study of a Reflector-Separated Light Dispersion-Compensated 3D Microscopy System
Abstract
1. Introduction
2. Theoretical Design
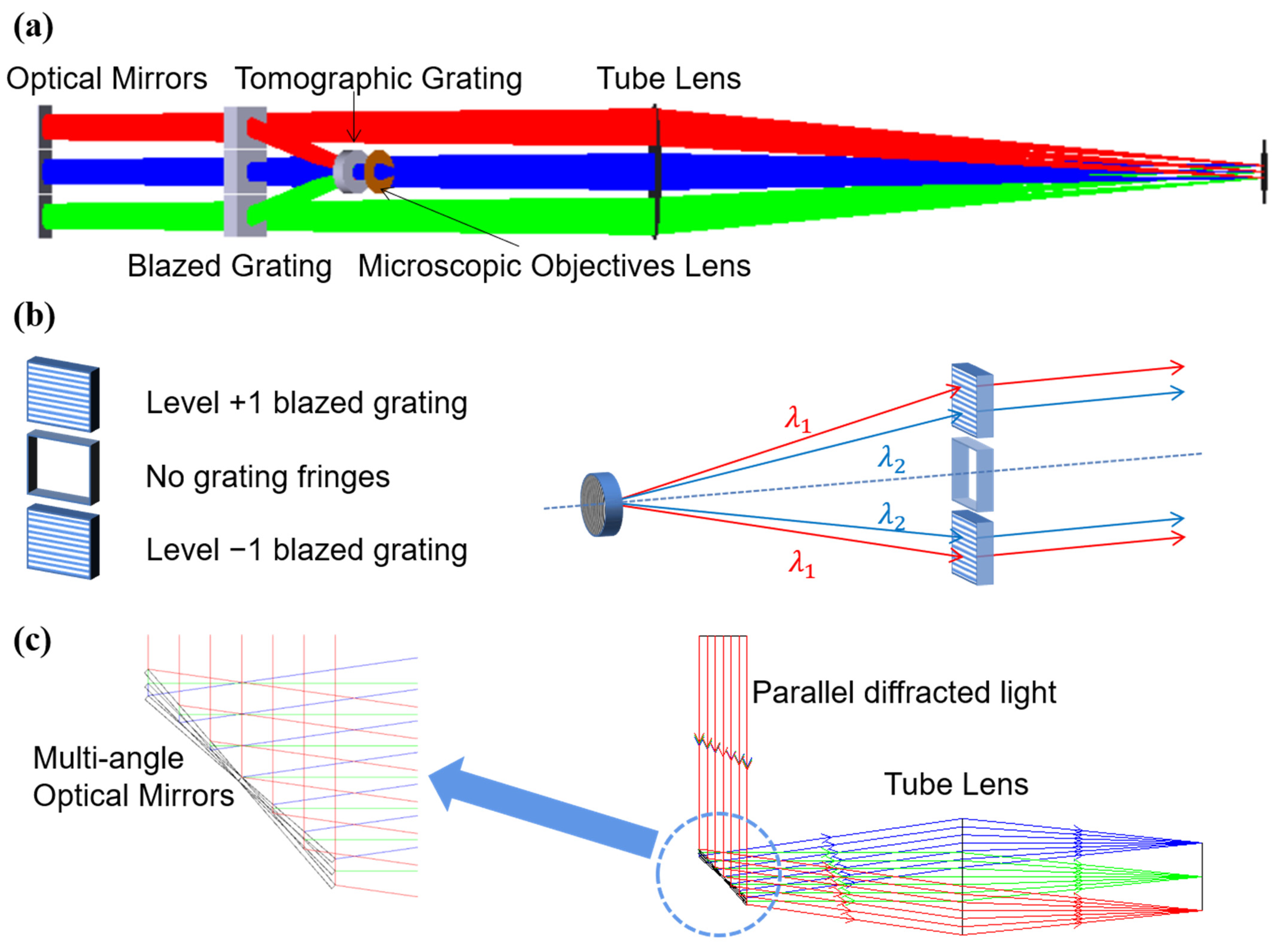
2.1. Composition of Reflector-Separated Light Dispersion-Compensated 3D Microscopy System
2.2. Theoretical Design and Parameter Selection of the Reflector-Separated Light Dispersion-Compensation Module
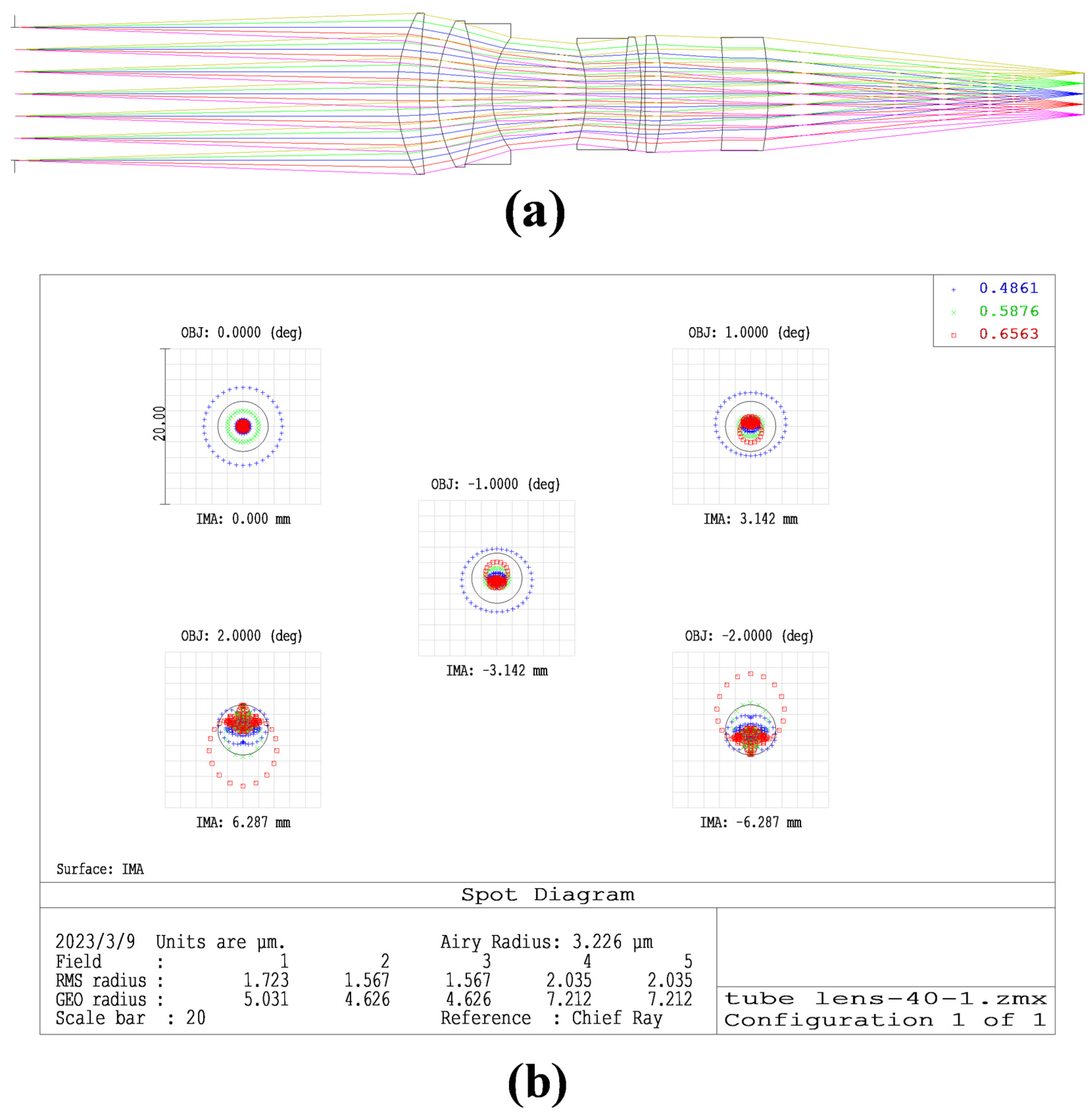
2.3. Imaging Module Parameters Selection
3. Results and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrahamsson, S.; Ilic, R.; Wisniewski, J.; Mehl, B.; Yu, L.; Chen, L.; Davanco, M.; Oudjedi, L.; Fiche, J.B.; Hajj, B.; et al. Multifocus microscopy with precise color multi-phase diffractive optics applied in functional neuronal imaging. Biomed. Opt. Express 2016, 7, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.J.; Zhao, T.; Yang, H.; Shaevitz, J.W. Multicolor multifocal 3D microscopy using in-situ optimization of a spatial light modulator. Sci. Rep. 2022, 12, 16343. [Google Scholar] [CrossRef]
- Alonso, J.R.; Silva, A.; Fernandez, A.; Arocena, M. Computational multifocus fluorescence microscopy for three-dimensional visualization of multicellular tumor spheroids. J. Biomed. Opt. 2022, 27, 066501. [Google Scholar] [CrossRef]
- Xiao, S.; Gritton, H.; Tseng, H.A.; Zemel, D.; Han, X.; Mertz, J. High-contrast multifocus microscopy with a single camera and z-splitter prism. Optica 2020, 7, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Laurent, J.; Frongia, C.; Cazales, M.; Mondesert, O.; Ducommun, B.; Lobjois, V. Multicellular tumor spheroid models to explore cell cycle checkpoints in 3D. BMC Cancer 2013, 13, 73. [Google Scholar] [CrossRef]
- Ram, S.; Kim, D.; Ober, R.J.; Ward, E.S. 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers. Biophys. J. 2012, 103, 1594–1603. [Google Scholar] [CrossRef]
- He, K.; Wang, Z.; Huang, X.; Wang, X.; Yoo, S.; Ruiz, P.; Gdor, I.; Selewa, A.; Ferrier, N.J.; Scherer, N.; et al. Computational multifocal microscopy. Biomed. Opt. Express. 2018, 9, 6477–6496. [Google Scholar] [CrossRef] [PubMed]
- Wolbromsky, L.; Dudaie, M.; Shinar, S.; Shaked, N.T. Multiplane imaging with extended field-of-view using a quadratically distorted grating. Opt. Commun. 2020, 463, 125399. [Google Scholar] [CrossRef]
- Jesacher, A.; Roider, C.; Ritsch-Marte, M. Enhancing diffractive multi-plane microscopy using colored illumination. Opt. Express 2013, 21, 11150–11161. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, J.; He, Y.; Tang, Y.; Hu, S.; Zhao, L. Quadratic grating apodized photon sieves for simultaneous multiplane microscopy. Opt. Lasers Eng. 2017, 97, 78–85. [Google Scholar] [CrossRef]
- Yang, Z.; Zhan, Q. Single-Shot Smartphone-Based Quantitative Phase Imaging Using a Distorted Grating. PLoS ONE 2016, 11, e0159596. [Google Scholar] [CrossRef]
- He, K.; Huang, X.; Wang, X.; Yoo, S.; Ruiz, P.; Gdor, I.; Ferrier, N.J.; Scherer, N.; Hereld, M.; Katsaggelos, A.K.; et al. Design and simulation of a snapshot multi-focal interferometric microscope. Opt. Express 2018, 26, 27381–27402. [Google Scholar] [CrossRef]
- Dalgarno, P.A.; Dalgarno, H.I.; Putoud, A.; Lambert, R.; Paterson, L.; Logan, D.C.; Towers, D.P.; Warburton, R.J.; Greenaway, A.H. Multiplane imaging and three dimensional nanoscale particle tracking in biological microscopy. Opt. Express 2010, 18, 877–884. [Google Scholar] [CrossRef]
- Hajj, B.; Oudjedi, L.; Fiche, J.B.; Dahan, M.; Nollmann, M. Highly efficient multicolor multifocus microscopy by optimal design of diffraction binary gratings. Sci. Rep. 2017, 7, 5284. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, Z.; Liu, S.; Jiao, Q.; Zhang, J.; Zhang, W.; Pei, J.; Li, H.; Li, Y.; Zou, Y.; et al. Study of the Off-Axis Fresnel Zone Plate of a Microscopic Tomographic Aberration. Sensors 2022, 22, 1113. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, S.; Nie, S.; Chang, C.; Ma, J.; Yuan, C. Multi-plane unequal interval imaging based on polarization multiplexing. Opt. Commun. 2019, 431, 126–130. [Google Scholar] [CrossRef]
- Chen, C.; Song, W.; Chen, J.W.; Wang, J.H.; Chen, Y.H.; Xu, B.; Chen, M.K.; Li, H.; Fang, B.; Chen, J.; et al. Spectral tomographic imaging with aplanatic metalens. Light. Sci. Appl. 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Dalgarno, P.A.; Lee, D.; Yang, Y.; Thomson, R.R.; Greenaway, A.H. Chromatically-corrected, high-efficiency, multi-colour, multi-plane 3D imaging. Opt. Express 2012, 20, 20705–20714. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, L.; Froner, E.; Antolini, R.; Taghizadeh, M.R.; Choudhury, A.; Pavone, F.S. Multiphoton multifocal microscopy exploiting a diffractive optical element. Opt. Lett. 2003, 28, 1918–1920. [Google Scholar] [CrossRef]
- Blanchard, P.M.; Greenaway, A.H. Broadband simultaneous multiplane imaging. Opt. Commun. 2000, 183, 29–36. [Google Scholar] [CrossRef]
- Oudjedi, L.; Fiche, J.B.; Abrahamsson, S.; Mazenq, L.; Lecestre, A.; Calmon, P.F.; Cerf, A.; Nollmann, M. Astigmatic multifocus microscopy enables deep 3D super-resolved imaging. Biomed. Opt. Express 2016, 7, 2163–2173. [Google Scholar] [CrossRef]
- Abrahamsson, S.; McQuilken, M.; Mehta, S.B.; Verma, A.; Larsch, J.; Ilic, R.; Heintzmann, R.; Bargmann, C.I.; Gladfelter, A.S.; Oldenbourg, R. MultiFocus Polarization Microscope (MF-PolScope) for 3D polarization imaging of up to 25 focal planes simultaneously. Opt. Express 2015, 23, 7734–7754. [Google Scholar] [CrossRef]
- Smith, C.S.; Preibisch, S.; Joseph, A.; Abrahamsson, S.; Rieger, B.; Myers, E.; Singer, R.H.; Grunwald, D. Nuclear accessibility of beta-actin mRNA is measured by 3D single-molecule real-time tracking. J. Cell. Biol. 2015, 209, 609–619. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, X.; Wang, Z.W.; Yi, H.; Scherer, N.F.; Katsaggelos, A.K.; Cossairt, O. Snapshot multifocal light field microscopy. Opt. Express 2020, 28, 12108–12120. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, S.; Chen, J.; Hajj, B.; Stallinga, S.; Katsov, A.Y.; Wisniewski, J.; Mizuguchi, G.; Soule, P.; Mueller, F.; Darzacq, C.D.; et al. Fast multicolor 3D imaging using aberration-corrected multifocus microscopy. Nat. Methods 2013, 10, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Liu, J.; Liu, J.; Sun, C.; Li, X.; Bayanheshig; Cui, J. Optical design of a short-wave infrared prism-grating imaging spectrometer. Appl. Opt. 2018, 57, F8–F14. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Liu, J.; Feng, S.; Li, X.; Cui, J. Optical design of a prism-grating-based lenslet array integral field spectrometer. Opt. Express 2018, 26, 19456–19469. [Google Scholar] [CrossRef]







| Parameters | Value |
|---|---|
| Microscope objective focal length | 18 mm |
| Object-side numerical aperture | 0.3 |
| Design wavelength | F, D, C |
| Tomographic grating center period | 2.5 μm |
| Blazed grating period | 2.5 μm |
| The angle of inclination of the main reflector | 21.5° |
| Parameters | Value |
|---|---|
| Focal length of the tube lens | 180 mm |
| Half-field of view | 2° |
| Diameter of the pupil | 40 mm |
| MTF | >0.4@144 lp/mm |
| Detector image element size | 3.45 μm |
| Detector size | 8.4 mm × 6.6 mm |
| Tilt angle between reflectors | ±0.5° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tan, X.; Jiao, Q.; Li, Y.; Liu, S.; Pei, J.; Zhang, J.; Zhang, W.; Xu, L. Design and Study of a Reflector-Separated Light Dispersion-Compensated 3D Microscopy System. Sensors 2023, 23, 4516. https://doi.org/10.3390/s23094516
Li H, Tan X, Jiao Q, Li Y, Liu S, Pei J, Zhang J, Zhang W, Xu L. Design and Study of a Reflector-Separated Light Dispersion-Compensated 3D Microscopy System. Sensors. 2023; 23(9):4516. https://doi.org/10.3390/s23094516
Chicago/Turabian StyleLi, Hui, Xin Tan, Qingbin Jiao, Yuhang Li, Siqi Liu, Jian Pei, Jiahang Zhang, Wei Zhang, and Liang Xu. 2023. "Design and Study of a Reflector-Separated Light Dispersion-Compensated 3D Microscopy System" Sensors 23, no. 9: 4516. https://doi.org/10.3390/s23094516
APA StyleLi, H., Tan, X., Jiao, Q., Li, Y., Liu, S., Pei, J., Zhang, J., Zhang, W., & Xu, L. (2023). Design and Study of a Reflector-Separated Light Dispersion-Compensated 3D Microscopy System. Sensors, 23(9), 4516. https://doi.org/10.3390/s23094516





