Near-Infrared Light-Responsive Hydrogels for Highly Flexible Bionic Photosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Biochemicals
2.2. Preparation of Hydrogel Sensors
2.3. Fabrication of the Photo-Responsive Devices
2.4. Photoelectric and Photothermal Measurements
2.5. Light Stimulation of the Nerve Cells
3. Results
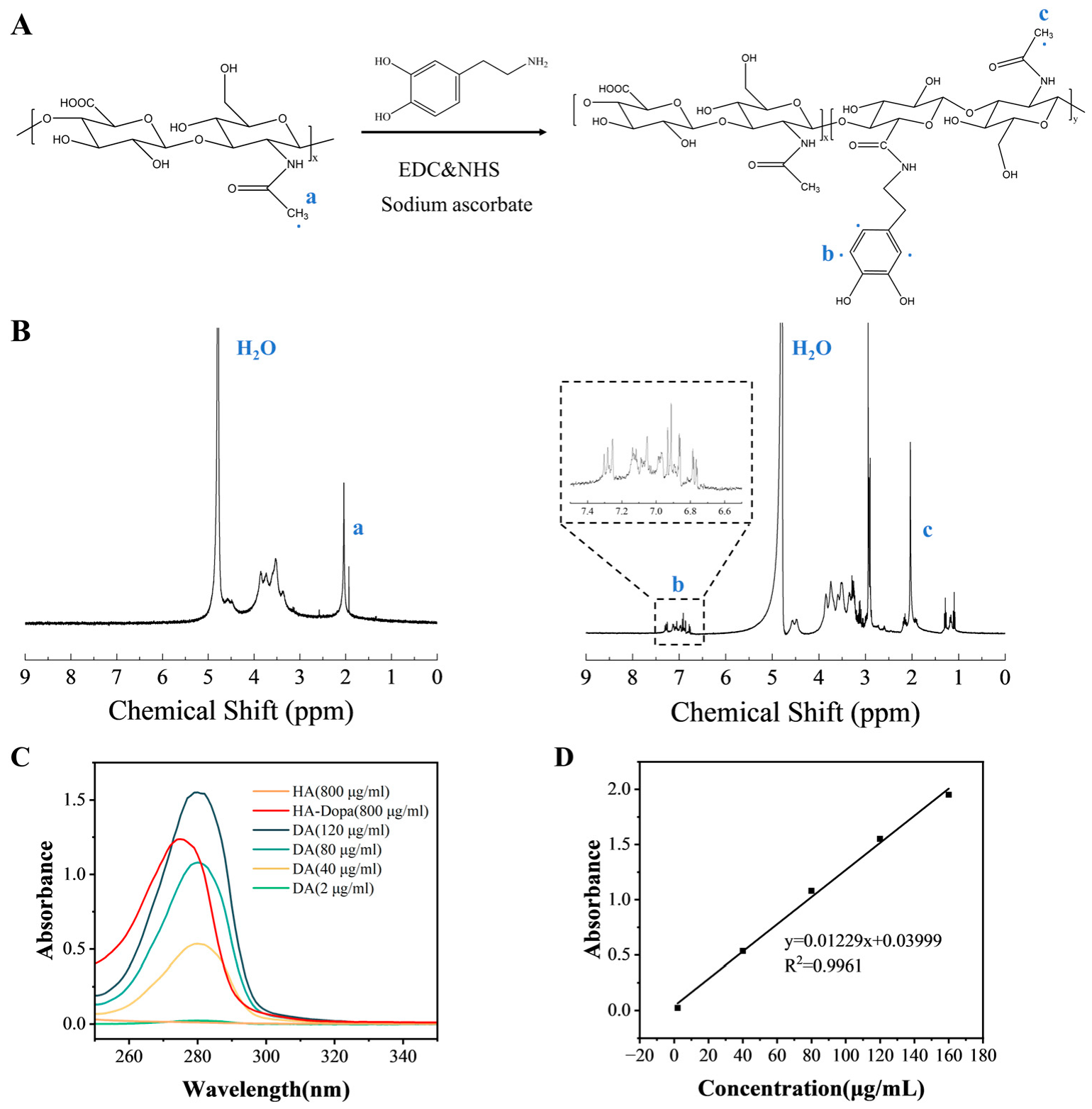
3.1. Design and Preparation of Biomimetic Photosensors
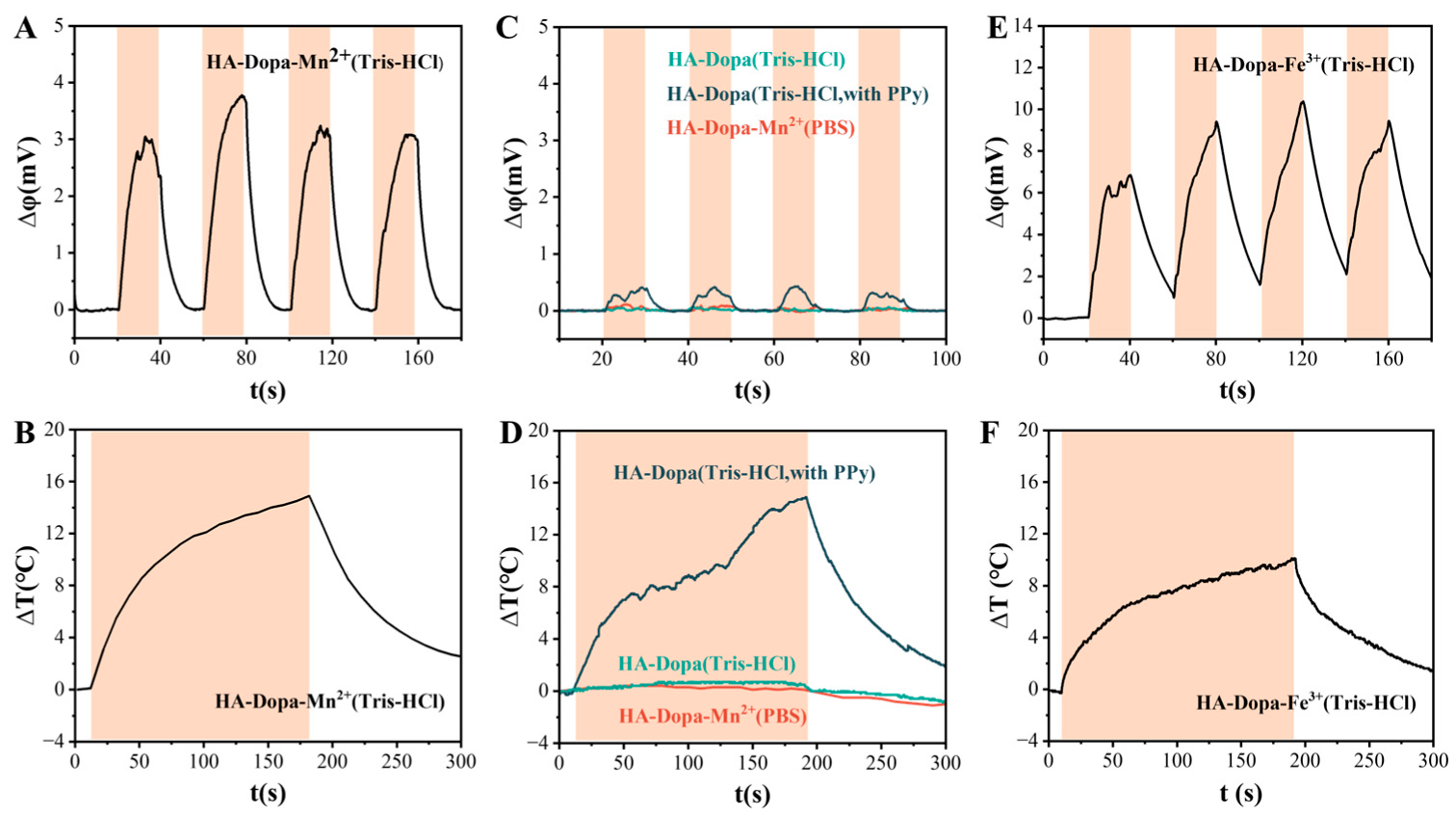
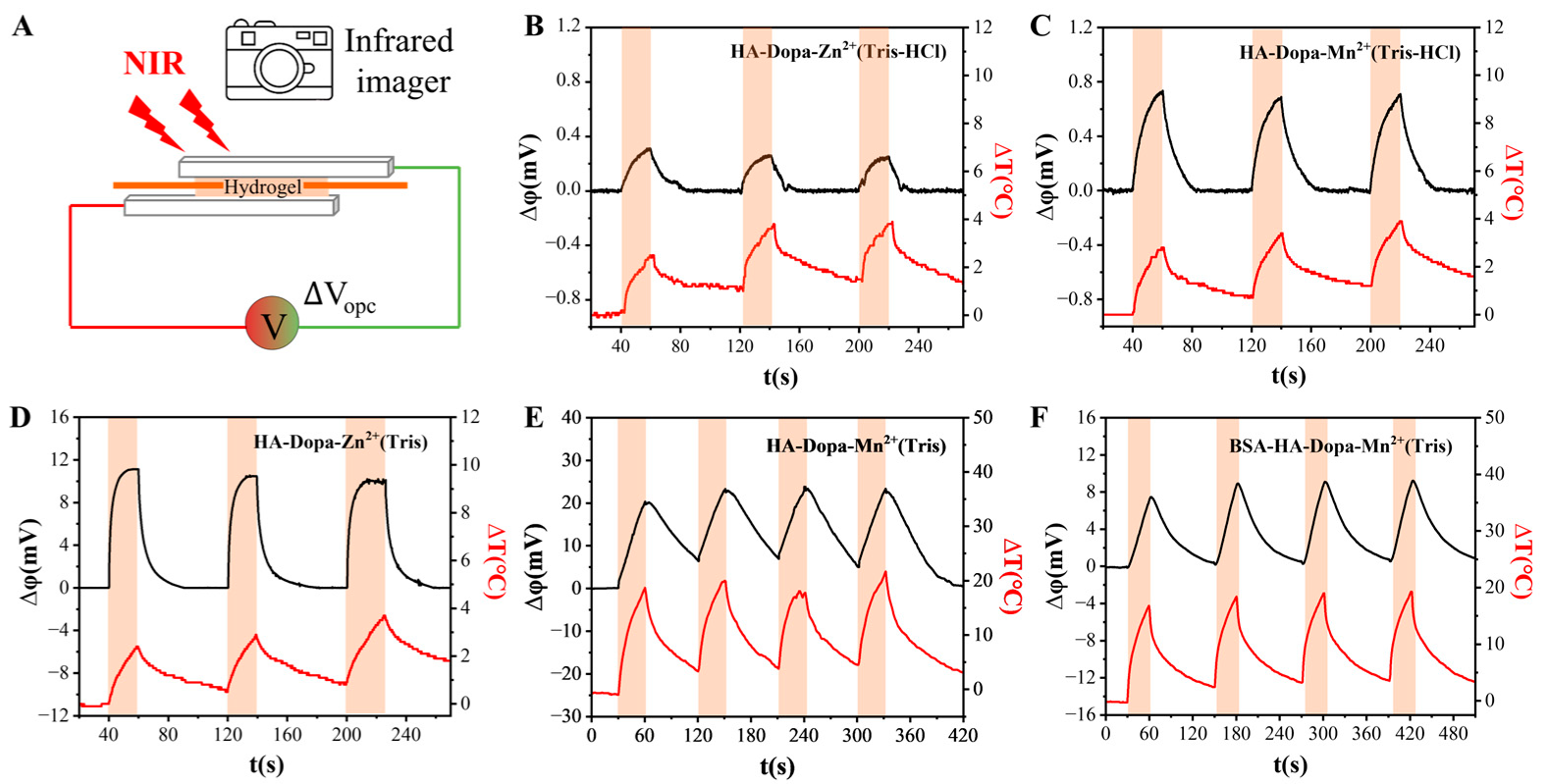
3.2. Photo-Response of the Hydrogel and Mechanism
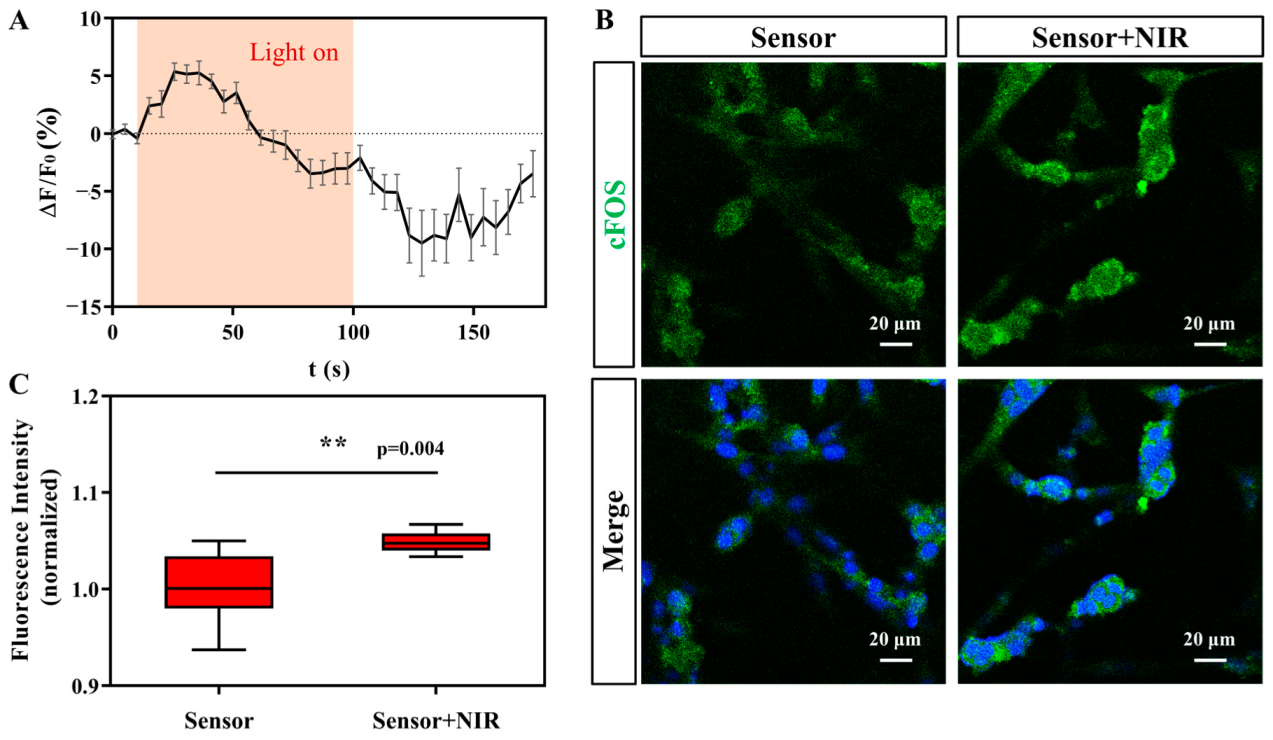
3.3. Signal Communication between the Photosensor and Nerve Cells
4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.-G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-G.; Li, Y.-Q.; Zhu, W.-B.; Huang, P.; Wang, T.-T.; Hu, N.; Fu, S.-Y. A wearable strain sensor based on a carbonized nano-sponge/silicone composite for human motion detection. Nanoscale 2017, 9, 6680–6685. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, D.; Hu, B.; Jiang, J.; Li, M.; Zhao, D.; Zhai, W. Eco-friendly biogenic hydrogel for wearable skin-like iontronics. J. Mater. Chem. A 2021, 9, 4692–4699. [Google Scholar] [CrossRef]
- Zhu, M.; Biswas, S.; Dinulescu, S.I.; Kastor, N.; Hawkes, E.W.; Visell, Y. Soft, Wearable Robotics and Haptics: Technologies, Trends, and Emerging Applications. Proc. IEEE 2022, 110, 246–272. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Jiang, K.; Shen, G. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 2017, 46, 6764–6815. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Y.; Xu, F.; Duan, Y.; Yin, Z. Energy Harvesters for Wearable and Stretchable Electronics: From Flexibility to Stretchability. Adv. Mater. 2016, 28, 9881–9919. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.-Y.; Chen, X. Silk Fibroin for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4250–4265. [Google Scholar] [CrossRef]
- Won, P.; Kim, K.K.; Kim, H.; Park, J.J.; Ha, I.; Shin, J.; Jung, J.; Cho, H.; Kwon, J.; Lee, H.; et al. Transparent Soft Actuators/Sensors and Camouflage Skins for Imperceptible Soft Robotics. Adv. Mater. 2021, 33, 2002397. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Wang, L.; Zhang, Q. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria. Nano Lett. 2020, 20, 5149–5158. [Google Scholar] [CrossRef]
- Dong, M.; Shi, B.; Liu, D.; Liu, J.-H.; Zhao, D.; Yu, Z.-H.; Shen, X.-Q.; Gan, J.-M.; Shi, B.-l.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Li, S.; Cong, Y.; Fu, J. Tissue adhesive hydrogel bioelectronics. J. Mater. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef]
- Kim, C.-C.; Lee, H.-H.; Oh, K.H.; Sun, J.-Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef]
- Dobashi, Y.; Yao, D.; Petel, Y.; Nguyen, T.N.; Sarwar, M.S.; Thabet, Y.; Ng, C.L.W.; Scabeni Glitz, E.; Nguyen, G.T.M.; Plesse, C.; et al. Piezoionic mechanoreceptors: Force-induced current generation in hydrogels. Science 2022, 376, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, M.; Li, Y.; Cao, Y.; Wang, W. Single Molecule Evidence for the Adaptive Binding of DOPA to Different Wet Surfaces. Langmuir 2014, 30, 4358–4366. [Google Scholar] [CrossRef] [PubMed]
- Barkade, S.S.; Pinjari, D.V.; Singh, A.K.; Gogate, P.R.; Naik, J.B.; Sonawane, S.H.; Ashokkumar, M.; Pandit, A.B. Ultrasound Assisted Miniemulsion Polymerization for Preparation of Polypyrrole–Zinc Oxide (PPy/ZnO) Functional Latex for Liquefied Petroleum Gas Sensing. Ind. Eng. Chem. Res. 2013, 52, 7704–7712. [Google Scholar] [CrossRef]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Levitz, J.; Broichhagen, J.; Gaub, B.M.; Visel, M.; Stanley, C.; Aghi, K.; Kim, Y.J.; Cao, K.; et al. Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 2017, 8, 1862. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef]
- Zhao, D.; Martinelli, A.; Willfahrt, A.; Fischer, T.; Bernin, D.; Khan, Z.U.; Shahi, M.; Brill, J.; Jonsson, M.P.; Fabiano, S.; et al. Polymer gels with tunable ionic Seebeck coefficient for ultra-sensitive printed thermopiles. Nat. Commun. 2019, 10, 1093. [Google Scholar] [CrossRef]
- Xu, Z. Mechanics of metal-catecholate complexes: The roles of coordination state and metal types. Sci. Rep. 2013, 3, 2914. [Google Scholar] [CrossRef]
- Alawi, K.; Keeble, J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol. Ther. 2010, 125, 181–195. [Google Scholar] [CrossRef]
- Zhang, Z.; Ferretti, V.; Güntan, İ.; Moro, A.; Steinberg, E.A.; Ye, Z.; Zecharia, A.Y.; Yu, X.; Vyssotski, A.L.; Brickley, S.G.; et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat. Neurosci. 2015, 18, 553–561. [Google Scholar] [CrossRef]
- Cui, Y. Wireless Biological Electronic Sensors. Sensors 2017, 17, 2289. [Google Scholar] [CrossRef]
- Han, M.; Yildiz, E.; Kaleli, H.N.; Karaz, S.; Eren, G.O.; Dogru-Yuksel, I.B.; Senses, E.; Şahin, A.; Nizamoglu, S. Tissue-Like Optoelectronic Neural Interface Enabled by PEDOT:PSS Hydrogel for Cardiac and Neural Stimulation. Adv. Healthc. Mater. 2022, 11, 2102160. [Google Scholar] [CrossRef]
- Akbar, Z.A.; Jeon, J.-W.; Jang, S.-Y. Intrinsically self-healable, stretchable thermoelectric materials with a large ionic Seebeck effect. Energy Environ. Sci. 2020, 13, 2915–2923. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Q.; Xiao, S.; Feng, J.; Zhang, X.; Wang, T. Giant negative thermopower of ionic hydrogel by synergistic coordination and hydration interactions. Sci. Adv. 2021, 7, eabi7233. [Google Scholar] [CrossRef]
- Malci, A.; Lin, X.; Sandoval, R.; Gundelfinger, E.D.; Naumann, M.; Seidenbecher, C.I.; Herrera-Molina, R. Ca2+ signaling in postsynaptic neurons: Neuroplastin-65 regulates the interplay between plasma membrane Ca2+ ATPases and ionotropic glutamate receptors. Cell Calcium 2022, 106, 102623. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Fan, Z.; Xue, B.; Ma, J.; Shen, Q. Near-Infrared Light-Responsive Hydrogels for Highly Flexible Bionic Photosensors. Sensors 2023, 23, 4560. https://doi.org/10.3390/s23094560
Huang R, Fan Z, Xue B, Ma J, Shen Q. Near-Infrared Light-Responsive Hydrogels for Highly Flexible Bionic Photosensors. Sensors. 2023; 23(9):4560. https://doi.org/10.3390/s23094560
Chicago/Turabian StyleHuang, Rui, Zhenhua Fan, Bin Xue, Junpeng Ma, and Qundong Shen. 2023. "Near-Infrared Light-Responsive Hydrogels for Highly Flexible Bionic Photosensors" Sensors 23, no. 9: 4560. https://doi.org/10.3390/s23094560
APA StyleHuang, R., Fan, Z., Xue, B., Ma, J., & Shen, Q. (2023). Near-Infrared Light-Responsive Hydrogels for Highly Flexible Bionic Photosensors. Sensors, 23(9), 4560. https://doi.org/10.3390/s23094560





