Development of Liquid-Phase Plasmonic Sensor Platforms for Prospective Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
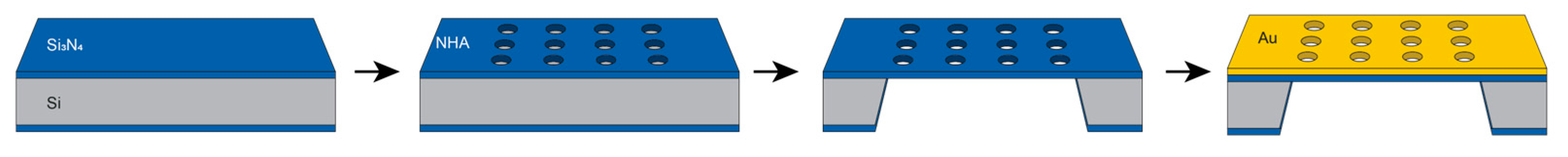
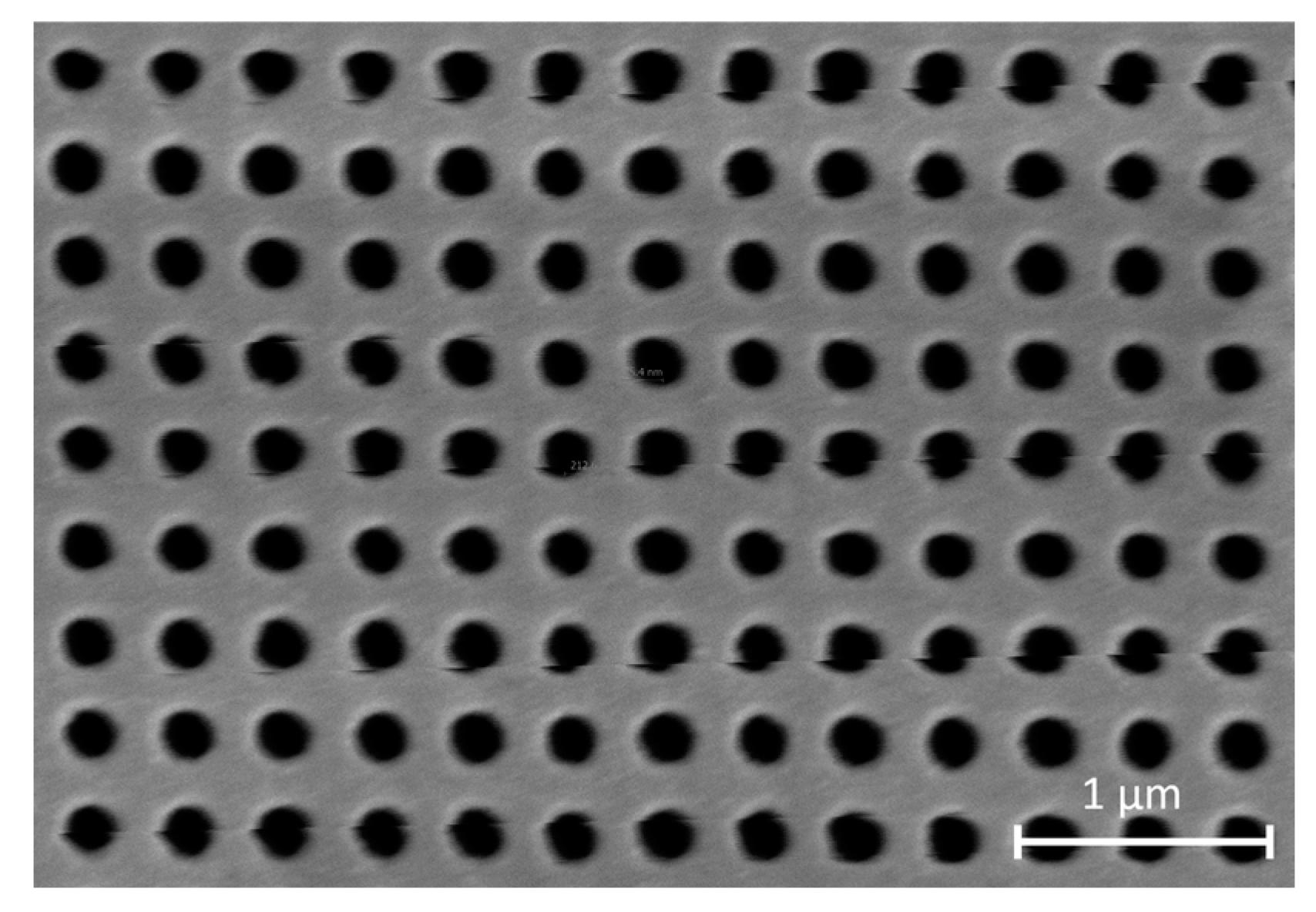
2.1. NHA Design and Fabrication
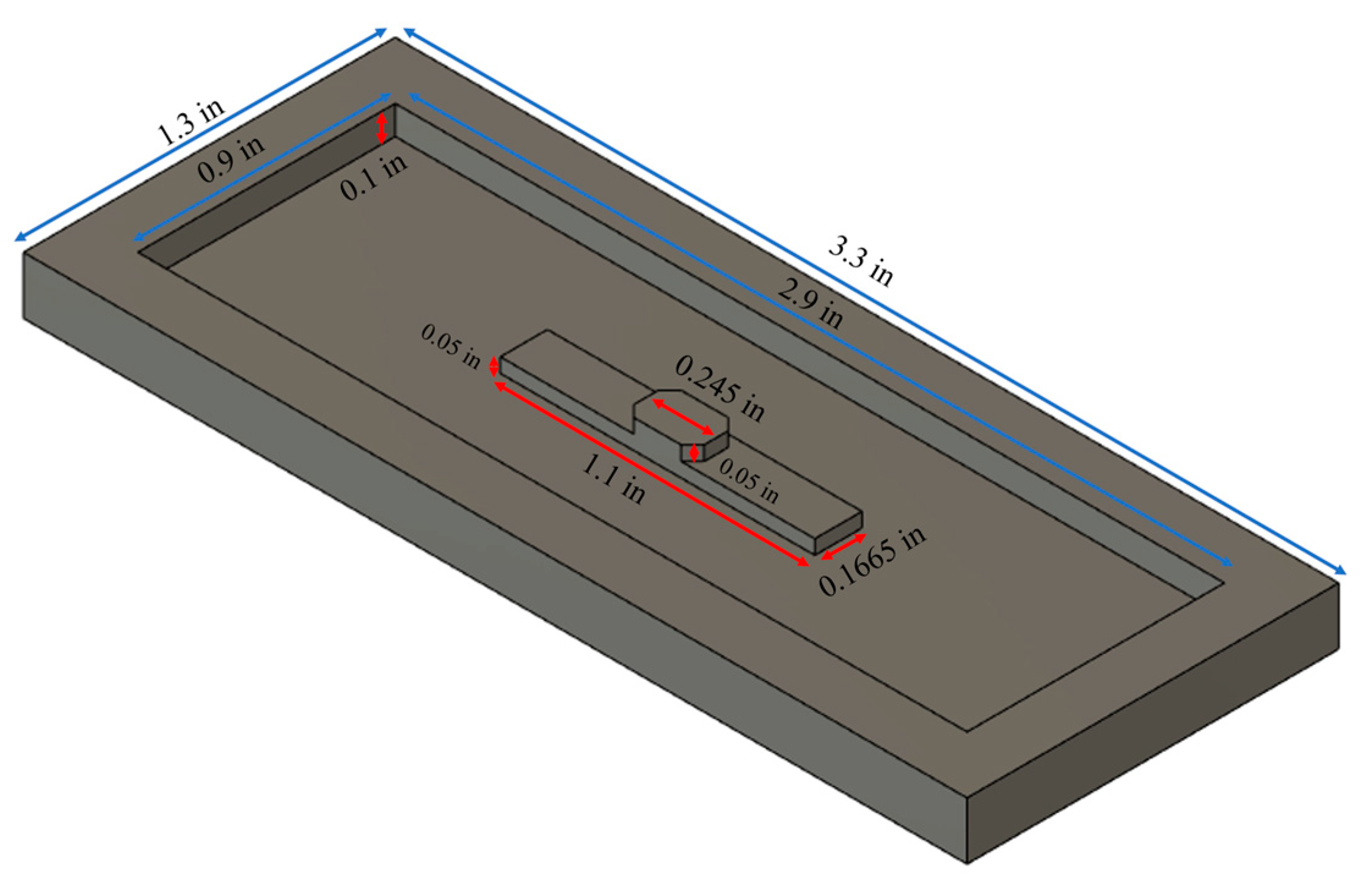
2.2. PDMS Well Fabrication
2.3. Spectrometer Setup and Measurements
3. Results and Discussion
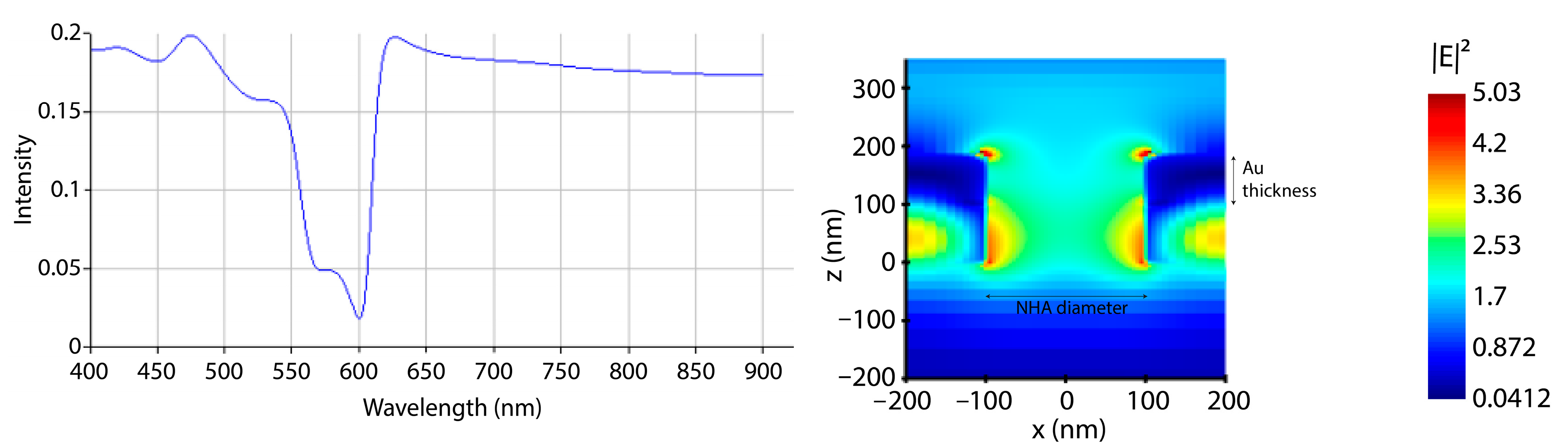
3.1. Au Evaporation Thickness Optimization and NHA Fabrication
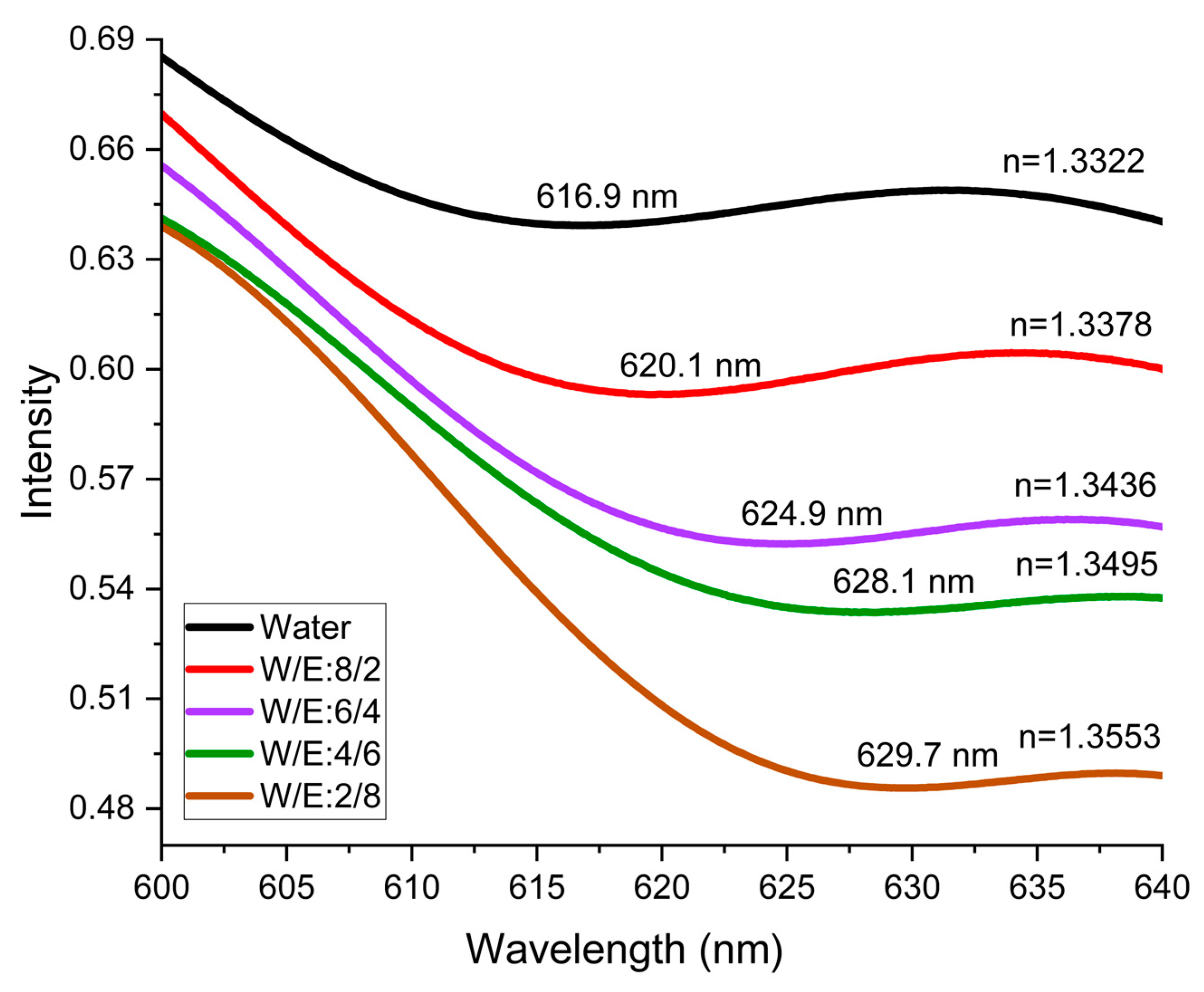
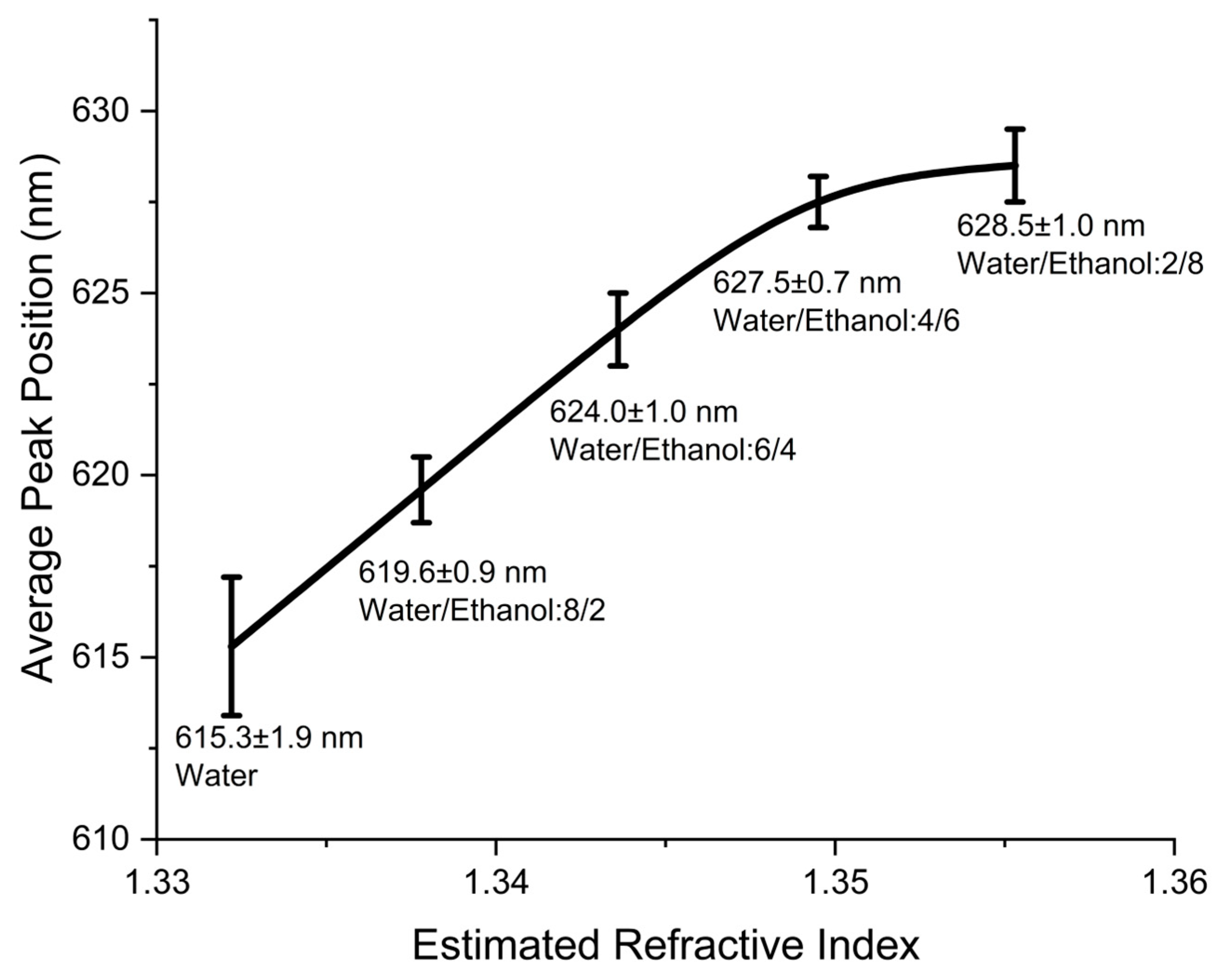
3.2. Control Response Demonstration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strobbia, P.; Languirand, E.; Cullum, B.M. Recent advances in plasmonic nanostructures for sensing: A review. Opt. Eng. 2015, 54, 100902. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Houngkamhang, N.; Vongsakulyanon, A.; Peungthum, P.; Sudprasert, K.; Kitpoka, P.; Kunakorn, M.; Srikhirin, T. ABO blood-typing using an antibody array technique based on surface plasmon resonance imaging. Sensors 2013, 13, 11913–11922. [Google Scholar] [CrossRef] [PubMed]
- Kurt, H.; Pishva, P.; Pehlivan, Z.S.; Arsoy, E.G.; Saleem, Q.; Bayazıt, M.K.; Yüce, M. Nanoplasmonic biosensors: Theory, structure, design, and review of recent applications. Anal. Chim. Acta 2021, 1185, 338842. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.S.; Raghuwanshi, S.K.; Shadab, A.; Ansari, M.T.I.; Tiwari, U.K.; Kumar, S. SPR based biosensing chip for COVID-19 diagnosis-A review. IEEE Sens. J. 2022, 22, 13800–13810. [Google Scholar] [CrossRef] [PubMed]
- Akib, T.B.A.; Mou, S.F.; Rahman, M.M.; Rana, M.M.; Islam, M.R.; Mehedi, I.M.; Kouzani, A.Z. Design and numerical analysis of a graphene-coated SPR biosensor for rapid detection of the novel coronavirus. Sensors 2021, 21, 3491. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.A.; Chowdhury, S.S.; Kabir, E. Numerical analysis of a highly sensitive surface plasmon resonance sensor for SARS-CoV-2 detection. Plasmonics 2021, 16, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Murray, J.; Haverstick, J.; Tripp, R.A.; Zhao, Y. Silver nanotriangle array based LSPR sensor for rapid coronavirus detection. Sens. Actuators B Chem. 2022, 359, 131604. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, M. The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles. Biosensors 2023, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wong, T.I.; Sun, L.L.; Deng, J. Point-of-Care Device with Plasmonic Gold Nanoarray Sensing Chip for Biomarker Detections. In Next Generation Point-of-care Biomedical Sensors Technologies for Cancer Diagnosis; Springer: Singapore, 2017; pp. 323–343. [Google Scholar]
- Lee, H.; Xu, L.; Koh, D.; Nyayapathi, N.; Oh, K.W. Various on-chip sensors with microfluidics for biological applications. Sensors 2014, 14, 17008–17036. [Google Scholar] [CrossRef]
- Wang, D.S.; Fan, S.K. Microfluidic surface plasmon resonance sensors: From principles to point-of-care applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, X.; Liu, T.; Gao, Y.; Huang, Y.; Wen, W. Real-time concentration monitoring in microfluidic system via plasmonic nanocrescent arrays. Biosens. Bioelectron. 2016, 77, 385–392. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, P.F.; Lin HT, H.; Huang, N.T. A Localized surface plasmon resonance (LSPR) sensor integrated automated microfluidic system for multiplex inflammatory biomarker detection. Analyst 2020, 145, 7654–7661. [Google Scholar] [CrossRef]
- Zhao, Y.; Mukherjee, K.; Benkstein, K.D.; Sun, L.; Steffens, K.L.; Montgomery, C.B.; Zaghloul, M.E. Miniaturized nanohole array based plasmonic sensor for the detection of acetone and ethanol with insights into the kinetics of adsorptive plasmonic sensing. Nanoscale 2019, 11, 11922–11932. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.E.; Etezadi, D.; Galarreta, B.C.; Busson, M.P.; Eksioglu, Y.; Altug, H. Plasmonic nanohole arrays on a robust hybrid substrate for highly sensitive label-free biosensing. ACS Photonics 2015, 2, 1167–1174. [Google Scholar] [CrossRef]
- Sun, L.; Conrad, D.; Hall, D.A.; Benkstein, K.D.; Semancik, S.; Zaghloul, M.E. Plasmonic sensing studies of a gas-phase cystic fibrosis marker in moisture laden air. Sensors 2021, 21, 3776. [Google Scholar] [CrossRef] [PubMed]
- Vala, M.; Ertsgaard, C.T.; Wittenberg, N.J.; Oh, S.H. Plasmonic sensing on symmetric nanohole arrays supporting high-Q hybrid modes and reflection geometry. ACS Sens. 2019, 4, 3265–3274. [Google Scholar] [CrossRef]
- Arif, M.F.H.; Biddut, M.J.H.; Babu, M.S.I.; Rahman, H.M.; Rahman, M.M.; Jahan, B.; Chaity, M.S.; Khaled, S.M. Photonic crystal fiber based sensor for detecting binary liquid mixture. Opt. Photonics J. 2017, 7, 221–234. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayin, S.; Zhou, Y.; Wang, S.; Acosta Rodriguez, A.; Zaghloul, M. Development of Liquid-Phase Plasmonic Sensor Platforms for Prospective Biomedical Applications. Sensors 2024, 24, 186. https://doi.org/10.3390/s24010186
Sayin S, Zhou Y, Wang S, Acosta Rodriguez A, Zaghloul M. Development of Liquid-Phase Plasmonic Sensor Platforms for Prospective Biomedical Applications. Sensors. 2024; 24(1):186. https://doi.org/10.3390/s24010186
Chicago/Turabian StyleSayin, Sezin, You Zhou, Sheng Wang, Andres Acosta Rodriguez, and Mona Zaghloul. 2024. "Development of Liquid-Phase Plasmonic Sensor Platforms for Prospective Biomedical Applications" Sensors 24, no. 1: 186. https://doi.org/10.3390/s24010186
APA StyleSayin, S., Zhou, Y., Wang, S., Acosta Rodriguez, A., & Zaghloul, M. (2024). Development of Liquid-Phase Plasmonic Sensor Platforms for Prospective Biomedical Applications. Sensors, 24(1), 186. https://doi.org/10.3390/s24010186




